Abstract
The objectives of this study were to explore the relation between body mass index (BMI) and prevalence of diabetes mellitus, hypertension and dyslipidaemia; examine BMI distributions among patients with these conditions; and compare results from two national surveys. The Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) 2004 screening questionnaire (mailed survey) and the National Health and Nutrition Examination Surveys (NHANES) 1999–2002 (interview, clinical and laboratory data) were conducted in nationally representative samples ≥ 18 years old. Responses were received from 127,420 of 200,000 households (64%, representing 211,097 adults) for SHIELD, and 4257 participants for NHANES. Prevalence of diabetes mellitus, hypertension and dyslipidaemia was estimated within BMI categories, as was distribution of BMI levels among individuals with these diseases. Mean BMI was 27.8 kg/m2 for SHIELD and 27.9 kg/m2 for NHANES. Increased BMI was associated with increased prevalence of diabetes mellitus, hypertension and dyslipidaemia in both studies (p < 0.001). For each condition, more than 75% of patients had BMI ≥ 25 kg/m2. Estimated prevalence of diabetes mellitus and hypertension was similar in both studies, while dyslipidaemia was substantially higher in NHANES than SHIELD. In both studies, prevalence of diabetes mellitus, hypertension and dyslipidaemia occurred across all ranges of BMI, but increased with higher BMI. However, not all overweight or obese patients had these metabolic diseases and not all with these conditions were overweight or obese. Except for dyslipidaemia prevalence, SHIELD was comparable with NHANES. Consumer panel surveys may be an alternative method to collect data on the relationship of BMI and metabolic diseases.
What's known
An increase in body fat is generally associated with increased risk of metabolic diseases such as type 2 diabetes mellitus, hypertension and dyslipidaemia. However, not all overweight or obese patients have metabolic diseases, and vice versa. While these concepts may be well-accepted, and assumed to be readily accessible in the literature, the authors are unaware of any single report presenting comprehensive data regarding the relationship between BMI and metabolic diseases.
What's new
Defining the relationship between body weight and metabolic diseases is critical toward better understanding of the underlying pathophysiological processes leading to these diseases. Data from the two national surveys reported here support the common clinical observation that patients with higher BMI are at higher risk for having diabetes mellitus, hypertension and dyslipidaemia. They also confirm the converse – the majority of patients with these metabolic diseases are either overweight or obese.
Introduction
An increase in body fat is generally associated with an increase in risk of metabolic diseases such as type 2 diabetes mellitus, hypertension and dyslipidaemia (1). Body mass index (BMI) criteria are currently the primary focus in obesity treatment recommendations, with different treatment cutoff points based upon the presence or absence of obesity-related comorbid disease (Table 1). In addition, many patients with these metabolic diseases are either overweight or obese. While these simple clinical concepts may be well-accepted among many clinicians and researchers, and assumed to be readily accessible in the medical literature, the authors are unaware of any previous reports in which data regarding the important relationship between BMI and metabolic disease are summarised in a comprehensive manner. Defining the relationship between body weight and metabolic disease is critical toward a better understanding of the underlying pathophysiological processes leading to excessive fat-related metabolic disease.
Table 1.
Adaptation of the 1998 National Heart, Lung and Blood Institute – National Institutes of Health Clinical Guidelines on the identification, evaluation and treatment of overweight and obesity in adults
| Risk category | BMI at which no intervention recommended (kg/m2) | BMI to initiate low-calorie diet, physical activity and behavioural therapy (kg/m2) | BMI to consider drug treatment* (kg/m2) | BMI to consider surgery† (kg/m2) |
|---|---|---|---|---|
| With comorbidities‡ | 18.5–24.9 | BMI ≥ 25 | BMI ≥ 27 | ≥ 35 |
| Without comorbidities | 18.5–24.9 | BMI ≥ 25§ | BMI ≥ 30 | ≥ 40 |
Reprinted with permission from (24).
Drug treatment can be considered if after 6 months of lifestyle therapy, there is inadequate weight loss. Drugs should be used only as part of a programme that includes diet, physical activity and behaviour therapy.
Although not mandatory, surgery for obesity is considered a treatment option, if medically appropriate, and is reserved for patients in whom efforts at medical therapy have failed and who are suffering from the complications of extreme obesity.
Comorbidities include two or more of obesity-related hypertension, dyslipidaemia, CHD, type 2 diabetes mellitus and obstructive sleep apnoea.
If no comorbidities are present, and BMI ≥ 25 but < 30 kg/m2, low-calorie diet, physical activity and behavioural therapy is recommended only if patient ‘wants’ to lose weight. Otherwise, the patient is advised to maintain weight and address other risk factors. BMI, body mass index.
