Abstract
The mechanisms by which phosphorus homeostasis is preserved in mammals are not completely understood. We demonstrate the presence of a mechanism by which the intestine detects the presence of increased dietary phosphate and rapidly increases renal phosphate excretion. The mechanism is of physiological relevance because it maintains plasma phosphate concentrations in the normal range after ingestion of a phosphate-containing meal. When inorganic phosphate is infused into the duodenum, there is a rapid increase in the renal fractional excretion of phosphate (FE Pi). The phosphaturic effect of intestinal phosphate is specific for phosphate because administration of sodium chloride does not elicit a similar response. Phosphaturia after intestinal phosphate administration occurs in thyro-parathyroidectomized rats, demonstrating that parathyroid hormone is not essential for this effect. The increase in renal FE Pi in response to the intestinal administration of phosphate occurs without changes in plasma concentrations of phosphate (filtered load), parathyroid hormone, FGF-23, or secreted frizzled related protein-4. Denervation of the kidney does not attenuate phosphaturia elicited after intestinal phosphate administration. Phosphaturia is not elicited when phosphate is instilled in other parts of the gastrointestinal tract such as the stomach. Infusion of homogenates of the duodenal mucosa increases FE Pi, which demonstrates the presence of one or more substances within the intestinal mucosa that directly modulate renal phosphate reabsorption. Our experiments demonstrate the presence of a previously unrecognized phosphate gut–renal axis that rapidly modulates renal phosphate excretion after the intestinal administration of phosphate.
Keywords: 1,25-dihydroxyvitamin D; FGF-23; fractional excretion of phosphate; intestinal phosphate absorption; secreted frizzled related protein-4
Phosphorus is important in numerous vital biological processes, including cell signaling, nucleic acid synthesis, membrane function, energy metabolism, and bone mineralization (1–7). Understanding how phosphorus homeostasis is controlled is thus of considerable importance. Phosphorus balance in mammals is controlled by the absorption of phosphorus from the diet in the duodenum and jejunum and the tubular reabsorption of inorganic phosphate by the kidney (8–13). The efficiency of these processes is controlled by a number of hormones and peptides including the vitamin D–endocrine system (14–19), parathyroid hormone (PTH) (20–22), and the phosphatonins such as FGF-23 and secreted frizzled related protein-4 (sFRP-4) (8, 12, 23, 24), which act to increase intestinal phosphate absorption or reduce the amount of phosphate retained by the kidney. The efficiency of intestinal phosphate absorption is increased by 1α, 25-dihydroxyvitamin D [1α, 25(OH)2D], the hormonal form of vitamin D, as well as by vitamin D-independent processes that depend on the amount of phosphorus in the diet (14–16, 18, 25). The synthesis of 1α, 25(OH)2D is regulated by serum phosphate concentrations such that decreases in serum phosphate are associated with an increase in the synthesis of the hormone (18). The efficiency of renal phosphate reabsorption is reduced by PTH (20, 21), FGF-23, and sFRP-4, all of which cause a redistribution of sodium phosphate cotransporters from the surface of the proximal tubular cell to compartments within the cell, thereby decreasing the efficiency of phosphate uptake in the kidney (8, 10, 12, 13, 26–29).
Several lines of evidence suggest that renal adaptations to changes in dietary phosphate occur independently of the known regulators of renal phosphate transport. First, adaptations to changes in dietary phosphate mediated by the vitamin D endocrine system, PTH, and the phosphatonins generally occur over a period of hours to days (24) and cannot account for the modulation of renal phosphate excretion that rapidly occurs after a phosphate-containing meal (30). Second, long-term changes observed in the concentrations of various hormones such as PTH and 1α, 25(OH)2D after the administration of phosphate are generally modest in magnitude and suggest the presence of other mechanisms that operate independent of these hormonal systems (31–33). Third, evidence suggests that, in the chronically parathyroidectomized state, renal adaptations (e.g., an increase in the fractional excretion of phosphate) to dietary phosphate still occurs in an efficient manner (34). These data suggest that previously unrecognized phenomena are influencing renal phosphate excretion after the administration of phosphate.
In this study, we present information that demonstrates the presence of a previously unrecognized gut–renal axis that rapidly responds to changes in intestinal luminal phosphate concentrations by increasing the renal excretion of phosphate within minutes of the administration of phosphate into the intestine. The signal originates in the duodenum and jejunum, and is independent of serum phosphate concentrations, PTH, FGF-23, sFRP-4, and the activity of renal nerves. The infusion of sodium chloride into the duodenum and jejunum of rats is not associated with such a response. Homogenates of the duodenal mucosa contain one or more substances that increase renal phosphate excretion. We suggest that this rapid renal response to increased dietary phosphate concentrations dampens subsequent large increases in serum phosphate concentrations that could have a deleterious effect by enhancing the precipitation of calcium phosphate salts in soft tissues. The intestinal mediator of this response might also be important in the chronic regulation of renal phosphate reabsorption.
Results and Discussion
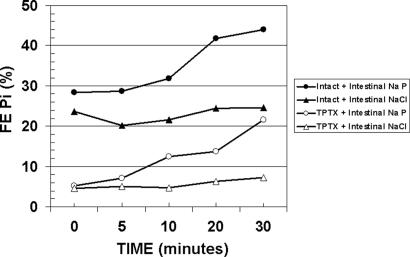
The Duodenal Infusion of Sodium Phosphate, but Not Sodium Chloride, Rapidly Increases Renal Fractional Excretion of Phosphate (Fig. 1 and Table 1, Groups A and B).
Fig. 1.
Mean FE Pi in intact or thyro-parathyroidectomized rats after the intestinal administration of sodium phosphate or sodium chloride. Groups of rats were administered either sodium phosphate or sodium chloride and FE Pi was measured at 0, 5, 10, 20, and 30 min after commencement of the infusion. Filled circles, intact rats given intestinal sodium phosphate; filled triangles, intact rats given intestinal sodium chloride; open circles, TPTX rats given intestinal sodium phosphate; open triangles, TPTX rats given intestinal sodium chloride.
