Abstract
Mitochondrial carriers are believed widely to be dimers both in structure and function. However, the structural fold is a barrel of six transmembrane α-helices without an obvious dimerisation interface. Here, we show by negative dominance studies that the yeast mitochondrial ADP/ATP carrier 2 from Saccharomyces cerevisiae (AAC2) is functional as a monomer in the mitochondrial membrane. Adenine nucleotide transport by wild-type AAC2 is inhibited by the sulfhydryl reagent 2-sulfonatoethyl-methanethiosulfonate (MTSES), whereas the activity of a mutant AAC2, devoid of cysteines, is unaffected. Wild-type and cysteine-less AAC2 were coexpressed in different molar ratios in yeast mitochondrial membranes. After addition of MTSES the residual transport activity correlated linearly with the fraction of cysteine-less carrier present in the membranes, and so the two versions functioned independently of each other. Also, the cysteine-less and wild-type carriers were purified separately, mixed in defined ratios and reconstituted into liposomes. Again, the residual transport activity in the presence of MTSES depended linearly on the amount of cysteine-less carrier. Thus, the entire transport cycle for ADP/ATP exchange is carried out by the monomer.
Keywords: mechanism, membrane protein, negative dominance studies, oligomeric state, transport
Mitochondrial carriers are believed widely to exist and function as homo-dimers (1–23). However, structural data are inconsistent with biochemical and biophysical studies that have been used to provide support for the existence of dimers in membranes and in detergents. First, the projection structure of the mitochondrial yeast ADP/ATP carrier demonstrated that the structural fold consists of six transmembrane (TM) α-helices rather than an intercalated bundle of twelve α-helices (24). Second, there are no extensive protein-protein interfaces between neighboring carriers in the crystals, as would be expected for proteins that require cooperative interaction to function (25). Third, the density distribution had 3-fold pseudo symmetry in agreement with the 3-fold sequence repeats found in all mitochondrial carriers (26, 27). Fourth, the substrate translocation pathway appeared to be in the centre of the protein, rather than at the interface between two monomers (24). Fifth, although the proteins formed two-dimensional crystals of dimers in rows in both the P22121 and P2 crystal forms (24), it was monomeric in detergent before crystal formation (28). Sixth, the atomic structure of the bovine ADP/ATP carrier in detergent determined by x-ray crystallography of P21212 and C2221 crystal forms showed that the structural fold was a six-α-helical bundle with three additional short α-helices in the mitochondrial matrix (13). Seventh, the P21212 crystal did not contain pairs of interacting proteins with the same orientation (13), but the C2221 crystal contained two types of dimers, one of which has been proposed to be biologically significant (11, 29). However, all of the protein-protein interactions in these crystals are mediated by proteins with different orientations, and so the dimers probably have been formed during crystallization. Eight, the pseudo 3-fold axis of symmetry in the C2221 crystal is at ≈10° to the plane of the membrane, which is incompatible with sequence and structural conservation of symmetry, and the density distribution in the projection structure of AAC3 (24). Ninth, homo-dimer formation in the C2221 crystal is mediated entirely by cardiolipins (11), and it is difficult to see how this type of interaction could provide a means for cooperativity and for the formation of specific homo-dimers in the presence many structurally related but functionally different carriers in mitochondrial membranes. Tenth, as with the P22121 and P2 crystals (24), both monomers of the dimer bind atractylosides and so they are in the same rather than in the opposed state. Finally, the dimer interface in the P22121 crystals (24) differs from that in the C2221 crystal (11), and so there is no consistent interaction interface. Thus, there is no structural explanation of how the dimers might form. Recently, we have shown that the yeast ADP/ATP carrier is monomeric in a wide range of detergents (28), but the question of whether the monomers are associated into homo-dimers in the membrane has remained unresolved.
Here, we have investigated by negative dominance studies whether a yeast ADP/ATP carrier in the mitochondrial membrane needs to be associated to function. This approach is based on dimers becoming dysfunctional when one protomer is disabled. It has been used to show that lactose permease LacY functions as a monomer (30), that the small drug transporter EmrE functions as an oligomer (31), and that maltoporin functions as a trimer with three distinct selectivity filters (32, 33). To establish the functional interaction between monomers, it must be possible to inactivate one protomer and to leave the second monomer unmodified and active. The yeast ADP/ATP carrier can be inactivated by sulfhydryl reagents, but replacement of its four cysteine residues by alanine provides a fully active carrier (34). Wild-type and cysteine-less ADP/ATP carriers were coexpressed in various molar ratios in mitochondrial membranes. The function of the cysteine-less ADP/ATP carrier 2 from Saccharomyces cerevisiae (AAC2) was unaffected by the inhibition of the coexpressed wild-type carriers, and so the two forms function independently. In addition, the cysteine-less and wild-type carriers were purified, mixed in defined ratios and reconstituted into liposomes. After addition of the sulfhydryl reagent, the residual transport rate correlated linearly with the fraction of cysteine-less AAC2, indicating that there is no functional association between AAC2 protomers. Thus, the yeast mitochondrial ADP/ATP carriers function as monomers in membranes.