Health information regarding such relationships is often obtained through the use of surveys. Population surveys are a well-recognised, and much utilised method to assess the prevalence of diseases as well as obtain other health-related information (2–4). One of the more recognised survey measures are the National Health and Nutrition Examination Surveys (NHANES) (4, 5), which have incorporated subjective survey data obtained from interviews, along with additional data derived from objective clinical assessment and laboratory data. Patient-reported surveys have the advantage of obtaining health data from large sample sizes and providing access to data that may otherwise be difficult to obtain.
In this study, data from two national surveys were evaluated to determine the relationship between different BMI categories and the prevalence of diabetes mellitus, hypertension and dyslipidaemia. The Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) was a self-reported survey (with no clinical or laboratory evaluation) conducted in 2004 that assessed the association of different BMI categories with diabetes mellitus, hypertension and dyslipidaemia. The comparator survey was the NHANES 1999–2002, which obtained data through interviewer-administered surveys, as well as clinical evaluations and laboratory assessments (4, 5).
The objectives of this study were to: (i) explore the relation between BMI level and prevalence of diabetes mellitus, hypertension and dyslipidaemia; (ii) examine the distribution of BMI levels among people with these conditions; and (iii) compare the results on these measures between these two national surveys (SHIELD and NHANES).
Methods
SHIELD survey
The SHIELD screening survey was mailed in April 2004 to a stratified random sample of 200,000 US households who were part of the Taylor Nelson Sofres National Family Opinion, Inc. (TNS NFO, Greenwich, CT, USA) household survey panel (no monetary or other inducement was offered for completing and returning the screening questionnaire). TNS NFO is a market research firm that has collected a survey panel of more than 600,000 households, whose only requirements for participation include being ≥18 years of age and having a telephone and mailing address. Stratified random samples of households (selected to be representative of the US population based on US census data for age, gender, income, household size, urban density and census region) are invited to enrol in the panel, and demographic information is obtained from those who enrol (and updated every 2 years). This panel methodology both minimises sample bias because of high response rates, and allows us to understand the demographics of survey non-responders (which random population sampling would not allow). Households who agree to participate are invited to take part in periodic surveys.
Because prospective stratification was performed to ensure that the survey panel represented the US population in terms of geographic residence, age of the head of household, and household size and income, the SHIELD screening survey could provide self-reported prevalence estimates at a national level. Previous NFO panel surveys have been used to calculate the population prevalence of conditions such as migraine (6) and bipolar disorder (2).
Once received, the screening survey was completed by the head of household, who answered for up to four adult (18 years of age or older) household members. This survey consisted of a 12-item questionnaire developed by a diversified panel of experts (the SHIELD Survey Group). Demographic information was requested as well as other data about the respondent and other adult family members, such as whether a healthcare professional had ever told them they had diabetes mellitus, high blood pressure or problems with cholesterol. Judging that many, if not most respondents to a self-administered questionnaire may be unable to recall their actual fasting plasma glucose (FPG) results, blood pressure or cholesterol levels, respondents were asked if they had ever been diagnosed as having, or were currently taking prescription medications for, diabetes mellitus, high blood pressure or cholesterol problems. Respondents were also asked to provide their weight and height, which were used to calculate BMI.
Once the screening questionnaires were completed and returned, samples of respondents with diabetes mellitus or risk factors for diabetes mellitus were then sent a longer, more detailed survey in August 2004 (with annual follow-up assessments planned over the subsequent 4 years) to determine the longitudinal relationship of demographics, comorbid conditions, health status, knowledge, attitudes, current behaviours and treatments toward the progression or onset of diabetes mellitus. The analysis described in this study focused only on the SHIELD data obtained from the 2004 screening survey.
NHANES 1999–2002 survey
Data from the SHIELD survey were compared with data derived from NHANES 1999–2002, which represents the fourth round of this national survey. NHANES is an annual survey that produces nationally representative data about the health and nutritional status of the US civilian non-institutionalised population. In NHANES, potential participants are selected through a complex statistical process using the most current US Census information. After agreeing to take part in the survey, and after it has been determined that they qualify, NHANES participants undergo a 1-h ‘in-home’ interview consisting of subjective survey questions regarding health, disease history and diet. Afterward, participants go to a local Mobile Exam Center, where objective health measurements, physical examinations and laboratory tests are performed based upon age and gender (7). Thus, NHANES includes both self-reported diagnosed conditions as well as clinical evaluation and laboratory testing to confirm diagnoses and to identify undiagnosed conditions. For example, the prevalence of diabetes mellitus can be calculated based on both the interview data (e.g. Have you ever been told by a physician that you have diabetes?) as well as laboratory glucose values. Details of the NHANES data collection are disseminated by the National Center for Health Statistics (NCHS) (4, 5). Because the NHANES data include laboratory values along with diagnoses and treatments, it can be used with a weighting system to estimate actual national prevalence of various conditions.