Table 1.
Fractional excretion of phosphate after infusion of sodium phosphate or sodium chloride in groups of rats
| Group (n) | FE Pi %, mean (SD) |
Slope/min (SD) | P value: slope = 0 | ||||
|---|---|---|---|---|---|---|---|
| Time, min | |||||||
| 0 | 5 | 10 | 20 | 30 | |||
| A: Intact + intestinal | 28.41 | 28.73 | 31.90 | 41.84 | 44.00 | 0.597 | 0.0037 |
| Na phosphate infusion (7) | (8.60) | (7.94) | (7.59) | (9.91) | (11.39) | (0.343) | |
| B: Intact + intestinal | 23.66 | 20.24 | 21.64 | 24.50 | 24.62 | 0.096 | 0.066 |
| NaCl infusion (5) | (7.19) | (3.99) | (6.85) | (8.52) | (5.42) | (0.086) | |
| C: Intact + gastric | 26.31 | 25.94 | 27.44 | 27.81 | 28.95 | 0.083 | 0.46 |
| Na phosphate infusion (7) | (6.56) | (7.97) | (7.76) | (6.64) | (5.80) | (0.278) | |
| D: TPTX + intestinal | 5.17 | 7.08 | 12.50 | 13.78 | 21.58 | 0.521 | 0.0027 |
| Na phosphate infusion (5) | (1.75) | (2.07) | (5.31) | (5.70) | (6.83) | (0.175) | |
| E: TPTX + intestinal | 4.56 | 5.06 | 4.80 | 6.28 | 7.18 | 0.107 | 0.14 |
| NaCl infusion (5) | (3.32) | (2.94) | (2.82) | (4.00) | (6.15) | (0.131) | |
| F: Denervated + intestinal | 22.25 | 23.53 | 31.83 | 38.05 | 40.10 | 0.647 | 0.037 |
| Na Pi infusion (4) | (4.75) | (3.30) | (4.08) | (8.45) | (12.99) | (0.360) | |
The infusion of sodium phosphate into the duodenum was associated with a rapid increase in the renal fractional excretion of phosphate (FE Pi) (Fig. 1, filled circles, and Table 1, group A, P value for slope = 0.0037). The amount of phosphate administered was equivalent to the estimated phosphate intake of a rat during nocturnal feeding. The increase in phosphate excretion was well established at 10, 20, and 30 min after the administration of phosphate. The fractional excretion of sodium (FE Na) did not change significantly in group A (Table 2). Glomerular filtration rate diminished slightly during this time (Table 3). Plasma phosphate increased over time in all groups studied (Table 4), with maximal changes observed at 30 min irrespective of whether phosphate was administered or whether there was a change in FE Pi (Table 4). Groups A–E had similar mean slopes in plasma phosphate (range 0.015–0.021 per min), whereas the slope for group F was somewhat higher (0.033 per min). The data show that that the increase in urinary phosphate is due to a decrease in phosphate reabsorption by the tubule, and that the change in filtered load of phosphate cannot account for the increase in urinary phosphate excretion.
Table 2.
Fractional excretion of sodium after infusion of sodium phosphate or sodium chloride in groups of rats
| Group (n) | FE Pi %, mean (SD) |
Slope/min | P value: slope = 0 | ||||
|---|---|---|---|---|---|---|---|
| Time, min | |||||||
| 0 | 5 | 10 | 20 | 30 | |||
| A: Intact + intestinal | 2.06 | 2.28 | 2.01 | 2.11 | 2.85 | 0.014 | 0.36 |
| Na phosphate infusion (7) | (1.18) | (1.16) | (0.86) | (1.04) | (1.38) | (0.037) | |
| B: Intact + intestinal | 2.78 | 2.31 | 2.54 | 2.76 | 3.54 | 0.030 | 0.031 |
| NaCl infusion (5) | (2.13) | (1.20) | (1.31) | (1.81) | (1.99) | (0.020) | |
| C: Intact + gastric | 2.25 | 2.92 | 2.69 | 2.31 | 3.01 | 0.006 | 0.68 |
| Na phosphate infusion (7) | (0.41) | (0.58) | (0.64) | (0.75) | (1.29) | (0.037) | |
| D: TPTX + intestinal | 1.84 | 2.14 | 2.52 | 2.47 | 2.97 | 0.033 | 0.016 |
| Na phosphate infusion (5) | (0.71) | (0.42) | (0.81) | (0.61) | (0.62) | (0.018) | |
| E: TPTX + intestinal | 2.11 | 1.50 | 2.33 | 2.30 | 2.99 | 0.048 | 0.059 |
| NaCl infusion (5) | (1.02) | (0.74) | (1.11) | (1.01) | (1.79) | (0.041) | |
| F: Denervated + intestinal | 2.41 | 3.10 | 3.68 | 3.80 | 3.38 | 0.029 | 0.16 |
| Na phosphate infusion (4) | (1.15) | (1.25) | (0.64) | (0.63) | (0.41) | (0.031) | |
Table 3.