Results
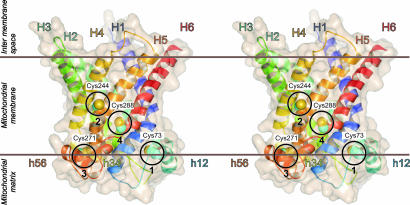
The yeast ADP/ATP carrier AAC2 has cysteines at residues 73, 244, 271, and 288 in matrix α-helix h12, in TM α-helix H5, in matrix α-helix h56, and in TM α-helix H6, respectively (Fig. 1). Transport activity of AAC2 can be inhibited by the sulfhydryl reagent eosin-5-maleimide, whereas the function of a cysteine-less AAC2, in which the cysteines were replaced by alanines, is unaffected (34). Similarly, the transport of ADP by wild-type AAC2 was inhibited fully by the membrane-impermeable sulfhydryl reagent, 2-sulfonatoethyl-methanethiosulfonate (MTSES), whereas ADP transport by the cysteine-less form was unaffected (Fig. 2C). The effect of MTSES on the transport activity was attributed mainly to Cys-73 and Cys-271, and to a much lesser extent to Cys-244 and Cys-288 [see supporting information (SI) Figs. 5 and 6 and http://www.pnas.org/cgi/content/full/0703969104/DC1SI Results].
Fig. 1.
Stereoview of the comparative structural model of the yeast ADP/ATP carrier AAC2. The model (courtesy of A. Robinson, Medical Research Council, Cambridge, U.K.) is based on coordinates of the bovine ADP/ATP carrier (13) and the alignment in SI Fig. 4. The structure is shown in cartoon and surface representation as generated by PyMOL (DeLano Scientific, Palo Alto, CA). The TM α-helices are numbered according to their appearance in the sequence. The matrix helices are preceded by h and named by the two TM α-helices they connect (13). The four encircled cysteines are numbered by their order in the sequence and they are shown as van der Waals spheres.
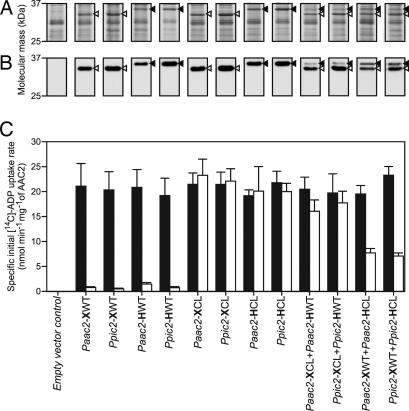
Fig. 2.
The transport activities in the presence and absence of MTSES of untagged and/or His-tagged AAC2 versions expressed individually or in combinations. (A) Coomassie blue-stained sodium-dodecylsulfate polyacrylamide gel of isolated mitochondrial membranes with ≈7.5 μg of protein loaded per lane. (B) Western blot of the same samples with α-AAC2 antibody with ≈0.75 μg of protein loaded per lane. Closed and open arrow heads indicate the positions of the His-tagged and untagged AAC2, respectively. (C) The effect of MTSES on the specific initial uptake rate of wild-type and cysteine-less AAC2, expressed individually or in combinations. The black and the white bars indicate the specific initial uptake rate in the absence and the presence of MTSES, respectively. Paac2 and Ppic2 are the promoters. X and H indicate untagged and His-tagged AAC2, respectively, and CL and WT indicate the cysteine-less and wild-type AAC2, respectively.
Coexpression of Cysteine-Less and Wild-Type AAC2 in Mitochondrial Membranes.
The cysteine-less and wild-type AAC2 were expressed at different and defined molar ratios in mitochondrial membranes (Fig. 2), and the effect of MTSES on transport activity was determined (Fig. 2 and 3). If the two versions of AAC2 functioned independently, the residual transport activity after addition of MTSES would correlate linearly with the fraction of cysteine-less AAC2 (Fcl) according to:
where Vwith MTSES and Vwithout MTSES are the specific initial transport rates in presence and absence of MTSES, respectively.
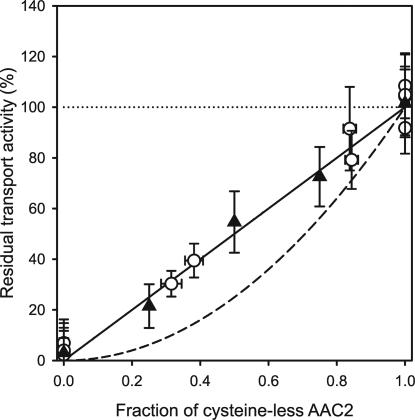
Fig. 3.