In this study, NHANES data on adults 18 years of age or older were analysed to determine the prevalence (self-reported plus laboratory test confirmed) of diabetes mellitus, hypertension and dyslipidaemia across different BMI ranges, as well as the distribution of BMI among those with these metabolic diseases.
Definitions used to identify conditions
For SHIELD, the diagnoses of diabetes mellitus (type 1 or 2), hypertension and dyslipidaemia were identified solely on the basis of self-reporting by respondents who recorded on the screener that a healthcare professional had diagnosed the condition (i.e. conditions that you/other adult household members have ever been told you have by a doctor or nurse). For comparison with NHANES, which does not distinguish between type 1 and 2 diabetes mellitus, the total self-reported diabetes mellitus prevalence is reported here. BMI was calculated as weight in kilograms divided by the square of height in metres, again, using only self-reported height and weight.
For NHANES, definitions were intended to be consistent with those used in prior analyses (8, 9). In building each analysis variable, cases with missing data on any of the components that were used to create the analysis variable were excluded. The NHANES definitions utilised in this study are listed below.
Body mass index
Calculated from height and weight (kg/m2) measured using standardised examination protocols (7).
Diabetes mellitus
Defined to include both previously diagnosed and undiagnosed diabetes mellitus (type 1 or 2). Diagnosed diabetes mellitus was based on self-reported responses (i.e. respondent answered yes to ‘Has a doctor ever told you that you have diabetes?’). Undiagnosed diabetes mellitus was defined using the American Diabetes Association criterion of FPG > 125 mg/dl (7.0 mmol/l) (10).
Hypertension
Defined to include patients with history of taking antihypertensive medication, or elevated blood pressure (using Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure criteria of systolic pressure of at least 140 mmHg or diastolic pressure of at least 90 mmHg) (11). Blood pressure measures in NHANES were based on the average of blood pressure measurements taken; in 1999–2002, 78% of NHANES participants had at least three blood pressure readings taken (the remaining 22% had fewer than three readings available).
Dyslipidaemia
Defined to include any one of the following: total cholesterol (TC) ≥ 240 mg/dl (6.22 mmol/l), triglycerides (TG) > 200 mg/dl (2.26 mmol/l), low-density lipoprotein cholesterol (LDL-C) ≥ 160 mg/dl (4.14 mmol/l) or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dl (1.04 mmol/l), as well as those ever told by a doctor or other healthcare professional that their blood cholesterol level was high. Other lipid parameters, such as non-HDL-C, apolipoprotein B, lipoprotein (a), abnormalities in lipoprotein particle size and subclass distribution, etc., were not reported in NHANES.
No consideration of coronary heart disease (CHD) risk factors was included in the definition of dyslipidaemia with NHANES because data on several specific risk factors was not available from SHIELD data. Other categories, such as prehypertension or hyperinsulinaemia, were also not examined because these conditions were not collected in the SHIELD survey.
Statistical analysis
The prospective stratification sampling used in SHIELD allowed performance of postweighting of the data to correct for over- or under-sampling of some demographic groups and to ensure that the respondents represented the US Census population (12) in terms of geographic residence, age of the head of household, and household size and income. No attempt was made to remove outliers from the self-reported data. Similarly, NHANES prevalence estimates were calculated using NCHS sampling weights (based on age, income and race/ethnicity) to represent the US adult population. The entire dataset time span (1999–2002) was used to create national prevalence estimates because estimates based on individual survey phases may vary. Neither survey was postweighted for parameters other than demographic data.
Using the weighted responses for this analysis, we constructed a matrix to compare BMI levels with the prevalence of diabetes mellitus, hypertension and dyslipidaemia, as well as to identify the population distribution of BMI levels among individuals with these metabolic diseases. BMI was categorised using cut-points derived from the 1998 National Heart, Lung and Blood Institute Guidelines (13) with respondents allocated into one of seven categories (< 18.5, 18.5–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35.0–39.9 and ≥ 40 kg/m2). We then compared the population distributions of BMI derived from SHIELD and NHANES.
For each survey, tests for linear trend across BMI categories were performed using a series of logistic regression analyses, with each condition as the dependent variable and the midpoint of each BMI category as the only independent variable. These analyses tested whether prevalence of each condition increased as BMI increased, by examining the Wald statistic for the BMI coefficient in each regression model (p-values <0.05 were considered statistically significant).
For each condition, prevalence estimates from SHIELD and NHANES within each BMI category were compared using chi-squared tests, with p-values <0.05 considered significant. Analyses of the SHIELD data were conducted using SPSS for Windows (release 13.0.1; SPSS Inc., Chicago, IL) and WesVar (version 4.2; Westat, Rockville, MD). Analyses in NHANES were performed using SUDAAN (SUDAAN: Software for the Statistical Analysis of Correlated Data, release 9.0; Research Triangle Institute, Research Triangle Park, NC). The WesVar and SUDAAN software programs account for the complex stratification procedures and clustering (e.g. at the household level) used in these surveys, to ensure proper sample weighting and estimation of variance. Variance estimation methods were used to calculate the standard errors, accounting for both the complex sample design and, in NHANES, the use of both interview and morning examination sample data in combination (4, 5).