Glomerular filtration rate (GFR) after infusion of sodium phosphate or sodium chloride in groups of rats
| Group (n) | GFR, ml/min, mean (SD) |
Slope/min | P value: slope = 0 | ||||
|---|---|---|---|---|---|---|---|
| Time, min | |||||||
| 0 | 5 | 10 | 20 | 30 | |||
| A: Intact + intestinal | 4.21 | 3.92 | 4.04 | 3.63 | 3.60 | −0.020 | 0.0082 |
| Na phosphate infusion (7) | (1.15) | (0.82 | (0.73) | (0.94) | (0.64) | (0.014) | |
| B: Intact + intestinal | 4.01 | 4.99 | 4.29 | 4.34 | 3.35 | −0.031 | 0.012 |
| NaCl infusion (5) | (1.70) | (0.98 | (1.31) | (1.82) | (1.02) | (0.016) | |
| C: Intact + gastric | 4.13 | 4.55 | 5.06 | 5.38 | 4.03 | 0.009 | 0.63 |
| Na phosphate infusion (7) | (1.01) | (1.67) | (1.97) | (1.58) | (0.97) | (0.045) | |
| D: TPTX + intestinal | 6.01 | 5.17 | 4.34 | 4.09 | 3.58 | −0.074 | 0.0015 |
| Na phosphate infusion (5) | (2.15) | (1.24) | (1.78) | (1.78) | (1.23) | (0.021) | |
| E: TPTX + intestinal | 4.45 | 6.19 | 4.23 | 3.93 | 3.69 | −0.069 | 0.091 |
| NaCl infusion (5) | (1.97) | (3.95) | (1.92) | (1.52) | (0.98) | (0.069) | |
| F: Denervated + intestinal | 1.42 | 1.84 | 1.61 | 1.50 | 1.60 | −0.001 | 0.94 |
| Na phosphate infusion (4) | (0.38) | (0.75) | (0.53) | (0.56) | (0.83) | (0.207) | |
Table 4.
Plasma phosphate concentrations after the infusion of sodium phosphate or sodium chloride in groups of rats
| Group (n) | Plasma phosphate, mmol/l, mean (SD) |
Slope/min | P value: slope = 0 | ||||
|---|---|---|---|---|---|---|---|
| Time, min | |||||||
| 0 | 5 | 10 | 20 | 30 | |||
| A: Intact + intestinal | 1.46 | 1.55 | 1.59 | 1.67 | 2.21 | 0.017 | 0.0013 |
| Na phosphate infusion (7) | (0.31) | (0.16) | (0.22) | (0.32) | (0.24) | (0.008) | |
| B: Intact + intestinal | 1.72 | 1.76 | 1.78 | 1.77 | 2.22 | 0.015 | 0.0004 |
| NaCl infusion (5) | (0.21) | (0.20) | (0.24) | (0.39) | (0.21) | (0.003) | |
| C: Intact + gastric | 1.48 | 1.62 | 1.68 | 1.68 | 2.05 | 0.016 | 0.0073 |
| Na phosphate infusion (7) | (0.45) | (0.46) | (0.41) | (0.27) | (0.39) | (0.011) | |
| D: TPTX + intestinal | 1.73 | 1.92 | 2.00 | 2.06 | 2.44 | 0.021 | 0.0074 |
| Na phosphate infusion (5) | (0.24) | (0.23) | (0.38) | (0.41) | (0.47) | (0.009) | |
| E: TPTX + intestinal | 1.64 | 1.69 | 1.76 | 1.90 | 2.29 | 0.017 | 0.018 |
| NaCl infusion (5) | (0.17) | (0.29) | (0.35) | (0.19) | (0.34) | (0.010) | |
| F: Denervated + intestinal | 1.67 | 1.86 | 1.98 | 2.25 | 2.71 | 0.033 | 0.015 |
| Na phosphate infusion (4) | (0.27) | (0.42) | (0.27) | (0.39) | (0.70) | (0.013) | |
To determine whether the change in the FE Pi is specific for the administration of phosphate, we administered sodium chloride in equimolar amounts into the intestine and measured changes in FE Pi and FE Na. The infusion of sodium chloride is not associated with a significant change in FE Pi (Fig. 1, filled triangles, and Table 1, group B; P value for slope = 0.066). There was a small increase in the FE Na (Table 2, P value for slope = 0.031).
The increase in FE Pi in group A was significantly greater than that obtained in group B, in which sodium chloride was infused instead of sodium phosphate (P = 0.0076) (Table 5). Similarly, there was no significant difference in the change in plasma phosphate levels in groups A and B (P = 0.55, Table 6).
Table 5.
Group comparisons for FE Pi % shown in Table 1
| Groups | P value: equal slopes |
|---|---|
| A vs. B | 0.0076 |
| A vs. C | 0.0096 |
| A vs. F | 0.82 |
| D vs. E | 0.0029 |
Table 6.
Group comparisons for plasma phosphate shown in Table 4
| Group | P value: equal slope |
|---|---|
| A vs. B | 0.5459 |
| A vs. C | 0.9099 |
| A vs. F | 0.0279 |
| D vs. E | 0.5724 |
The Infusion of Sodium Phosphate into the Stomach Does Not Elicit Changes in the Renal Fractional Excretion of Phosphate (Table 1, Group C).
Specific organs of the gastrointestinal tract specifically sense different nutrients. To ascertain whether changes in the concentration of luminal phosphate were sensed by other organs of the gastrointestinal tract, such as the stomach, we measured renal FE Pi after the infusion of sodium phosphate into the stomach. The efflux of phosphate into the duodenum and jejunum was prevented by placing a ligature at the gastro–duodenal junction. As shown in Table 1, group C, the infusion of sodium phosphate into the stomach did not significantly increase renal FE Pi (P value for slope = 0.46), thus showing that increases in gastric luminal phosphate do not elicit the same response seen after the infusion of sodium phosphate into the duodenum and jejunum. Furthermore, comparison of the change in FE Pi in group A was statistically greater than that in group C (P = 0.0096, Table 5). These data, together with those noted above, show that the duodenum and jejunum detect the presence of increased luminal phosphate.
Duodenal Phosphate Infusion Increases Renal Fractional Phosphate Excretion in Parathyroidectomized Rats (Fig. 1 and Table 1, Groups D and E).
