Abstract
Herpesviruses must traverse the nuclear envelope to gain access to the cytoplasm and, ultimately, to exit cells. It is believed that herpesvirus nucleocapsids enter the perinuclear space by budding through the inner nuclear membrane (NM). To reach the cytoplasm these enveloped particles must fuse with the outer NM and the unenveloped capsids then acquire a second envelope in the trans-Golgi network. Little is known about the process by which herpesviruses virions fuse with the outer NM. Here we show that a herpes simplex virus (HSV) mutant lacking both the two putative fusion glycoproteins gB and gH failed to cross the nuclear envelope. Enveloped virions accumulated in the perinuclear space or in membrane vesicles that bulged into the nucleoplasm (herniations). By contrast, mutants lacking just gB or gH showed only minor or no defects in nuclear egress. We concluded that either HSV gB or gH can promote fusion between the virion envelope and the outer NM. It is noteworthy that fusion associated with HSV entry requires the cooperative action of both gB and gH, suggesting that the two types of fusion (egress versus entry) are dissimilar processes.
Keywords: egress, nuclear envelope, deenvelopment, perinuclear space
The nuclear envelope (NE) is composed of inner and outer nuclear membrane (NM) separated by the perinuclear space and connected by nuclear pore complexes. A dense network of lamins forms a rigid girdle underneath the inner NM, organizing nuclear pores and chromatin (reviewed in ref. 1). For viruses that replicate their genomes in the nucleus, the NE often serves to confine and orchestrate nucleic acid synthesis and assembly of virus particles. However, the NE can also present a formidable barrier to virus egress from cells. Nonenveloped viruses such as SV40 and adenoviruses rupture the NE, as well as the plasma membrane, to gain release from cells (2, 3). Herpesviruses assemble large capsids in the nucleus but, because they are enveloped viruses, have evolved the capacity to cross membranes by budding and fusion mechanisms.
There has been extensive debate over how herpesviruses exit cells (4–8). Earlier models suggested that perinuclear enveloped virions are ferried from the perinuclear space/endoplasmic reticulum through the Golgi apparatus to the cell surface in exocytic transport vesicles (reviewed in ref. 9). However, a substantial body of genetic, biochemical, and morphologic evidence has substantiated the envelopment→deenvelopment→reenvelopment model for egress, at least for α-herpesviruses (reviewed in refs. 4 and 6). In this model, capsids become enveloped at the inner leaflet of the NE (primary envelopment) and are deenveloped at the outer NM releasing unenveloped cytoplasmic capsids, which acquire other tegument proteins and a second envelope by budding into the trans-Golgi network. For herpes simplex virus (HSV), this secondary envelopment requires glycoproteins gD or gE, and removal of both abolishes this process, but removal of either individually has little effect (10). Recently, it was suggested that capsids pass across the NE through enlarged nuclear pores into the cytoplasm as an alternative to envelopment→ reenvelopment (5). However, this conclusion relied entirely on EM, there was no genetic or biochemical corroboration, and other groups have not observed enlargement of nuclear pores or virus particles crossing nuclear pores (6).
Some aspects of primary envelopment have been elucidated. The HSV UL31 and UL34 proteins interact with one another and with the NE and partially disrupt or thin the nuclear lamina (11, 12). This provides sites on the inner NM where HSV nucleocapsids become enveloped. Similarly, the murine cytomegalovirus m50 and m53 proteins dock onto the inner NM and recruit protein kinase C that phosphorylates lamins causing dissolution of the nuclear lamina (13). Tegument-coated capsids apparently interact with modified regions of the inner NM, and virions bud into the perinuclear space. Based on what is known about secondary envelopment (10), it seems likely that primary envelopment might also involve interactions between tegument-coated capsids and viral glycoproteins. There is evidence that HSV glycoproteins gB, gD, and gM are present in the NE and perinuclear virions (14–16).
Almost nothing is known about the next step in egress, the process by which herpesvirus particles cross the outer NM. Certain viral proteins are incorporated into perinuclear enveloped virions but are not found in mature extracellular particles (reviewed in refs. 4 and 7). This suggests that virus particles are deenveloped at the outer NM, implying that there is fusion between the virion envelope and the outer NM. Given their capacity to mediate membrane fusion during entry, we hypothesized that herpesvirus glycoproteins promote fusion during egress. Three HSV membrane glycoproteins (gD, gB, and a heterodimer, gH/gL) are required for virus entry into cells (17–20). gD binds several classes of cell surface receptors, preceding the action of gB and gH/gL, which apparently directly or indirectly promote fusion between the virion envelope and cellular membranes (reviewed in ref. 21).