Results
The SHIELD screener questionnaire was sent to 200,000 households; 127,420 were returned with usable surveys, yielding a response rate of 64% (published reviews have found mean response rates of approximately 67–68% for mailed surveys) (14, 15). Each questionnaire was completed for up to four adults per household; therefore, the returned screener questionnaires contained data on 211,097 adults. The response rate for completed examinations in NHANES 1999–2002 was 76.3% (9282/12,160). A subsample of NHANES respondents (n = 4257) were selected to fast eight or more hours (up to 24) for laboratory testing (e.g. FPG).
Comparison of SHIELD and NHANES data
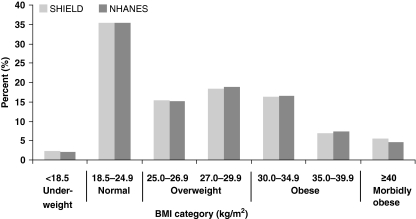
As Figure 1 shows, the patients represented by these surveys had similar distributions of BMI, with mean (± standard deviation) of 27.8 kg/m2 (± 6.8) (median = 26.6 kg/m2) for SHIELD and 27.9 kg/m2 (± 6.2) (median = 26.8 kg/m2) for NHANES. The estimated prevalence of diabetes mellitus and hypertension within each BMI category was similar in the SHIELD and NHANES participants (Figures 2 and 3), although the prevalence of hypertension was slightly higher in NHANES than in SHIELD. All comparisons between SHIELD and NHANES estimates within BMI category were statistically significant (p < 0.001 in chi-squared tests), largely because of the large sample sizes involved, but the practical significance of these differences for diabetes mellitus and hypertension is minimal. In contrast, the prevalence of dyslipidaemia was substantially higher across all BMI levels in NHANES compared with SHIELD (Figure 4).
Figure 1.
Distributions of body mass index (BMI) in Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES)
Figure 2.
Prevalence of diabetes mellitus (types 1 and 2) by body mass index (BMI) level*. *p < 0.001 in tests of linear trend across BMI groups within each study [Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES)]; p < 0.001 in tests comparing SHIELD with NHANES estimates (for each BMI category)
Figure 3.
Prevalence of hypertension by body mass index (BMI) level*. *p < 0.001 in tests of linear trend across BMI groups within each study [Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES)]; p < 0.001 in tests comparing SHIELD with NHANES estimates (for each BMI category)
Figure 4.
Prevalence of dyslipidaemia by body mass index (BMI) level*. *p < 0.001 in tests of linear trend across BMI groups within each study [Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES)]; p < 0.001 in tests comparing SHIELD with NHANES estimates (for each BMI category)
Relationship of BMI level to prevalence of diabetes mellitus, hypertension and dyslipidaemia
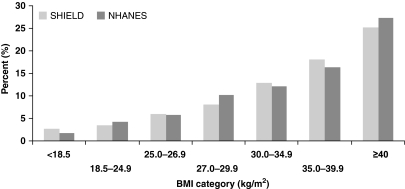
Both surveys showed that an increase in BMI is generally associated with a significant increase in prevalence of diabetes mellitus, hypertension and dyslipidaemia (p < 0.001 for all in tests for linear trend across BMI groups). However, these metabolic diseases were present at all levels of BMI (Figures 2–4). The prevalence of diabetes mellitus and hypertension increased in an observable, linear fashion as BMI levels increased. The prevalence of diabetes mellitus was highest among morbidly obese individuals (BMI ≥ 40 kg/m2), with rates of 25% (SHIELD) and 27% (NHANES). The same was true for hypertension, with highest prevalence among morbidly obese individuals (49% in SHIELD; 51% in NHANES).
Somewhat in contrast, while there was a significant increasing trend towards higher prevalence of dyslipidaemia as BMI increased, once BMI reached 30 kg/m2 or more, this increasing trend was blunted and the likelihood of participants having self-reported or laboratory-confirmed dyslipidaemia had less direct relationship to increasing BMI category than was seen for diabetes mellitus or hypertension (Figure 4). At the very highest levels of BMI, the prevalence of dyslipidaemia levelled off in the obese and morbidly obese groups in the SHIELD data (35–36%) and actually declined in the NHANES data (68–63%).
These increasing trends meant that conditions also tended to co-occur at higher BMI levels. For example, in NHANES 1999–2002 data, approximately 80% of those with BMI ≥ 35 kg/m2 had one or more of these metabolic diseases, compared with only 36% of those with BMI < 18.5 kg/m2 having one or more of these metabolic diseases.