We next ascertained the mechanisms responsible for changes in renal FE Pi after duodenal phosphate infusion. PTH is known to play an important role in altering the reabsorption of phosphate in the renal proximal tubule. To determine whether changes in PTH are responsible for the changes in renal FE Pi after the infusion of sodium phosphate into the duodenum, we performed experiments in acutely thyro-parathyroidectomized (TPTX) rats. Despite the absence of parathyroid hormone as evidenced by the low basal FE Pi (Table 1, groups D and E), the infusion of sodium phosphate into the duodenum was associated with an increase in the FE Pi (Fig. 1, open circles, and Table 1, group D) albeit from a lower FE Pi (P value for slope = 0.0027). As in the intact rats, intestinal sodium chloride infusion did not result in a significant change in the FE Pi (Fig. 1, open triangles, and Table 1, group E; P value for slope = 0.14). FE Pi increased at a greater rate with phosphate infusion (Table 1, group D) than with sodium chloride infusion (group E, P = 0.0029, Table 5). These data demonstrate that rapid increases in renal phosphate excretion after duodenal infusion of sodium phosphate occur in the absence of PTH.
Duodenal Phosphate Infusion Increases Renal Fractional Phosphate Excretion Independently of Changes in Plasma PTH, FGF-23, or sFRP-4.
PTH and several peptides collectively known as the “phosphatonins” (FGF-23 and sFRP-4) have been shown to alter the renal excretion of phosphate in various phosphate wasting conditions. To determine whether these substances were responsible for the acute change in renal phosphate reabsorption after the infusion of sodium phosphate into the duodenum of intact/normal rats, we measured full-length (intact) PTH, FGF-23, and sFRP-4 after the infusion of sodium phosphate into the duodenum. PTH, FGF-23, and sFRP-4 did not change significantly after the administration of sodium phosphate into the duodenum and jejunum (PTH pg/ml: 0 min, mean ± SD, 46.14 ± 22.60; 5 min, 33.81 ± 22.21; 10 min, 43.47 ± 30.36; 20 min, 40.24 ± 18.49; 30 min, 59.14 ± 10.17; P = 0.051 for slope. FGF-23 pg/ml: 0 min, mean ± SD, 93.67 ± 30.08; 5 min, 84.36 ± 31.37; 10 min, 97.55 ± 34.09; 20 min, 92.66 ± 28.30; 30 min, 120.39 ± 65.91; P = 0.23 for slope. SFRP-4 ng/ml: 0 min, mean ± SD, 30.65 ± 8.65; 5 min, 31.10 ± 8.99; 10 min, 35.55 ± 14.02; 20 min, 35.44 ± 7.17; 30 min, 35.54 ± 11.12; P = 0.30 for slope), thereby demonstrating that these factors are likely not responsible for the acute change in renal phosphate excretion in our experiments.
Duodenal Phosphate Infusion Increases Renal Fractional Phosphate Excretion in the Denervated Kidney (Table 1, Group F).
Renal sympathetic efferent nerves are known to alter electrolyte excretion (35, 36). To determine whether renal nerves are responsible for the change in renal phosphate excretion after the administration of sodium phosphate in the duodenum, we transected the renal nerves at the renal hilum, and measured phosphate excretion after duodenal phosphate infusion. Renal denervation did not prevent the changes in renal phosphate excretion seen after the infusion of sodium phosphate into the duodenum (Table 1, group F; P value for slope = 0.037). Therefore, it is unlikely that this reflex is neurally mediated. The response in FE Pi to the intestinal infusion of sodium phosphate in the denervated kidney (group F) was similar to that observed in the intact kidney with normal nerve supply (group A, P = 0.82, Table 5).
Duodenal Extracts Contain a Phosphaturic Factor.
To determine whether phosphaturic factors are present in the duodenal mucosa, we prepared saline extracts of duodenal mucosa, and infused them into five rats in vivo. Infusion of these extracts was associated with increase in FE Pi (mean ± SD, control period = 13.12 ± 5.03% vs. mean ± SD, experimental period 31.54 ± 13.54%, P = 0.025). Sodium excretion increased as well (mean ± SD, control period 1.12 ± 1.02% vs. mean ± SD, experimental period 2.29 ± 0.60%, P = 0.02). Glomerular filtration rate did not change (P = 0.48).
In sum, our data support the existence of a heretofore undescribed reflex that rapidly mediates changes in renal phosphate reabsorption on the introduction of phosphate into the intestinal lumen. The reflex is not neurally mediated and is independent of PTH and the phosphatonins. The change in renal phosphate reabsorption only occurs when phosphate is infused in the intestine and not when it is infused in the stomach, suggesting that a phosphate “sensor” exists in the duodenum and/or jejunum. Duodenal homogenates contain a factor or factors that alter renal phosphate reabsorption after release and entry into the portal circulation. The nature of this factor is undetermined at present.
The data suggest the presence of a sensing mechanism within the duodenum that recognizes an increase in luminal phosphate concentrations. The intestine is capable of recognizing and responding to a variety of nutrients by diverse mechanisms (37–39), and therefore its ability to respond to luminal phosphate concentrations is not without precedent. The precise cellular localization or structure of the “phosphate sensor” is not known at present. The sensor might be localized on enterocytes, entero-endocrine, or neuronal cells, where it might exist as a membrane-bound receptor or may reside within the cell itself. Other mineral sensors, such as the calcium-sensing receptor, are widely distributed in gastrointestinal tract epithelial and neural cells, and modulate fluid and electrolyte secretion in the colon and small intestine and gastrin secretion in the stomach (40–44). Once increases in phosphate are recognized, the release of a factor within the mucosa that modulates phosphate excretion is likely to occur based on our finding of a phosphaturic substance within the duodenal mucosa. The steps between the recognition of the phosphate signal and the release of the phosphaturic factor are unknown. It is also possible that factors might be released from other organs, such as the liver, that regulate phosphate transport. Hypophosphatemia is seen frequently after liver injury and hepatic resection, and it is possible that a variety of hepatic mediators might be involved in this process (45–49).