However, there is also evidence arguing against the notion that the HSV glycoproteins play important roles in nuclear egress. HSV mutants lacking any one of gB, gD, gH, or gL (essential for entry) are not substantially compromised in virus egress yet cannot enter cells (17–20). Additionally, the related herpesvirus pseudorabies virus produces perinuclear virions that are morphologically distinct from mature virions (22), and it has been suggested that perinuclear particles do not contain viral glycoproteins (7) (T. Mettenleiter, personal communication). However, the inference that HSV glycoproteins do not participate in nuclear egress, based on gB−, gD−, and gH− mutants, ignores the important notion of redundancy. Critical steps in herpesvirus morphogenesis are performed by more than a single protein; e.g., HSV gD or gE suffices for secondary envelopment (10). Here we investigated nuclear egress of HSV by characterizing a panel of double mutants lacking pairs of HSV glycoproteins. An HSV mutant lacking both gB and gH accumulated as enveloped virions in the perinuclear space and in the nucleoplasm. Mutants lacking just gB or gH showed much less pronounced defects in nuclear egress. We concluded that either gB or gH is required for fusion between the virion envelope and the outer NM and that they act in a redundant manner.
Results
Construction of Mutant HSV.
To characterize the roles of HSV glycoproteins we constructed a panel of mutant viruses by using a bacterial artificial chromosome (BAC) copy of the genome of HSV-1 strain F. A kanamycin resistance gene (kanr) cassette was used to replace glycoprotein genes as previously described for the construction of a BAC lacking both gD and gE sequences (23). This began with the construction of BAC mutants lacking individual genes, gD, gB, gH, or gE, and, subsequently, mutants lacking (i) gB, gE, and gI; (ii) gE and gI; (iii) gD, gE, and gI; (iv) gE and gH; and (v) gB and gH were constructed. Because glycoproteins gB, gD, and gH are essential for virus entry, viruses lacking these glycoproteins were propagated on VB38 cells (Vero cells that express gB), VD60 cells that express gD (19), or F6 cells that express gH (18). A cell line, denoted F6/gB+12 cells, that expresses both gB and gH was constructed from F6 cells by transfection with the gB gene and a selectable marker. F6/gB+12 cells were transfected with BAC gB−/gH− DNA producing a virus denoted F-BAC gB−/gH− that did not express gB and gH in Vero cells [supporting information (SI) Fig. 5].
An HSV Mutant Lacking gB and gH Accumulates in Nuclear Herniations in HaCaT Cells.
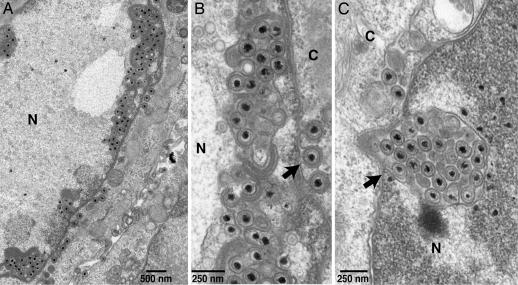
As previously observed (10), enveloped virions produced by wild-type HSV F-BAC were most commonly found on the surfaces of HaCaT (keratinocyte) cells, with smaller numbers in the cytoplasm and more rarely in the perinuclear space (SI Fig. 6 A and B). Other single and double HSV mutants (gB−, gD−, gE−, gH−, gE−/gI−, and gE−/gH− HSV) exited cells in a relatively normal fashion (data not shown). The gD−/gE−/gI− mutant was defective in secondary envelopment as described (10). By contrast, F-BAC gB−/gH− produced numerous enveloped virions that accumulated in the nucleus or in the perinuclear space (Fig. 1 and SI Fig. 6C). Membrane vesicles filled with enveloped virions were frequently observed in the nucleoplasm usually adjacent to the inner NM (Fig. 1 and SI Fig. 6C). These structures were denoted “herniations” based on the presumption that they were produced by budding of enveloped