Distribution of BMI levels among those with diabetes mellitus, hypertension and dyslipidaemia
The above data reflect the prevalence of metabolic disease with increasing BMI. But patients were also evaluated with regard to the relative distribution of BMI levels among those who had metabolic diseases: specifically diabetes mellitus, hypertension and dyslipidaemia. In general, the BMI ranges of patients with diabetes mellitus, hypertension and dyslipidaemia were similar between SHIELD and NHANES.
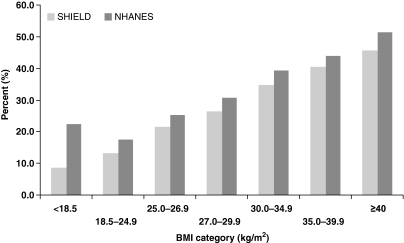
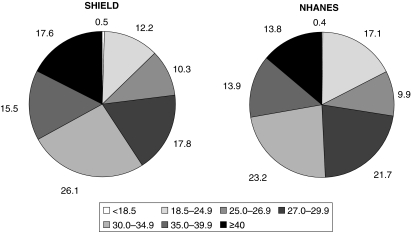
Figure 5 (Table 2) shows the BMI distributions observed in SHIELD and NHANES among adults with diabetes mellitus. The majority of adults with diabetes mellitus were obese (BMI ≥ 30 kg/m2; 59% for SHIELD and 51% for NHANES). When BMI ≥ 25 kg/m2 (the cut-off point for ‘overweight’) was applied, this percentage increased to 87% for SHIELD and 82% for NHANES, meaning that 13% of SHIELD and 18% NHANES diabetes mellitus patients were not overweight or obese.
Figure 5.
Relative distributions of body mass index (BMI) in Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES) respondents with diabetes mellitus (types 1 and 2)
Table 2.
Relative distributions of body mass index (BMI) in SHIELD and NHANES respondents with diabetes mellitus (types 1 and 2), hypertension and dyslipidaemia
| Diabetes mellitus (types 1 and 2) | Hypertension | Dyslipidaemia | ||||
|---|---|---|---|---|---|---|
| BMI category (kg/m2) | SHIELD | NHANES | SHIELD | NHANES | SHIELD | NHANES |
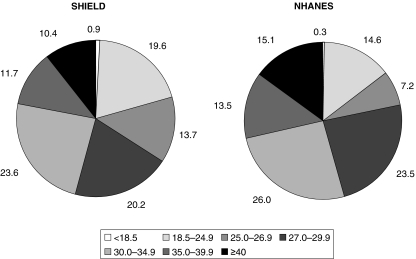
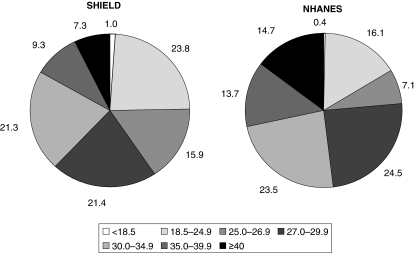
| < 18.5 | 0.5 | 0.4 | 0.9 | 0.3 | 1.0 | 0.4 |
| 18.5–24.9 | 12.2 | 17.1 | 19.6 | 14.6 | 23.8 | 16.1 |
| 25.0–26.9 | 10.3 | 9.9 | 13.7 | 7.2 | 15.9 | 7.1 |
| 27.0–29.9 | 17.8 | 21.7 | 20.2 | 23.5 | 21.4 | 24.5 |
| 30.0–34.9 | 26.1 | 23.2 | 23.6 | 26.0 | 21.3 | 23.5 |
| 35.0–39.9 | 15.5 | 13.9 | 11.7 | 13.5 | 9.3 | 13.7 |
| ≥ 40.0 | 17.6 | 13.8 | 10.4 | 15.1 | 7.3 | 14.7 |
NHANES, National Health and Nutrition Examination Surveys; SHIELD, Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes.
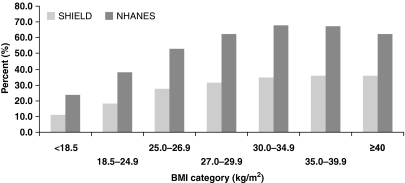
Similarly, the prevalence of excessive body weight was also high in patients with hypertension (Figure 6, Table 2). Approximately 46% of SHIELD and 55% of NHANES hypertensive patients were obese, and 80% of SHIELD and 85% of NHANES hypertensive patients were overweight or obese. Twenty per cent of SHIELD and 15% of NHANES hypertensive patients had a BMI < 25 kg/m2, and therefore would be considered to be normal weight or underweight (1, 13).
Figure 6.