Correlation between the fraction of cysteine-less AAC2 and the residual initial transport rate after addition of MTSES. The residual initial transport rate in the presence of MTSES is expressed as a percentage of the rate in the absence of MTSES. Open circles represent the residual rate of coexpressed wild-type and/or cysteine-less AAC2 in mitochondrial membranes. The average rate in the absence of MTSES (100%, dotted line) was 20.6 ± 2.7 nmol·min−1·mg−1 of AAC2 (raw data from Fig. 2). The closed triangles indicate the residual transport rate of wild-type and/or cysteine-less AAC2, purified separately and mixed in defined molar ratios and then reconstituted into liposomes. In this case, the average rate was 16.4 ± 4.6 nmol·min−1·mg−1 of AAC2 in the absence of MTSES (100%, dotted line). The initial uptake rates of [14C]-ADP were determined in quintuplicate in the first 15 s of linear uptake. The amount of AAC2 in the fused mitochondrial membranes or proteoliposomes was quantified in triplicate by using Western blot analyses and known amounts of purified AAC2 as standard. The continuous and dashed lines represent the theoretical correlation for the independent and dependent functional interactions of the cysteine-less AAC2 and wild-type AAC2, respectively.
If two functional monomers had to dimerize to transport, both homo-dimers of the wild-type AAC2 and hetero-dimers of wild-type and cysteine-less protomers would be affected by the addition of MTSES. If so, the residual specific initial uptake rate in the presence of MTSES would depend on the square of the fraction of the cysteine-less AAC2 (see SI Fig. 7 for an explanation).
Cysteine-less and wild-type AAC2 were expressed from eight different expression cassettes, each consisting of a constitutive promoter followed by a version of the aac2 gene and a transcription terminator. The promoters were Paac2, the promoter of aac2, and Ppic2, the promoter of the mitochondrial phosphate carrier from S. cerevisiae. Each version of AAC2 was expressed with and without a six-histidine tag and a protease Xa cleavage site. Also, the wild-type and cysteine-less AAC2 were coexpressed from four tandem cassettes with the same promoter in both cassettes, so as to provide simultaneous expression of the two versions of AAC2. In the latter experiments, either the wild-type or the cysteine-less version of AAC2 was tagged. The tagged version was less mobile than the untagged version in sodium-dodecylsulfate polyacrylamide gels, allowing the two forms to be resolved and the amounts of wild-type and cysteine-less AAC2 expressed in the membrane to be quantified by Western blot analysis.
The tagged and untagged versions of wild-type and cysteine-less AAC2 were expressed in mitochondrial membranes separately or in all combinations (Fig. 2 A and B). The cysteine-less AAC2 was produced at similar levels to the wild-type AAC2, and the Ppic2 promoter was stronger than the Paac2 promoter. The average expression levels of the His-tagged versions were 77% that of the untagged versions. The fraction of cysteine-less carriers coexpressed by the tandem constructs was 0.843 for Paac2-XCL+Paac2-HWT, 0.838 for Ppic2-XCL+Ppic2-HWT, 0.382 for Paac2-XWT+Paac2-HCL, and 0.315 for Ppic2-XWT+Ppic2-HCL (Fig. 2 A and B). The molar ratio was not affected by the promoter or by the version of AAC2 and the presence of the His-tag was the main factor that reduced expression in both single and tandem constructs.
The specific initial uptake rate of ADP was determined in mitochondrial membranes in the presence and absence of MTSES. In the absence of MTSES, it had a similar value (average of 20.6 ± 2.7 nmol min−1 mg−1 of AAC2) for all version of AAC2 whether they were expressed as single or as tandem combinations (Fig. 2C). The average specific initial uptake rate for the cysteine-less carrier was similar to that of the wild-type AAC2 (20.2 ± 1.2 versus 20.9 ± 1.2 nmol·min−1·mg−1 of AAC2), confirming that the alanine substitutions did not affect the function of the carrier (Fig. 2C). The introduction of a six-histidine tag had no effect on function either, because the average values in the absence of MTSES were 20.6 ± 1.0 and 20.4 ± 1.5 nmol·min−1·mg−1 of AAC2 for untagged and tagged AAC2, respectively. Wild-type AAC2 was inhibited fully by the addition of MTSES, whereas cysteine-less AAC2 was unaffected (Fig. 2C). The specific initial uptake transport rates of the coexpressed Ppic2-XCL+Ppic2-HWT and Paac2-XCL+Paac2-HWT were reduced by the addition of MTSES to 90 and 78%, respectively. The addition of MTSES affected coexpressed Paac2-XWT+Paac2-HCL and Ppic2-XWT+Ppic2-HCL to a greater extent, leading to a reduction of transport rate to 39 and 30%, respectively. The residual transport activity correlated linearly with the fraction of cysteine-less AAC2 present in the membrane (Fig. 3). Therefore, the cysteine-less and wild-type carriers functioned independently.