virions into the inner NE followed by bulging back into the nucleoplasm. In some instances we could trace continuities between the inner NM and the outer membrane of these vesicles (see arrow in Fig. 1C). A substantial fraction (≈25–40%) of F-BAC gB−/gH− infected HaCaT cells exhibited numerous herniations (Fig. 1A), another fraction (≈30%) had one to four herniations in a given section, and others contained no obvious herniations. Those gB−/gH− infected HaCaT cells with larger or more numerous herniations tended to have very few, or no, cell surface virions (Fig. 1A and SI Fig. 6C). When we counted virus particles, there was a 60-fold increase in enveloped nuclear virions (herniations) and a 34-fold increase in perinuclear virions comparing F-BAC gB−/gH− with F-BAC (wild type) (Table 1). More than 70% of the enveloped gB−/gH− virions were found in the perinuclear space and nucleus, whereas >70% of wild-type HSV enveloped particles were on cell surfaces. There were no obvious defects in the numbers or morphology of nuclear capsids in F-BAC gB−/gH− infected cells compared with wild type HSV-infected cells (Fig. 1); nuclear capsids represented ≈50% of the total capsids. There were reduced numbers of enveloped particles in the cytoplasm of F-BAC gB−/gH− infected HaCaT cells (Table 1) but no evidence of increased ratios of unenveloped:enveloped capsids in the cytoplasm (data not shown). The total numbers of enveloped virions in cells in gB−/gH− infected cells was not substantially different from those in F-BAC-infected cells (data not shown). Herniations were also observed, more rarely in HaCaT cells infected with wild-type F-BAC, and these tended to be small (SI Fig. 6B, arrow).
Fig. 1.
EM of HaCaT cells infected with F-BAC gB−/gH− for 18 h. The arrow in B points to a virion within the perinuclear space and protruding into the cytoplasm. The arrow in C points to a continuity between the inner NE and the membrane surrounding a herniation.
Table 1.
Distribution of enveloped virus particles in HaCaT and Vero cells infected with HSV mutants lacking gB and gH, gB, or gH
| Virus | Cells | No. of enveloped perinuclear virions | No. of enveloped nuclear virions* | No. of cytoplasmic enveloped virions† | No. of cell surface virions | Total |
|---|---|---|---|---|---|---|
| F-BAC (wild type) | HaCaT | 9 (0.7) | 10 (0.8) | 303 (24) | 923 (74) | 1,245 |
| F-BAC gB−/gH− | HaCaT | 315 (24) | 631 (49) | 157 (12) | 194 (15) | 1,297 |
| F-BAC gB− | HaCaT | 31 (4.5) | 45 (6.5) | 152 (22) | 461 (67) | 689 |
| F-BAC gB−/gE−/gI− | HaCaT | 16 (3) | 32 (6) | 196 (36) | 298 (55) | 542 |
| F-BAC gH− | HaCaT | 14 (1.0) | 36 (2.4) | 378 (26) | 1,038 (71) | 1,466 |
| F-BAC (wild type) | Vero | 6 (1.0) | 23 (4.0) | 63 (11) | 509 (85) | 601 |
| F-BAC gB−/gH− | Vero | 412 (50) | 70 (8.5) | 173 (21) | 166 (20) | 821 |
| F-BAC (wild type) | F6/gB+12 | 7 (1.4) | 2 (0.4) | 131 (27) | 345 (71) | 485 |
| F-BAC gB−/gH− | F6/gB+12 | 14 (2.2) | 0 (0) | 143 (23) | 457 (75) | 614 |
| KOS (wild type) | HaCaT | 5 (1.2) | 8 (2.0) | 120 (25) | 277 (67) | 410 |
| KO82 gB− | HaCaT | 49 (8.2) | 48 (8.0) | 186 (31) | 311 (52) | 594 |
| SC16 (wild type) | HaCaT | 10 | 21 | ND | 602 | 633 |
| SC16 gH− | HaCaT | 22 | 45 | ND | 535 | 602 |
Enveloped HSV particles were counted in 10–15 cells. Numbers in parentheses are the percentage of total particles counted. ND, not counted.
*Enveloped particles found in herniations surrounded by a membrane within the nucleoplasm.
†The majority (>90%) of HSV particles in the cytoplasm were enveloped.