Relative distributions of body mass index (BMI) in Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES) respondents with hypertension
With regard to dyslipidaemia (Figure 7, Table 2), again, a high prevalence of obesity was found in patients who had at least one of four criteria for the diagnosis of ‘dyslipidaemia’ (as described above in ‘Definitions used to identify conditions’); 38% of SHIELD and 52% of NHANES dyslipidaemic patients were obese, while 75% of SHIELD and 84% of NHANES dyslipidaemic patients were overweight or obese. However, 25% of SHIELD and 16% of NHANES dyslipidaemic patients were not overweight.
Figure 7.
Relative distributions of body mass index (BMI) in Study to Help Improve Early evaluation and management of risk factors Leading to Diabetes (SHIELD) and National Health and Nutrition Examination Surveys (NHANES) respondents with dyslipidaemia
Comment
Data from both the SHIELD and NHANES surveys reported here reflect and support the common clinical observation that patients with higher BMI are at higher risk for having diabetes mellitus, hypertension and dyslipidaemia. It also confirms the converse – that the majority of patients with these metabolic diseases are either overweight or obese. These results provide nationally representative data regarding the important relationship between BMI and these metabolic diseases. Finally, this analysis suggests that a self-reported only survey such as SHIELD may often provide useful and reasonably reliable information when compared with a ‘gold standard’ survey that also includes clinical evaluation and laboratory confirmation, such as NHANES. The exception to this is information that is highly dependent upon laboratory values, especially when multiple defined variables are involved, as in the data reported here concerning dyslipidaemia.
The BMI distributions in SHIELD and NHANES were remarkably similar (Figure 1), in large part likely because of the fact that both the SHIELD and NHANES responses were weighted to match the US adult population, and because other epidemiologic studies have demonstrated that self-reported height and weight accurately correlated with measured height and weight (16, 17). Similarly, both SHIELD and NHANES consistently demonstrated an increase in prevalence of type 2 diabetes mellitus and hypertension with increasing BMI, with reported percentage rates across various BMI ranges that were also remarkably similar (Figures 2 and 3).
Additionally, both SHIELD and NHANES demonstrated gradual increases in dyslipidaemia until the BMI reached above 30 kg/m2 (Figure 4). Beyond this, the prevalence peaked, and in fact estimates began to decline in NHANES. The prevalence of dyslipidaemia in NHANES was higher at each cut-off point when compared with SHIELD. This is likely related to the fact that the definition of dyslipidaemia included not only history, but also four laboratory values (TC, TG, LDL-C and HDL-C levels). An abnormality of any of these individual variables would have been recorded as ‘dyslipidaemia’. Given that NHANES included laboratory testing and SHIELD did not, it is thus not surprising that the prevalence of dyslipidaemia was reportedly higher in NHANES. Had dyslipidaemia been defined as one variable, such as an increase in LDL-C level only, then the prevalence of dyslipidaemia in NHANES would likely have been less, and the differences in the reported prevalence of dyslipidaemia between SHIELD and NHANES would have been closer. Conversely, it should be noted that the lipid level values that were chosen to define ‘dyslipidaemia’ in this study were conservative. Had more aggressive cut-off levels been used to define ‘dyslipidaemia,’ such as TC ≥ 200, TG ≥ 150, LDL-C ≥ 100 or HDL-C < 60 mg/dl, instead of the definition of dyslipidaemia used in this analysis (TC ≥ 240, TG > 200, LDL-C ≥ 160 or HDL-C < 40 mg/dl), then the diagnosis of dyslipidaemia in NHANES would have been greater, and the differences between the prevalence of dyslipidaemia in SHIELD and NHANES would have been even greater. Hence, the degree of correlation of ‘dyslipidaemia’ in a self-reported survey (such as SHIELD) compared with that of an objective survey that includes laboratory assessment (such as NHANES), and that is performed on a wide spectrum of participants (without regard to their CHD risk) is thus largely dependent upon how the dyslipidaemia is defined.
With regard to the analysis of patients with diabetes mellitus, hypertension or dyslipidaemia, whether it was data collected through a self-reported survey only (such as SHIELD) or through a more detailed evaluation (NHANES), 75% or more of patients with each of these individual metabolic diseases (often thought to be ‘obesity related’) were overweight or obese, while about 10–25% were not overweight. In fact, some prevalence of metabolic diseases was reported at all BMI levels. Collectively, the findings presented here document that, while often directly related, not all overweight or obese patients have diabetes mellitus, hypertension or dyslipidaemia, and that not all patients with these metabolic diseases are overweight or obese. This simple message has profound implications as to the pathophysiologic relationship between fat and metabolic disease (18–20), such as whether, from the standpoint of excessive fat-related metabolic diseases, it is best to focus on fat mass (adiposity) alone (Table 1), or whether a focus on the pathogenic potential of adipose tissue (adiposopathy) might also be warranted (20–27).