The long-term adaptation to dietary phosphate over a period of days is likely to involve factors such as PTH and the phosphatonins (23, 24, 31–33). In the short-term, however, intestinal factors described in this report are likely to play a role in the rapid increase in renal phosphate excretion. Our data concerning the short-term response to phosphate feeding are consistent with the results of Nishida et al. (30), who showed brisk and rapid increases in renal phosphate excretion but no changes in serum FGF-23 concentrations after feeding of meals containing increasing amounts of phosphate.
The gastrointestinal tract produces peptides and hormones that modulate a variety of physiological functions such as hunger/satiety, among others, after the ingestion of a meal (50–56). Lennane et al. (57) demonstrated that orally administered sodium chloride produces a more profound natriuresis than sodium chloride administered intravenously, and, based on these observations, suggested that the intestine produces a factor that is responsible for this natriuresis. Subsequently, the intestine was shown to produce and release a small peptide, guanylin, in response to oral salt loading (58). Guanylin and the closely related peptide uroguanylin are secreted into the intestine lumen and stimulate enterocytes via membrane-bound guanylate cyclase to increase cGMP production, which, via a cascade of biochemical events, increases the secretion of chloride, bicarbonate, and water via the cystic fibrosis transmembrane regulator (58–66). Guanylin and uroguanylin also increase the secretion of sodium, potassium and water in the kidney without changes in the glomerular filtration rate (66–69). Expression of uroguanylin messenger RNA is increased in the kidney during salt loading (70). The importance of uroguanylin in mediating natriuresis after oral salt loading has been demonstrated by the absence of a natriuretic response after oral salt loading in uroguanylin knockout mice (71). Our data support the existence of a similar intestinal-renal axis specific for phosphate that is mediated by an unknown factor: an intestinal “phosphatonin.” The exact nature of this mediator is unknown. We believe that further analysis of this pathway, including the identification of a phosphate sensor and the mediator of this response, will be helpful in completely understanding phosphate homeostasis.
Methods
General Analytical Methods.
Plasma and urine phosphate concentrations were determined by using the method of Chen (72). Inulin concentrations in plasma and urine were determined by using the anthrone method (73). Sodium concentrations in urine and plasma were determined by an ion selective electrode (EasyLyte Plus Analyzer; Medica, Bedford, MA). Protein concentrations were measured by using the Bio-Rad protein assay with BSA standard (Bio-Rad Laboratories, Hercules, CA). Plasma-intact parathyroid hormone was measured by using an analytical kit from Alpco Diagnostic Laboratories (Salem, NH). Plasma-intact FGF-23 was measured by using a kit from Kainos Laboratories (Tokyo, Japan). Secreted frizzled related protein-4 was measured as reported earlier by using an ELISA method (27). Spectroscopy was carried out by using a Beckman (Fullerton, CA) DU-7400 spectrophotometer or a Coleman (Pittsburgh, PA) Model 35 spectrophotometer. ELISA plates were read by using a Molecular Devices (Sunnyvale, CA) Thermo Max microplate reader.
Animals and Animal Protocols.
All animal protocols were approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Surgical methods.
Preparation of rats for renal clearance experiments.
Male Sprague–Dawley rats weighing ≈300 g were purchased from Harlan Sprague–Dawley (Madison, WI). They were fed a rodent diet containing 0.7% phosphate and 0.5% calcium. On the day of the experiment, rats were anesthetized with an i.p. injection of 100–150 mg/kg body weight of 5-sec-butyl-ethyl-2-thiobarbituricacid (Inactin; Byk Gulden Konstanz, Hamburg, Germany). The rats were placed on a heated table to maintain body temperature between 36–38°C. After a tracheostomy, a PE 50 catheter was placed in the left carotid artery to monitor mean arterial blood pressure (MAP), and to collect blood samples. Catheters were also placed in the left and right jugular veins for infusions. 2% inulin in 0.9% NaCl plus 4.5% BSA were infused at a rate of 3.0 ml/hr in one catheter, and 0.9% NaCl was infused in the other catheter at a rate of 2.5 ml/hr. Catheters (PE 50) were placed in the right and left ureter for urine collection. This protocol was used for all experiments except those described below.
In experiments in which the effect of infusion of intestinal homogenates on renal phosphate and sodium excretion was tested, the above-mentioned protocol was modified so that the i.v. infusion rate of 2% inulin in 0.9% NaCl plus 4.5% BSA was 1.2 ml/hr via one catheter, and that of 0.9% NaCl was 1.2 ml/hr via the other catheter. Urine was collected from the urinary bladder by using a catheter made of PE-90 tubing.
Preparation of rats for the administration of solutes into the duodenum.
Rats were prepared as described above for measurement of renal phosphate and sodium excretion. For the administration of sodium phosphate or sodium chloride into the duodenum, a small incision in the duodenum was made ≈1 cm from the pylorus. A PE 90 catheter was then inserted into the duodenum for a distance of 0.5 cm. The catheter was anchored in place by a ligature placed around the duodenum.
Preparation of rats for the administration of sodium phosphate into the stomach.
For the administration of sodium phosphate into the stomach, a suture was passed behind the gastro-duodenal junction at the pylorus, and tied so as to prevent the flow of stomach contents into the duodenum. A small incision was then made in the body of the stomach, and a PE 90 catheter was introduced into the stomach, and secured with a suture.
Denervation of the kidney.
In rats prepared as described above, the left kidney was exposed and renal nerves were carefully identified and cut. To assure complete absence of neural transmission, the renal pedicle was further treated with a solution of 2% phenol. Successful denervation was confirmed by observing an increase in urine flow and sodium excretion in the denervated kidney.
Acute thyroparathyroidectomy.
Acute thyroparathyroidectomy was carried out by identifying and removing the thyroid together with the parathyroids from the para-tracheal region of the neck at the time of catheter placement described in the first paragraph.
Preparation of duodenal mucosal extract.