Given these observations we reassessed whether HSV gB− and gH− mutants exit cells normally. There were some increases in perinuclear enveloped virus particles and smaller herniations comparing F-BAC gB− with F-BAC (Table 1 and SI Fig. 7A). It is important to note that the herniations and perinuclear virions shown in SI Fig. 7 were not nearly as common as with gB−/gH− infected cells and that there were not diminished numbers of cell surface virions with F-BAC gB− (Table 1). Similar results were obtained with another gB− mutant, KO82 (17), and with F-BAC gB−/gE−/gI− (Table 1 and SI Fig. 7C), suggesting that gE/gI does not add to this defect. With the gB−, gB−/gE−/gI−, and gB−/gH− mutants we also observed enveloped virions within extensions from the NE protruding into the cytoplasm (see arrows in Fig. 1B and SI Fig. 7C). These structures were rarely observed in cells infected with wild-type HSV and likely represented reduced or stalled fusion between virions and the outer NM. There were also vesicles containing enveloped particles immersed in an electron-dense material (akin to the nucleoplasm) in the cytoplasm of gB−/gH− infected cells (SI Fig. 6D). Cells infected with BAC gH− and another gH− mutant, SC16gHZ (18), displayed minor increases in the numbers of nuclear and perinuclear enveloped virions, but in most cases cells primarily displayed cell surface virions (Table 1 and SI Fig. 7D).
HSV gB−/gH− Enveloped Virions Accumulate in the Perinuclear Space of Vero Cells.
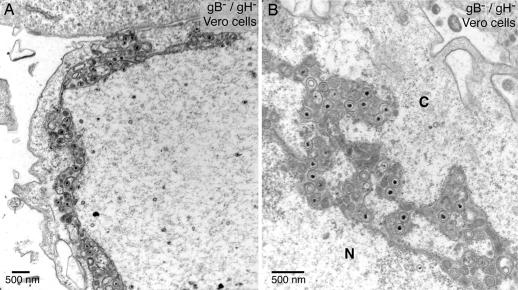
Defects in nuclear egress were also observed with Vero cells infected with F-BAC gB−/gH−, and, again, herniations were observed (Fig. 2, Table 1, and SI Fig. 8B). Herniations in Vero cells again exhibited continuities with the inner NM (see arrow in SI Fig. 8B). However, more F-BAC gB−/gH− particles accumulated in a modified version of the perinuclear space with dramatic separations between the inner and outer NM and branched extensions filled with vesicles, electron-dense material, and enveloped virions (Fig. 2). We characterized egress in F6/gB+12 cells (Vero cells that express gB and gH) to verify that the defects observed with F-BAC gB−/gH− did not result from mutations outside the gB and gH genes. F-BAC gB−/gH− did not exhibit obvious defects in egress in F6/gB+12 cells (Table 1 and SI Fig. 8 C and D).
Fig. 2.
EM of Vero cells infected with F-BAC gB−/gH− for 18 h. N, nucleus; C, cytoplasm.
Accumulation of gB and gH in the NE.
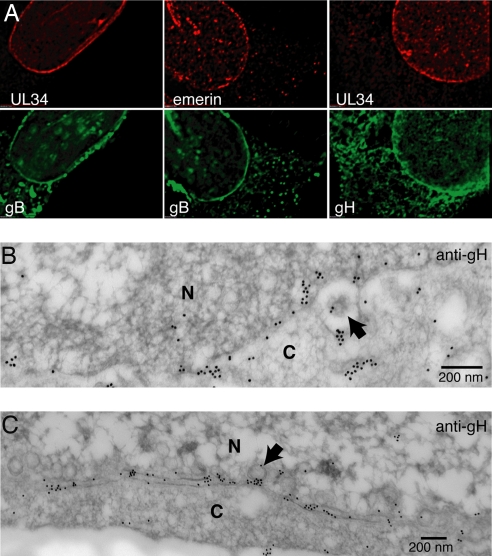
To begin to try to understand how gB and gH promote HSV egress across the NE, we used immunofluorescence and immunoelectron microscopy to characterize the NE and perinuclear virions. HaCaT cells were infected with HSV TsProtA, a mutant that produces a temperature-sensitive protease and immature capsids that accumulate in the nucleus and are not enveloped (24). By blocking egress, we anticipated that gB and gH might accumulate more extensively in the NE. In TsProtA-infected cells at 39°C, we observed a strong staining of the NE with gB- and gH-specific antibodies, colocalizing with NE proteins emerin and HSV UL34 (Fig. 3A). In wild-type HSV-infected cells, there was also substantial accumulation of gB and gH in the NE, although more of these glycoproteins were in cytoplasmic and surface membranes (data not shown). In immunoelectron microscopy experiments, gH− specific antibodies decorated both the NE and perinuclear virions (Fig. 3 B and C). As in previous reports (15, 16), we observed gB in the inner NE and perinuclear virions (SI Fig. 9). Together, these results show that gB and gH traffic into the NE, are incorporated into perinuclear virions, and accumulate in the NE when egress is blocked.