In other words, whether or not weight gain may cause or worsen metabolic disease (25) and whether or not weight loss may improve metabolic disease (26) are very much dependent upon the effects on the pathogenic potential of adipose tissue. For example, positive caloric balance is most likely to result in metabolic disease when accompanied by: (i) impaired adipogenesis, which limits energy storage potential, resulting in excessive adipocyte hypertrophy which adversely affects adipocyte/adipose tissue dysfunction; (ii) accumulation and hypertrophy of visceral fat, hypertrophy of peripheral fat and increases in intra-organ fat (such as in the liver, muscle or pancreas), which result in adverse metabolic and immunologic consequences; (iii) impaired nutrient metabolism such as a net increase in free fatty acids, which is lipotoxic to body organs such as muscle, liver and pancreas; (iv) adipocyte and adipose tissue dysfunction which results in adverse metabolic consequences, because adipose tissue is an active endocrine organ (v) adipocyte and adipose tissue dysfunction which results in adverse immunological consequences, because adipose tissue is an active immune organ, and (vi) disruption of optimal interorgan ‘cross-talk’ of adipose tissue with other body organs, because metabolic diseases associated with positive caloric balance are most often caused by a pathologic partnership between the dysfunction and/or limitations of adipose tissue and the dysfunction and/or limitations of other body organs (25–27). Adiposopathy is a term used to describe pathogenic adipose tissue whose adverse clinical consequences may be promoted and exacerbated by adipocyte hypertrophy, visceral adipose tissue accumulation, and sedentary lifestyle in genetically and environmentally susceptible patients, and which represents an underlying, root physiological process leading to metabolic diseases such as type 2 diabetes mellitus, hypertension and dyslipidaemia. The results of this survey study demonstrate that while generally and directly associated with one another, the relationship between BMI and metabolic disease is not an absolute one, and further lends support to the adipocentric paradigm wherein pathologenic adipose tissue (adiposopathy) is a more rational treatment target than BMI (adiposity) alone (23).
With regard to the survey itself, the SHIELD study represents the largest such initiative ever taken. However, consumer panel surveys such as SHIELD do have limitations. First of all, SHIELD relied only on self-reporting of medical data without clinical or laboratory confirmation. Furthermore, only a small percentage (5–8%) of consumers initially invited to participate in the NFO panel (the step prior to mailing of the screener questionnaire) elect to do so, leading to the possibility of bias because of self-selection. Also, household panels also tend to under-represent the very wealthy and very poor segments of the population, and do not include military or institutionalised individuals (6, 28). However, NFO survey response rates are generally high (60–75%) and the demography of non-responders is known and can be controlled for in analyses. Another potential confounder includes the potential for misreporting of parameters such as height and weight in a self-reported survey.
Nonetheless, this report demonstrates that a self-reported survey can often acquire data that reasonably approximates surveys that also include clinical and laboratory evaluations. This is important because population assessments of the frequency of the associations of obesity with metabolic diseases such as diabetes mellitus, hypertension and dyslipidaemia have great epidemiological value, but are impaired by the logistical difficulties in obtaining reasonable and reliable data to make these assessments. For the SHIELD screener survey, a large number of questionnaires were sent, with a high return rate (64%), providing a sample that is generally representative of the overall US population. Thus, the SHIELD survey appeared to be a relatively cost-effective method to collect data on many aspects of the relationship of self-reported data and metabolic diseases.
Another potential utility of a self-reported survey is that the use of a volunteer panel that is accustomed to completing surveys also allows for the collection of much data that are otherwise difficult to collect (e.g. quality-of-life data for those with diabetes mellitus). Furthermore, longitudinal surveys are more easily obtained. For example, subsamples of the SHIELD screener respondents are currently under way, using a longer, more detailed survey assessing individual health status, health knowledge, and attitudes as well as current behaviours and treatments. Annual follow-up assessments are planned over the next 4 years, which will allow further exploration of relationships between these variables and metabolic diseases.
The SHIELD screener was a useful tool to identify individuals with metabolic risk factors. Mailed consumer panel surveys such as this one may represent a timely alternative to in-person interviews and examinations for identifying populations with certain conditions, such as diabetes mellitus, but may be less useful for others, such as dyslipidaemia.
Acknowledgments
The SHIELD Investigators’ Group includes the following individuals: Harold E. Bays, MD (chair), Benjamin J. Ansell, MD, Debbra D. Bazata, RD, LD, MA, Nathaniel G. Clark, MD, Andrew J. Green, MD, Sandra J. Lewis, MD, Michael L. Reed, PhD and Walter Stewart, PhD. The following individuals also contributed to the work reported in this manuscript: Tina Fanning (data collection and analysis) and Cheryl Prasad (data analysis). This study was presented as a poster at the North American Association for the Study of Obesity 2005 Annual Scientific Meeting, Vancouver, BC, Canada, 15–19 October, 2005.