Reconstitution of Cysteine-Less and Wild-Type AAC2 in Defined Molar Ratios.
The cysteine-less AAC2 and wild-type AAC2 in complex with ATR were purified separately in dodecylmaltoside, where AAC2 is monomeric (28), mixed in a defined ratio and reconstituted into liposomes. After reconstitution of the carriers into liposomes, ATR was displaced with ADP. The specific initial rate of uptake of ADP in the absence of MTSES was 16.4 ± 4.6 nmol·min−1·mg−1 of AAC2, which is slightly lower than the value found for the carrier in mitochondrial membranes, and so the reconstitution procedure was efficient. The slightly lower value may be due to the loss of small amounts of functional material during insertion, or to incomplete reversal of ATR inhibition or to the lipid environment, not mimicking the mitochondrial membrane. Again, the residual transport activity after addition of MTSES correlated linearly with the fraction of cysteine-less AAC2 that had been added to the mixture before reconstitution (Fig. 3).
Discussion
Here, the possible association of the monomers of the yeast ADP/ATP carrier AAC2 to form functional dimers was tested by negative dominance studies. The approach exploits the sensitivity of the wild-type AAC2 to sulfhydryl reagents (35), and the finding that the four cysteines in the wild-type AAC2 can be replaced by alanine without affecting its activity. When the cysteine-less and wild-type carriers were coexpressed in mitochondrial membranes, or mixed in defined ratios before being reconstituted in liposomes, the residual transport rate after addition of MTSES depended on the fraction of cysteine-less carrier in the membrane in a linear way (Fig. 3). The residual transport rates in the presence of MTSES fitted a power function, according to Vwith MTSES = a × Fclb. The fitted values were 102% and 1.03 for a and b, respectively, confirming that the cysteine-less and wild-type AAC2 functioned independently. The root mean square deviation was calculated between the experimental data and the theoretical curves taking the data points in the presence of MTSES (0 < Fcl < 1) (Fig. 3). The root mean square deviation is 2.8% for functionally independent carriers, which is within experimental error. In contrast, the root mean square deviation would have been 13.1% if they had functioned in a 1:1 dimer. Thus, the cysteine-less and wild-type AAC2 function independently. It might be argued that the results could be explained by homo-dimers of cysteine-less AAC2 or homo-dimers of wild-type AAC2 forming preferentially. For example, the presence of a His-tag could lead to the dimerisation of this version exclusively. However, this possibility was eliminated by the reconstitution experiments, in which both the cysteine-less AAC2 and wild-type AAC2 were His-tagged. In addition, if dimerisation by the His-tags occurred, it would have to have been stronger than dimerisation by a protein–protein interface, otherwise hetero-dimers of the two versions of AAC2 would have formed. However, then the specific transport rates would be expected to be low as dimerisation required for function would have been impaired (Fig. 2). Another possibility was that if cysteines were involved in dimer formation, the wild-type AAC2 might dimerize specifically to the exclusion of the cysteine-less AAC2. However, because cysteine-less carriers would be unable to dimerize, the specific uptake rate of the cysteine-less carriers would be low. Also, the overall transport rate of the coexpressed carriers would be negligible in the presence of MTSES, because the cysteine-less carriers would be unable to dimerize efficiently and the wild-type carriers would be inhibited. The simplest explanation of our data is that the yeast mitochondrial ADP/ATP carriers function as monomers rather than dimers. This conclusion is consistent with its structure (13, 24) and the dimensions and molecular mass of the ADP/ATP carrier in detergents (28).
Transporters of organic compounds vary considerably in size, oligomeric state, and the position of the translocation pathway. The TM domains of ABC transporters (10, but most commonly six TM α-helices) form dimers with the substrate transport pathway at their interface (36–38). Members of the major facilitator superfamily (12 TM α-helices) consist of two structurally similar protein domains (each of six TM α-helices) through which the substrate passes (39–42). The minimal functional unit for substrate binding in the multidrug transporter EmrE (four TM α-helices) is also a dimer with the substrate binding at the monomer-monomer interface (43, 44). Aquaporins and glycerol transporters (six TM α-helices plus two half-spanning α-helices) are tetramers with a substrate translocation pore in each monomer (45–47). The glutamate transporter (eight TM α-helices and two α-helical hairpins) (48), the ammonium channel (11 TM α-helices) (49), and the multidrug transporter AcrB (12 TM α-helices) (50) are trimers, whereas the leucine and aspartate transporters (10 TM α-helices plus two interrupted TM α-helices) are dimers (51, 52), but each monomer contains a substrate binding site.