Rats were used for preparation of duodenal mucosal extracts. Animals were anesthetized, and a 10-cm segment of the duodenum immediately distal to the pylorus was dissected and excised. The intestinal lumen was washed with ice-cold 0.9% sodium chloride. The intestine was longitudinally cut along the mesenteric border, and placed on a glass plate in an ice bucket. The duodenal mucosa was scraped with a glass slide, and immediately transferred to a tube, and weighed. An equal volume of ice-cold 0.9% sodium chloride (1:1 wt/vol) was immediately added. The mucosa was homogenized on ice with three, 30-second bursts of a Polytron homogenizer/sonicator (Beckman Instruments, Fullerton, CA) set at maximum. Between each homogenizer pulse, the homogenates were allowed to rest on ice at 4°C for one minute. The homogenates were centrifuged at 14,000 × g for 30 min in a microcentrifuge (Eppendorf, Westbury, NY) in a cold room at 4°C. The supernatant was collected and filtered through a 0.22-μm filter. Protein concentration was determined as described above, and 100 μg of the protein were added to the 4.5% BSA/0.9% saline solution for infusion as described above.
Experimental protocols.
The following groups of rats were studied.
Intact rats administered sodium phosphate into the duodenum and jejunum (n = 7).
After surgery as described above, and equilibration for a period of 60 min, a 15-min urine collection was made to determine basal excretion of phosphate, sodium and inulin. A midpoint collection of blood was made for determination of sodium, phosphate, inulin, PTH, FGF-23, and sFRP-4 in plasma. After the baseline collection period, 1 ml of 1.3 mM NaH2PO4 (pH 5) was infused via the duodenal catheter over a period of 15 s. Blood and urine samples were collected at 5, 10, 20, and 30 min. Clearances of sodium, phosphate, and inulin were made at these time points. The fractional excretions of phosphate and sodium were determined by dividing the clearance of phosphate or sodium by the clearance of inulin, and expressing it as a percentage. The clearance of inulin was used as an index of glomerular filtration rate.
Intact rats administered sodium chloride into the duodenum and jejunum (n = 5).
To determine whether the effect of duodenal sodium phosphate infusion was due to administration of sodium or phosphate ions, rats prepared as described above received sodium chloride at the same concentration and in the same manner as noted above.
Intact rats administered sodium phosphate into the stomach (n = 7).
To determine the site at which phosphate exerted its effects, we infused sodium phosphate as noted above into the stomach which had been isolated from the duodenum. Clearances were carried out as described as above.
Denervated rats administered sodium phosphate into the duodenum (n = 4).
These rats were prepared as described above. Each animal received sodium phosphate that was infused into the duodenum as described above. Clearances were carried out as described above.
Thyroparathyroidectomized rats administered sodium phosphate into the duodenum and jejunum (n = 5).
Thyroparathyroidectomized rats prepared as described above were administered sodium phosphate into the duodenum and jejunum. These experiments were identical to those above except that animals were thyroparathyroidectomized.
Thyroparathyroidectomized rats administered sodium chloride into the duodenum and jejunum (n = 5).
Thyroparathyroidectomized rats were prepared as described above, and sodium chloride was administered into the duodenum and jejunum. These experiments were identical to those described in paragraph 2 above except that animals were thyroparathyroidectomized.
Intact rats administered homogenates of duodenal mucosa (n = 5).
Rats were prepared as above. After an equilibration period, 100 μg of duodenal mucosal homogenate was infused for 1 h.
Statistical Analysis.
For each time point data are summarized by group using means and standard deviations (SDs). The primary interest was whether responses changed with time after injection, and whether those changes differed by study group. Within-animal linear regression was used to summarize time trends for each animal. This method uses all readings, yields independent observations, avoids issues associated with multiple testing at each time point, and is reasonably powerful if the trends are approximately linear (74). The individual slopes per minute from the regression were summarized for each group by using means and SDs. Multiplying the slope times 30 approximates the average change over the 30-min experiment. One-sample t tests were used to assess whether the mean within-group slope was different from zero (no time trend). Two-sample t tests were used for comparisons of slopes between selected groups. For differences between control and experimental time periods in duodenal homogenate infusions, a paired t test was used to assess the statistical significance of the differences between means. All tests were two-sided with an alpha level of 0.05.
Acknowledgments
This work was supported by National Institutes of Health Grants DK65830 and DK73369 (to R.K.).