References
- 1.World Health Organization. [March 2007];Obesity and Overweight Facts. http://www.who.int/hpr/NPH/docs/gs_obesity.pdf.
- 2.Hirschfeld RM, et al. Screening for bipolar disorder in the community. J Clin Psychiatry. 2003;64:53–9. doi: 10.4088/jcp.v64n0111. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267:64–9. [PubMed] [Google Scholar]
- 4.National Center for Health Statistics. [March 2007];NHANES 1999–2000 Data Files: Data, Docs, Codebooks, SAS Code. http://www.cdc.gov/nchs/about/major/nhanes/nhanes99_00.htm.
- 5.National Center for Health Statistics. [March 2007];NHANES 2001–2002 Data Files: Data, Docs, Codebooks, SAS Code. http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm.
- 6.Lipton RB. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–57. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. [March 2007];Mobile Exam Center Components Descriptions. http://www.cdc.gov/nchs/data/nhanes/meccomp.pdf.
- 8.Flegal KM. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. doi: 10.1001/jama.293.15.1861. [DOI] [PubMed] [Google Scholar]
- 9.Gregg EW, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–74. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–8. [PubMed] [Google Scholar]
- 11.Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. [March 2007];Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. http://www.nhlbi.nih.gov/guidelines/hypertension/jnc7full.pdf.
- 12.US Census Bureau. Annual Supplement to the Current Population Survey: Census Bureau Resident Population Estimates of the United States. Washington, DC: US Census Bureau; 2003. [Google Scholar]
- 13.National Heart, Lung, and Blood Institute in cooperation with National Institute of Diabetes and Digestive and Kidney Diseases. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: the Evidence Report. Rockville, MD, USA: National Institutes of Health; 1998. (NIH Publication No. 98–4083) [Google Scholar]
- 14.Asch DA. Response rates to mail surveys published in medical journals. J Clin Epidemiol. 1997;50:1129–36. doi: 10.1016/s0895-4356(97)00126-1. [DOI] [PubMed] [Google Scholar]
- 15.Sitzia J. Response rate in patient satisfaction research: an analysis of 210 published studies. Int J Qual Health Care. 1998;10:311–7. doi: 10.1093/intqhc/10.4.311. [DOI] [PubMed] [Google Scholar]
- 16.Spencer EA. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 17.Avila-Funes JA. Validity of height and weight in Mexican adults: results from the National Health and Aging Study. J Nutr Health Aging. 2004;8:355–67. [PubMed] [Google Scholar]
- 18.American Heart Association; National Heart, Lung, and Blood Institute. Grundy SM, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 19.Kahn R American Diabetes Association; European Association for the Study of Diabetes. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 20.Bays H. Adiposopathy, metabolic syndrome, quantum physics, general relativity, chaos and the Theory of Everything. Expert Rev Cardiovasc Ther. 2005;3:393–404. doi: 10.1586/14779072.3.3.393. [DOI] [PubMed] [Google Scholar]
- 21.Bays H. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–78. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 22.Bays H. Adiposopathy: sick fat causes high blood sugar, high blood pressure, and dyslipidemia. Future Cardiol. 2005;1:39–59. doi: 10.1517/14796678.1.1.39. [DOI] [PubMed] [Google Scholar]
- 23.Bays HE. Adiposopathy is a more rational treatment target for metabolic disease than obesity alone. Curr Atheroscler Rep. 2006;8:144–56. doi: 10.1007/s11883-006-0052-6. [DOI] [PubMed] [Google Scholar]
- 24.Bays HE. Antibesity drug development. Expert Opin Investig Drugs. 2002;11:1189–204. doi: 10.1517/13543784.11.9.1189. [DOI] [PubMed] [Google Scholar]
- 25.Bays H. Adiposopathy: why do adiposity and obesity cause metabolic disease? Future Lipidol. 2006;1:389–420. [Google Scholar]
- 26.Bays H. Adiposopathy: how do diet, exercise, weight loss and drug therapies improve metabolic disease in overweight patients? Expert Rev Cardiovasc Ther. 2006;4:871–95. doi: 10.1586/14779072.4.6.871. [DOI] [PubMed] [Google Scholar]
- 27.Bays H. Adiposopathy – defining, diagnosing, and establishing indications to treat ‘sick fat’: what are the regulatory considerations? [March 2007];US Endocr Dis. 2006 2 January 2007, 12–4. http://www.touchbriefings.com/cdps/cditem.cfm?cid=58nid=2403. [Google Scholar]
- 28.Ettinger A for the Epilepsy Impact Project Group. Depression and comorbidity in community-based patients with epilepsy or asthma. Neurology. 2004;63:1008–14. doi: 10.1212/01.wnl.0000138430.11829.61. [DOI] [PubMed] [Google Scholar]