In mitochondrial carriers the substrate is translocated via a pathway in the middle of the structural fold of the protein, which is formed by six TM α-helices (13, 24, 53, 54). Therefore, the ADP/ATP carrier is the smallest known functional transporter unit, and yet its substrates ADP and ATP are among the largest molecules that are actively transported across membranes. Other members of the mitochondrial carrier family transport even larger substrates like palmitoyl-carnitine (55, 56), CoA (57) or S-adenosyl-methionine (58). The transport of these substrates has to be achieved without any significant proton leak that would uncouple ATP synthesis from the proton motive force. These properties may explain why the mitochondrial ADP/ATP carrier transports substrates at a relatively low rate compared with members of other transporters families (59). Further studies into the structural mechanism of the mitochondrial carriers are required to understand how they accomplish this remarkable feat.
Methods
Construction of Expression Vectors.
A gene for the cysteine-less AAC2 was constructed by replacing all cysteine codons in wild-type aac2 by alanine codons by using PCR overlap extension with KOD polymerase (Novagen, Madison, WI and EMD Biosciences, San Diego, CA). Vectors for the expression of the His-tagged and untagged versions of the wild-type and cysteine-less AAC2 as single proteins were based on the pYES-Ppic2-aac2 and pYES-Ppic2-His-aac2 vectors (28) or the pYES-Paac2-His-aac2 and pYES-Paac2-aac2 vectors (60). To obtain vectors for coexpression, the aac2 gene (wild-type or cysteine-less), preceded by the aac2 or pic2 promoter region and followed by a transcription terminator, were introduced into the SpeI site at the start of the promoter region in the pYES-Ppic2-His-aac2 vector or pYES-Paac2-His-aac2 vector, containing the second type of aac2. SpeI sites were introduced by PCR with KOD polymerase (Novagen) at both ends of the promoter-aac2-transcription terminator cassette. Vectors and inserts were digested with SpeI (New England BioLabs, Ipswich, MA). The insert was ligated into the vector, and the vector was transformed into Escherichia coli. Because the insert could be inserted in both orientations, the desired version was identified by colony PCR and sequencing (Geneservice, Cambridge, U.K.). The vector containing the tandem expression cassette encoding wild-type and cysteine-less AAC2 was transformed into the S. cerevisiae strain WB-12 (MATα ade2-1 trp1-1 ura3-1 can1-100 aac1::LEU2 aac2::HIS3), lacking functional AAC1 and AAC2 carriers (21).
Preparation of Fused Mitochondrial Membranes for Transport Assays.
Yeast strains were grown as described in SI Methods and mitochondrial membranes were prepared as described in ref. 60. Liposomes were prepared with E. coli total lipid extract and egg yolk phosphatidylcholine (Avanti Polar Lipids, Alabaster, IL) mixed in a 3:1 (wt/wt) ratio in 50 mM potassium phosphate buffer (KPi), pH 7.0, at a final lipid concentration of 20 mg·ml−1. Liposomes (5 mg·ml−1) and mitochondrial membranes (1 mg of protein ml−1) were fused by mixing in 50 mM KPi, pH 7.0, containing 5 mM ADP, followed by freezing in liquid nitrogen and thawing at room temperature 7 times.
Inhibition and Purification of Yeast AAC2.
Mitochondrial membranes containing either wild-type or cysteine-less AAC2 were diluted to 20 mg·ml−1 with buffer, consisting of 0.65 M sorbitol and 0.02 M Tris·HCl, pH 7.4. The membranes were incubated for 20 min at 4°C with ATR (Calbiochem, San Diego, CA and EMD Biosciences, San Diego, CA) at a final concentration of 20 nmol·mg−1 of protein. Proteins were solubilized in 10 mM Tris·HCl (pH 7.4)/150 mM NaCl/10 mM imidazole/1% dodecyl-β-d-maltoside (Anatrace, Maumee, OH), and one tablet of Complete protease inhibitors minus EDTA (Roche Diagnostics, Mannheim, Germany) for 1 h at 4°C with stirring. Particulate material was removed by ultracentrifugation at 140,000 × g at 4°C for 45 min. The supernatant was passed through a nickel-nitrilotriacetic acid Superflow (Qiagen, Valencia, CA) column (flow rate, 0.5 ml·min−1). The column was washed with 10 mM Tris·HCl (pH 7.4)/150 mM NaCl/0.05% dodecylmaltoside/5 μM ATR, containing first 40 mM and then 60 mM imidazole. The carrier was eluted in the same buffer containing 200 mM imidazole. The eluate was concentrated to 2 mg·ml−1 in a YM-30 Centricon (Millipore, Billerica, MA) and purified on a Superdex 200 XK16/60 column (GE Healthcare, Little Chalfont, U.K.) in buffer containing 10 mM Tris·HCl (pH 7.4), 150 mM NaCl, 0.05% dodecylmaltoside, and 5 μM ATR.
Reconstitution of Purified AAC2.