Abbreviations
- FE Pi
fractional excretion of phosphate
- FE Na
fractional excretion of sodium
- sFRP-4
secreted frizzled related protein-4
- 1α
25(OH)2D, 1α, 25-dihydroxyvitamin D
- PTH
parathyroid hormone
- TPTX
thyro-parathyroidectomized.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cohen P. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard SR, Till JH. Annu Rev Biochem. 2000;69:373–398. doi: 10.1146/annurev.biochem.69.1.373. [DOI] [PubMed] [Google Scholar]
- 3.Hunter T, Cooper JA. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- 4.Kumar R, Riggs B. In: Fundamental and Clinical Bone Physiology. Urist M, editor. Philadelphia: Lippincott; 1980. pp. 394–406. [Google Scholar]
- 5.Krebs EG, Beavo JA. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- 6.Boyer PD. Annu Rev Biochem. 1997;66:717–749. doi: 10.1146/annurev.biochem.66.1.717. [DOI] [PubMed] [Google Scholar]
- 7.Hatefi Y. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 8.Berndt TJ, Schiavi S, Kumar R. Am J Physiol. 2005;289:F1170–F1182. doi: 10.1152/ajprenal.00072.2005. [DOI] [PubMed] [Google Scholar]
- 9.Berndt T, Knox F. In: The Kidney: Physiology and Pathophysiology. Seldin DW, Giebisch G, editors. New York: Raven; 1992. pp. 2511–2532. [Google Scholar]
- 10.Murer H, Hernando N, Forster I, Biber J. Curr Opin Nephrol Hypertens. 2001;10:555–561. doi: 10.1097/00041552-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dennis VW, Stead WW, Myers JL. Annu Rev Physiol. 1979;41:257–271. doi: 10.1146/annurev.ph.41.030179.001353. [DOI] [PubMed] [Google Scholar]
- 12.Berndt T, Kumar R. Annu Rev Physiol. 2007;69:341–359. doi: 10.1146/annurev.physiol.69.040705.141729. [DOI] [PubMed] [Google Scholar]
- 13.Murer H, Hernando N, Forster I, Biber J. Annu Rev Physiol. 2003;65:531–542. doi: 10.1146/annurev.physiol.65.042902.092424. [DOI] [PubMed] [Google Scholar]
- 14.Kabakoff B, Kendrick NC, DeLuca HF. Am J Physiol. 1982;243:E470–E475. doi: 10.1152/ajpendo.1982.243.6.E470. [DOI] [PubMed] [Google Scholar]
- 15.Chen TC, Castillo L, Korycka-Dahl M, DeLuca HF. J Nutr. 1974;104:1056–1060. doi: 10.1093/jn/104.8.1056. [DOI] [PubMed] [Google Scholar]
- 16.Williams KB, Deluca HF. Am J Physiol. 2007;292:E1917–E1921. doi: 10.1152/ajpendo.00654.2006. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Deluca HF. Proc Natl Acad Sci USA. 1974;71:1040–1044. doi: 10.1073/pnas.71.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka Y, Deluca HF. Arch Biochem Biophys. 1973;154:566–574. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- 19.DeLuca HF, Schnoes HK. Annu Rev Biochem. 1983;52:411–439. doi: 10.1146/annurev.bi.52.070183.002211. [DOI] [PubMed] [Google Scholar]
- 20.Aurbach GD, Keutmann HT, Niall HD, Tregear GW, O'Riordan JL, Marcus R, Marx SJ, Potts JT., Jr Recent Prog Horm Res. 1972;28:353–398. [PubMed] [Google Scholar]
- 21.Aurbach GD, Heath DA. Kidney Int. 1974;6:331–345. doi: 10.1038/ki.1974.118. [DOI] [PubMed] [Google Scholar]
- 22.Chase LR, Aurbach GD. Proc Natl Acad Sci USA. 1967;58:518–525. doi: 10.1073/pnas.58.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 24.Sommer S, Berndt T, Craig T, Kumar R. J Steroid Biochem Mol Biol. 2007;103:497–503. doi: 10.1016/j.jsbmb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Segawa H, Kaneko I, Yamanaka S, Ito M, Kuwahata M, Inoue Y, Kato S, Miyamoto K. Am J Physiol. 2004;287:F39–F47. doi: 10.1152/ajprenal.00375.2003. [DOI] [PubMed] [Google Scholar]
- 26.Karim-Jimenez Z, Hernando N, Biber J, Murer H. Proc Natl Acad Sci USA. 2000;97:12896–12901. doi: 10.1073/pnas.220394197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, Jan De Beur SM, Schiavi SC, Kumar R. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan X, Yokote H, Jing X, Yao L, Sawada T, Zhang Y, Liang S, Sakaguchi K. Genes Cells. 2005;10:489–502. doi: 10.1111/j.1365-2443.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 30.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 31.Van Den Berg CJ, Kumar R, Wilson DM, Heath H, III, Smith LH. J Clin Endocrinol Metab. 1980;51:998–1001. doi: 10.1210/jcem-51-5-998. [DOI] [PubMed] [Google Scholar]
- 32.Calvo MS, Kumar R, Heath H., III J Clin Endocrinol Metab. 1988;66:823–829. doi: 10.1210/jcem-66-4-823. [DOI] [PubMed] [Google Scholar]
- 33.Calvo MS, Kumar R, Heath H. J Clin Endocrinol Metab. 1990;70:1334–1340. doi: 10.1210/jcem-70-5-1334. [DOI] [PubMed] [Google Scholar]
- 34.Steele TH, DeLuca HF. J Clin Invest. 1976;57:867–874. doi: 10.1172/JCI108363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mann KJ, Dousa DM, Kerrigan RJ, Berndt TJ, Knox FG. Miner Electrolyte Metab. 1992;18:354–358. [PubMed] [Google Scholar]
- 36.Bello-Reuss E, Colindres RE, Pastoriza-Munoz E, Mueller RA, Gottschalk CW. J Clin Invest. 1975;56:208–217. doi: 10.1172/JCI108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchan AM. Am J Physiol. 1999;277:G1103–G1107. doi: 10.1152/ajpgi.1999.277.6.G1103. [DOI] [PubMed] [Google Scholar]
- 38.Furness JB, Kunze WA, Clerc N. Am J Physiol. 1999;277:G922–G928. doi: 10.1152/ajpgi.1999.277.5.G922. [DOI] [PubMed] [Google Scholar]