Fig. 3.
HSV gB and gH localize to the nuclear envelope. (A) Immunofluorescence studies of HaCaT cells infected with TsProtA at 39°C for 12 h then stained with chicken anti-UL34 or mouse anti-emerin antibodies and, simultaneously, with rabbit gB− or gH− antibodies. Secondary Texas red-conjugated anti-chicken IgY or anti-mouse IgG and FITC-conjugated anti-rabbit IgG secondary antibodies were used. (B and C) Immunoelectron microscopy of wild-type HSV-infected HaCaT cells stained with rabbit anti-gH antibodies and donkey anti-rabbit IgG conjugated onto 18-nm gold particles. Arrows point to perinuclear particles. N, nucleus; C, cytoplasm.
Immunoelectron Microscopic Analyses of Nuclear Herniations.
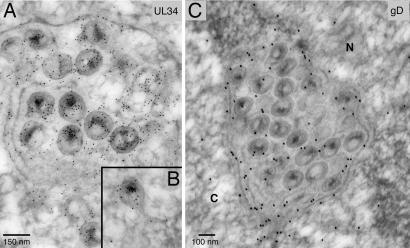
To further characterize the derivation of herniations, sections of F-BAC gB−/gH− infected cells were stained with UL34- and gD-specific antibodies. UL34 is largely localized to the NE, as well as to perinuclear virions (25). Anti-UL34 antibodies extensively decorated virions present within herniations, as well as the membranes surrounding herniations (Fig. 4A) and membranous protrusions extending from the NE into the cytoplasm (Fig. 4B). gD, which is part of the inner NM and perinuclear virions (14, 16), was present in herniation membranes and enclosed virions (Fig. 4C). These findings, combined with the observed continuities between herniation membranes and the inner NM, support our hypothesis that herniations are derived by inward bulging of the inner NM.
Fig. 4.
Immunoelectron microscopy of HaCaT cells infected with F-BAC gB−/gH− for 18 h then stained with chicken UL34-specific antibodies followed by donkey anti-chicken IgY conjugated onto 6-nm gold particles (A and B) or rabbit anti-gD antibodies followed by donkey anti-rabbit IgG conjugated onto 18-nm gold particles (C).
Discussion
Herpesviruses egress from host cells is a complex process, especially given that large capsids cross the NE without cell lysis. Genetic and biochemical studies support models in which envelopment of capsids at the inner NM is followed by deenvelopment, delivering capsids into the cytoplasm (4, 7). Deenvelopment would appear to involve fusion with the outer NM and is very poorly understood. No cellular or viral proteins that act directly in this fusion have been identified. HSV glycoprotein gK might regulate this process because enveloped perinuclear particles accumulate when gK is overexpressed (26). Herpesviruses employ viral glycoproteins to enter cells, a process that involves fusion of the virion envelope with cellular membranes. Thus, these glycoproteins are prime candidates to mediate egress across the NE. Confounding this notion, HSV mutants lacking individual viral glycoproteins do not exhibit substantial defects in nuclear egress (17–20). However, here we show that an HSV gB−/gH− mutant traverses the NE poorly, accumulating in the perinuclear space or in herniations. Viruses lacking only one of these glycoproteins were not as severely affected. We concluded that either gB or gH, acting in a largely redundant fashion, can mediate nuclear egress.