Liposomes were prepared by using E. coli total lipid extract and egg yolk phosphatidylcholine (Avanti Polar Lipids) in a 3:1 (wt/wt) ratio in 50 mM KPi buffer, pH 7.0, at a final lipid concentration of 20 mg·ml−1. Liposomes were passed nine times through a membrane with 1-μm pores (Whatman, Clifton, NJ) to make them unilamellar, and they were destabilized with Triton X-100 (SI Methods). Purified carrier protein (20 μg) and destabilized liposomes (10 mg) were added to 50 mM KPi buffer (pH 7.0), with 0.2% (vol/vol) Triton X-100 and 5 mM ADP (3-ml total volume), and the suspension was mixed. Detergent was removed with Biobeads (240 mg) (Bio-Rad, Hercules, CA) and gentle rotation for 2 h at 4°C. The Biobeads were removed by filtration and the procedure was repeated twice more. The proteoliposomes were collected by centrifugation at 300,000 × g for 30 min at 4°C. The pellet was resuspended in KPi buffer containing 5 mM ADP to a final volume of 1 ml.
Transport Assays.
Proteoliposomes or fused membranes were extruded 9 times in the presence of 5 mM ADP through a membrane with 1-μm pores (Whatman). When required, 3 mM MTSES (Anatrace) was added before extrusion. The extruded membranes were harvested by centrifugation at 300,000 × g for 30 min at 4°C. The pellet was resuspended in KPi buffer containing 5 mM ADP. The external ADP was removed on a Sephadex G-75 gel filtration column (3.5-ml bed volume) equilibrated with KPi buffer. The proteoliposomes or fused membranes were eluted in 1 ml of KPi buffer. Transport was initiated by diluting 100 μl of membranes in 300 μl of KPi buffer containing 1.35 μM [8-14C]-ADP (Perkin–Elmer, Waltham, MA). The experiments were performed at 12°C with constant stirring. At intervals, the uptake of radio-labeled substrate was quenched by adding ice cold KPi buffer (4 ml), and immediately the mixture was filtered through cellulose nitrate membranes (0.45 μm pore size). The filters were washed with ice-cold KPi buffer (2 ml), and incorporated radioactivity was determined by scintillation counting. The initial uptake rates of [14C]-ADP were determined in quintuplicate in the first 15 s of linear uptake. The amount of AAC2 in the fused mitochondrial membranes or proteoliposomes was quantified in triplicate by using Western blot analyses and known amounts of purified AAC2 as standard. The total protein concentrations of the yeast mitochondrial membranes were determined by the BCA protein assay kit (Pierce, Rockford, IL) with BSA as standard. The sodium-dodecylsulfate polyacrylamide gel electrophoresis and Western blot analysis are described in SI Methods.
Supplementary Material
Acknowledgments
We thank J.E.W. for his support and for editing the manuscript. This work was funded by the U.K. Medical Research Council and the European Membrane Protein consortium.
Abbreviations
- ATR
atractyloside
- AAC2
ADP/ATP carrier 2 from S. cerevisiae
- MTSES
2-sulfonatoethyl-methanethiosulfonate
- KPi
potassium phosphate buffer
- TM
transmembrane.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703969104/DC1.
References
- 1.Lin CS, Hackenberg H, Klingenberg EM. FEBS Lett. 1980;113:304–306. doi: 10.1016/0014-5793(80)80614-4. [DOI] [PubMed] [Google Scholar]
- 2.Klingenberg M. Nature. 1981;290:449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- 3.Klingenberg M, Appel M. Eur J Biochem. 1989;180:123–131. doi: 10.1111/j.1432-1033.1989.tb14622.x. [DOI] [PubMed] [Google Scholar]
- 4.Bisaccia F, Zara V, Capobianco L, Iacobazzi V, Mazzeo M, Palmieri F. Biochim Biophys Acta. 1996;1292:281–288. doi: 10.1016/0167-4838(95)00215-4. [DOI] [PubMed] [Google Scholar]
- 5.Riccio P, Aquila H, Klingenberg M. FEBS Lett. 1975;56:133–138. doi: 10.1016/0014-5793(75)80127-x. [DOI] [PubMed] [Google Scholar]
- 6.Riccio P, Aquila H, Klingenberg M. FEBS Lett. 1975;56:129–132. [PubMed] [Google Scholar]