- 39.Flemstrom G, Sjoblom M. Am J Physiol. 2005;289:G377–G380. doi: 10.1152/ajpgi.00093.2005. [DOI] [PubMed] [Google Scholar]
- 40.Hebert SC, Cheng S, Geibel J. Cell Calcium. 2004;35:239–247. doi: 10.1016/j.ceca.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Cheng SX, Geibel JP, Hebert SC. Gastroenterology. 2004;126:148–158. doi: 10.1053/j.gastro.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 42.Chattopadhyay N, Cheng I, Rogers K, Riccardi D, Hall A, Diaz R, Hebert SC, Soybel DI, Brown EM. Am J Physiol. 1998;274:G122–G130. doi: 10.1152/ajpgi.1998.274.1.G122. [DOI] [PubMed] [Google Scholar]
- 43.Cheng SX, Okuda M, Hall AE, Geibel JP, Hebert SC. Am J Physiol. 2002;283:G240–G250. doi: 10.1152/ajpgi.00500.2001. [DOI] [PubMed] [Google Scholar]
- 44.Conigrave AD, Brown EM. Am J Physiol. 2006;291:G753–G761. doi: 10.1152/ajpgi.00189.2006. [DOI] [PubMed] [Google Scholar]
- 45.Dawson DJ, Babbs C, Warnes TW, Neary RH. Br Med J (Clin Res Ed) 1987;295:1312–1313. doi: 10.1136/bmj.295.6609.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salem RR, Tray K. Ann Surg. 2005;241:343–348. doi: 10.1097/01.sla.0000152093.43468.c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smyrniotis V, Kostopanagiotou G, Katsarelias D, Theodoraki K, Hondros K, Kouskouni E. Int Surg. 2003;88:100–104. [PubMed] [Google Scholar]
- 48.Pomposelli JJ, Pomfret EA, Burns DL, Lally A, Sorcini A, Gordon FD, Lewis WD, Jenkins R. Liver Transpl. 2001;7:637–642. doi: 10.1053/jlts.2001.26287. [DOI] [PubMed] [Google Scholar]
- 49.George R, Shiu MH. Surgery. 1992;111:281–286. [PubMed] [Google Scholar]
- 50.Murphy KG, Dhillo WS, Bloom SR. Endocr Rev. 2006;27:719–727. doi: 10.1210/er.2006-0028. [DOI] [PubMed] [Google Scholar]
- 51.Murphy KG, Bloom SR. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 52.Schusdziarra V, Zyznar E, Rouiller D, Boden G, Brown JC, Arimura A, Unger RH. Science. 1980;207:530–532. doi: 10.1126/science.7352262. [DOI] [PubMed] [Google Scholar]
- 53.Tepperman BL, Evered MD. Science. 1980;209:1142–1143. doi: 10.1126/science.7403876. [DOI] [PubMed] [Google Scholar]
- 54.Badman MK, Flier JS. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 55.Holst JJ. Annu Rev Physiol. 1997;59:257–271. doi: 10.1146/annurev.physiol.59.1.257. [DOI] [PubMed] [Google Scholar]
- 56.Dockray GJ. Annu Rev Physiol. 1979;41:83–95. doi: 10.1146/annurev.ph.41.030179.000503. [DOI] [PubMed] [Google Scholar]
- 57.Lennane RJ, Peart WS, Carey RM, Shaw J. Clin Sci Mol Med. 1975;49:433–436. doi: 10.1042/cs0490433. [DOI] [PubMed] [Google Scholar]
- 58.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. Proc Natl Acad Sci USA. 1992;89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kita T, Kitamura K, Sakata J, Eto T. Am J Physiol. 1999;277:G960–G966. doi: 10.1152/ajpgi.1999.277.5.G960. [DOI] [PubMed] [Google Scholar]
- 60.Li Z, Knowles JW, Goyeau D, Prabhakar S, Short DB, Perkins AG, Goy MF. Gastroenterology. 1996;111:1714–1721. doi: 10.1016/s0016-5085(96)70037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamra FK, Forte LR, Eber SL, Pidhorodeckyj NV, Krause WJ, Freeman RH, Chin DT, Tompkins JA, Fok KF, Smith CE, et al. Proc Natl Acad Sci USA. 1993;90:10464–10468. doi: 10.1073/pnas.90.22.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. EMBO J. 1994;13:1065–1072. doi: 10.1002/j.1460-2075.1994.tb06355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaandrager AB, Bot AG, Ruth P, Pfeifer A, Hofmann F, De Jonge HR. Gastroenterology. 2000;118:108–114. doi: 10.1016/s0016-5085(00)70419-7. [DOI] [PubMed] [Google Scholar]
- 64.Vaandrager AB, Bot AG, De Jonge HR. Gastroenterology. 1997;112:437–443. doi: 10.1053/gast.1997.v112.pm9024297. [DOI] [PubMed] [Google Scholar]
- 65.Tien XY, Brasitus TA, Kaetzel MA, Dedman JR, Nelson DJ. J Biol Chem. 1994;269:51–54. [PubMed] [Google Scholar]
- 66.Sindic A, Schlatter E. Nephrol Dial Transplant. 2006;21:3007–3012. doi: 10.1093/ndt/gfl314. [DOI] [PubMed] [Google Scholar]
- 67.Sindic A, Schlatter E. Curr Opin Nephrol Hypertens. 2007;16:10–15. doi: 10.1097/MNH.0b013e328011cb4a. [DOI] [PubMed] [Google Scholar]
- 68.Sindic A, Hirsch JR, Velic A, Piechota H, Schlatter E. Kidney Int. 2005;67:1420–1427. doi: 10.1111/j.1523-1755.2005.00219.x. [DOI] [PubMed] [Google Scholar]
- 69.Sindic A, Schlatter E. Pflugers Arch. 2005;450:283–291. doi: 10.1007/s00424-005-1464-9. [DOI] [PubMed] [Google Scholar]
- 70.Potthast R, Ehler E, Scheving LA, Sindic A, Schlatter E, Kuhn M. Endocrinology. 2001;142:3087–3097. doi: 10.1210/endo.142.7.8274. [DOI] [PubMed] [Google Scholar]
- 71.Lorenz JN, Nieman M, Sabo J, Sanford LP, Hawkins JA, Elitsur N, Gawenis LR, Clarke LL, Cohen MB. J Clin Invest. 2003;112:1244–1254. doi: 10.1172/JCI18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen P, Toribara T, Warnner H. Anal Chem. 1956;28:1756–1758. [Google Scholar]
- 73.Führ J, Kazmarczyk J, Krüttgen CD. Klin Wochenschr. 1955;33:729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- 74.Matthews JN, Altman DG, Campbell MJ, Royston P. Br Med J. 1990;300:230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]