- 7.Hackenberg H, Klingenberg M. Biochemistry. 1980;19:548–555. doi: 10.1021/bi00544a024. [DOI] [PubMed] [Google Scholar]
- 8.Schroers A, Burkovski A, Wohlrab H, Kramer R. J Biol Chem. 1998;273:14269–14276. doi: 10.1074/jbc.273.23.14269. [DOI] [PubMed] [Google Scholar]
- 9.Kotaria R, Mayor JA, Walters DE, Kaplan RS. J Bionenerg Biomembr. 1999;31:543–549. doi: 10.1023/a:1005460810527. [DOI] [PubMed] [Google Scholar]
- 10.Block MR, Zaccai G, Lauquin GJ, Vignais PV. Biochem Biophys Res Commun. 1982;109:471–477. doi: 10.1016/0006-291x(82)91745-4. [DOI] [PubMed] [Google Scholar]
- 11.Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin G, Brandolin G, Pebay-Peyroula E. FEBS Lett. 2005;579:6031–6036. doi: 10.1016/j.febslet.2005.09.061. [DOI] [PubMed] [Google Scholar]
- 12.Pebay-Peyroula E, Brandolin G. Curr Opin Struct Biol. 2004;14:420–425. doi: 10.1016/j.sbi.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Pebay-Peyroula E, Dahout-Gonzalez C, Kahn R, Trezeguet V, Lauquin GJ, Brandolin G. Nature. 2003;426:39–44. doi: 10.1038/nature02056. [DOI] [PubMed] [Google Scholar]
- 14.Trezeguet V, Le Saux A, David C, Gourdet C, Fiore C, Dianoux A, Brandolin G, Lauquin GJ. Biochim Biophys Acta. 2000;1457:81–93. doi: 10.1016/s0005-2728(99)00115-2. [DOI] [PubMed] [Google Scholar]
- 15.Fiore C, Trezeguet V, Le Saux A, Roux P, Schwimmer C, Dianoux AC, Noel F, Lauquin GJ, Brandolin G, Vignais PV. Biochimie. 1998;80:137–150. doi: 10.1016/s0300-9084(98)80020-5. [DOI] [PubMed] [Google Scholar]
- 16.Postis V, De Marcos Lousa C, Arnou B, Lauquin GJ, Trezeguet V. Biochemistry. 2005;44:14732–14740. doi: 10.1021/bi051648x. [DOI] [PubMed] [Google Scholar]
- 17.Palmisano A, Zara V, Honlinger A, Vozza A, Dekker PJ, Pfanner N, Palmieri F. Biochem J. 1998;333:151–158. doi: 10.1042/bj3330151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capobianco L, Ferramosca A, Zara V. J Protein Chem. 2002;21:515–521. doi: 10.1023/a:1022473504904. [DOI] [PubMed] [Google Scholar]
- 19.Dyall SD, Agius SC, De Marcos Lousa C, Trezeguet V, Tokatlidis K. J Biol Chem. 2003;278:26757–26764. doi: 10.1074/jbc.M302700200. [DOI] [PubMed] [Google Scholar]
- 20.Huang SG, Odoy S, Klingenberg M. Arch Biochem Biophys. 2001;394:67–75. doi: 10.1006/abbi.2001.2520. [DOI] [PubMed] [Google Scholar]
- 21.Hatanaka T, Hashimoto M, Majima E, Shinohara Y, Terada H. Biochem Biophys Res Commun. 1999;262:726–730. doi: 10.1006/bbrc.1999.1283. [DOI] [PubMed] [Google Scholar]
- 22.Majima E, Ikawa K, Takeda M, Hashimoto M, Shinohara Y, Terada H. J Biol Chem. 1995;270:29548–29554. doi: 10.1074/jbc.270.49.29548. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto M, Majima E, Goto S, Shinohara Y, Terada H. Biochemistry. 1999;38:1050–1056. doi: 10.1021/bi9822978. [DOI] [PubMed] [Google Scholar]
- 24.Kunji ER, Harding M. J Biol Chem. 2003;278:36985–36988. doi: 10.1074/jbc.C300304200. [DOI] [PubMed] [Google Scholar]
- 25.Perutz MF. Q Rev Biophys. 1989;22:139–237. doi: 10.1017/s0033583500003826. [DOI] [PubMed] [Google Scholar]
- 26.Saraste M, Walker JE. FEBS Lett. 1982;144:250–254. doi: 10.1016/0014-5793(82)80648-0. [DOI] [PubMed] [Google Scholar]
- 27.Aquila H, Misra D, Eulitz M, Klingenberg M. Hoppe Seylers Z Physiol Chem. 1982;363:345–349. [PubMed] [Google Scholar]
- 28.Bamber L, Harding M, Butler PJ, Kunji ER. Proc Natl Acad Sci USA. 2006;103:16224–16229. doi: 10.1073/pnas.0607640103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nury H, Dahout-Gonzalez C, Trezeguet V, Lauquin GJ, Brandolin G, Pebay-Peyroula E. Annu Rev Biochem. 2006;75:713–741. doi: 10.1146/annurev.biochem.75.103004.142747. [DOI] [PubMed] [Google Scholar]
- 30.Sahin-Toth M, Lawrence MC, Kaback HR. Proc Natl Acad Sci USA. 1994;91:5421–5425. doi: 10.1073/pnas.91.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yerushalmi H, Lebendiker M, Schuldiner S. J Biol Chem. 1996;271:31044–31048. doi: 10.1074/jbc.271.49.31044. [DOI] [PubMed] [Google Scholar]
- 32.Marchal C, Hofnung M. EMBO J. 1983;2:81–86. doi: 10.1002/j.1460-2075.1983.tb01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferenci T, Lee KS. J Bacteriol. 1989;171:855–861. doi: 10.1128/jb.171.2.855-861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatanaka T, Kihira Y, Shinohara Y, Majima E, Terada H. Biochem Biophys Res Commun. 2001;286:936–942. doi: 10.1006/bbrc.2001.5498. [DOI] [PubMed] [Google Scholar]
- 35.Kihira Y, Majima E, Shinohara Y, Terada H. Biochemistry. 2005;44:184–192. doi: 10.1021/bi0488653. [DOI] [PubMed] [Google Scholar]
- 36.Pinkett HW, Lee AT, Lum P, Locher KP, Rees DC. Science. 2007;315:373–377. doi: 10.1126/science.1133488. [DOI] [PubMed] [Google Scholar]
- 37.Hollenstein K, Frei DC, Locher KP. Nature. 2007;446:213–216. doi: 10.1038/nature05626. [DOI] [PubMed] [Google Scholar]
- 38.Locher KP, Lee AT, Rees DC. Science. 2002;296:1091–1098. doi: 10.1126/science.1071142. [DOI] [PubMed] [Google Scholar]
- 39.Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 40.Hirai T, Heymann JA, Shi D, Sarker R, Maloney PC, Subramaniam S. Nat Struct Biol. 2002;9:597–600. doi: 10.1038/nsb821. [DOI] [PubMed] [Google Scholar]
- 41.Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Science. 2006;312:741–744. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Science. 2003;301:616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- 43.Ubarretxena-Belandia I, Baldwin JM, Schuldiner S, Tate CG. EMBO J. 2003;22:6175–6181. doi: 10.1093/emboj/cdg611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Butler PJ, Ubarretxena-Belandia I, Warne T, Tate CG. J Mol Biol. 2004;340:797–808. doi: 10.1016/j.jmb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Sui H, Han BG, Lee JK, Walian P, Jap BK. Nature. 2001;414:872–878. doi: 10.1038/414872a. [DOI] [PubMed] [Google Scholar]
- 46.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 47.Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, Stroud RM. Science. 2000;290:481–486. doi: 10.1126/science.290.5491.481. [DOI] [PubMed] [Google Scholar]
- 48.Yernool D, Boudker O, Jin Y, Gouaux E. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 49.Khademi S, O'Connell J, 3rd, Remis J, Robles-Colmenares Y, Miercke LJ, Stroud RM. Science. 2004;305:1587–1594. doi: 10.1126/science.1101952. [DOI] [PubMed] [Google Scholar]
- 50.Seeger MA, Schiefner A, Eicher T, Verrey F, Diederichs K, Pos KM. Science. 2006;313:1295–1298. doi: 10.1126/science.1131542. [DOI] [PubMed] [Google Scholar]
- 51.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 52.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 53.Kunji ER, Robinson AJ. Biochim Biophys Acta. 2006;1757:1237–1248. doi: 10.1016/j.bbabio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Robinson AJ, Kunji ER. Proc Natl Acad Sci USA. 2006;103:2617–2622. doi: 10.1073/pnas.0509994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huizing M, Iacobazzi V, Ijlst L, Savelkoul P, Ruitenbeek W, van den Heuvel L, Indiveri C, Smeitink J, Trijbels F, Wanders R, Palmieri F. Am J Hum Genet. 1997;61:1239–1245. doi: 10.1086/301628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Indiveri C, Tonazzi A, Palmieri F. Biochim Biophys Acta. 1990;1020:81–86. doi: 10.1016/0005-2728(90)90096-m. [DOI] [PubMed] [Google Scholar]
- 57.Prohl C, Pelzer W, Diekert K, Kmita H, Bedekovics T, Kispal G, Lill R. Mol Cell Biol. 2001;21:1089–1097. doi: 10.1128/MCB.21.4.1089-1097.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marobbio CM, Agrimi G, Lasorsa FM, Palmieri F. EMBO J. 2003;22:5975–5982. doi: 10.1093/emboj/cdg574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knirsch M, Gawaz MP, Klingenberg M. FEBS Lett. 1989;244:427–432. doi: 10.1016/0014-5793(89)80577-0. [DOI] [PubMed] [Google Scholar]
- 60.van der Giezen M, Slotboom DJ, Horner DS, Dyal PL, Harding M, Xue GP, Embley TM, Kunji ER. EMBO J. 2002;21:572–579. doi: 10.1093/emboj/21.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.