Our results are the first to solidly implicate herpesvirus membrane proteins in the deenvelopment process associated with nuclear egress. Given that gB and gH/gL are known to be intimately involved in entry fusion, it seems highly probable that gB and gH function directly in the fusion that occurs at the outer NM delivering capsids into the cytoplasm. However, it is also possible, although much less likely, that gB and gH are required to alter the outer NM in some manner, or that particles produced without gB and gH are somehow defective and lack the capacity to cross the NE.
In HaCaT cells infected with HSV gB−/gH−, most enveloped virions accumulated in herniations. In some instances, we could discern continuities between the outer membrane of the hernia and the inner NM. This suggested that there was bulging of the NM inward as virions filled the perinuclear space. Consistent with this hypothesis, UL34 and gD are present in perinuclear virions and the inner NM (14, 16, 25), as well as the outer membranes of herniations and virions inside herniations. We observed normal numbers of enveloped virions in gB−/gH− infected cells, suggesting that primary envelopment was not inhibited. Apparently, enveloped virions introduced into the perinuclear space, and unable to exit, herniate into the nucleus. In this view the NE is relatively fixed in volume or structurally rigid, so there is insufficient space for virions that accumulate and are unable to egress from the perinuclear space. We also observed enveloped virions present in membranous protuberances extending from the outer NM into the cytoplasm, consistent with the notion that fusion was slowed or inhibited. On the other hand, Vero cells infected with gB−/gH− HSV displayed mostly enveloped virions within the perinuclear space. Perhaps the NE is more flexible in Vero cells. Regardless of the mechanism, the accumulation of enveloped virions in the nucleus supports the hypothesis that deenvelopment or nuclear fusion is blocked when both of these glycoproteins are absent. This strongly argues that there is not substantial transit of HSV capsids across enlarged nuclear pores as an alternative for envelopment→deenvelopment.
An HSV US3− mutant accumulated structures similar to the herniations we observed (27). US3 is a serine/threonine kinase that phosphorylates UL34 (28) and likely regulates or facilitates nuclear egress in some way. Interestingly, accumulation of virions in US3-null-infected Vero cells was primarily in herniations, whereas our gB−/gH− mutant primarily accumulated as perinuclear virions in Vero cells. This suggests that US3 may have different effects on NE architecture compared with gB and gH. Structures morphologically similar to herniations have also been described in certain types of genetic diseases involving lamins (29, 30).
HSV glycoproteins gB and gH/gL have both been implicated as fusion-inducing proteins. gB has surprising structural similarity to vesicular stomatitis virus G protein, including putative fusion loops near the virion envelope (31). HSV gH contains an essential α-helical domain that has attributes of a fusion peptide and can be replaced by a vesicular stomatitis virus G protein fusion peptide (32). There is also recent evidence that gH/gL initiates hemifusion and that fusion is completed by gB (33). Our findings fit with the view that both gB and gH/gL are fusion proteins; i.e., gB or gH/gL can each (independent of the other) mediate fusion with the outer NM. Without both gB and gH, HSV exit from the perinuclear space is blocked and particles accumulate or herniate into the nucleus. The role of gL in this process is not yet clear. Export of gH from the endoplasmic reticulum requires gL (20), but this might not be required for gH to function in the nucleus.
All herpesviruses express gB and gH homologues. Thus, our observations may more universally describe how gB and gH function in this conserved process. There is evidence that Epstein–Barr virus gB is primarily localized to the NE and endoplasmic reticulum (34) and that Epstein–Barr virus and Kaposi's sarcoma-associated virus gB− mutants are defective in assembly or egress (35, 36). It is possible that other herpesviruses may rely more extensively on gB alone for nuclear fusion. There is also substantial probability that other HSV membrane glycoproteins play important roles in nuclear egress, e.g., participating in primary envelopment and/or fusion. Inhibition of HSV egress with the loss of gB and gH was not absolute. There were enveloped virions, albeit fewer, on the surfaces of gB−/gH− HSV-infected cells. gD could, for example, be involved in nuclear egress, and to uncover this it might require the construction of gB−/gD− or gD−/gH− mutants.
Materials and Methods
Cells.
The human HaCaT keratinocyte cell line and monkey Vero cells were described (10). F6 cells are Vero cells that express gH (18). VB38 cells are Vero cells that express gB and were constructed similar to VD60 cells (19). F6/gB+12 cells that express gB and gH were constructed by cotransfecting F6 cells with a plasmid containing gB sequences and pSV2HIS, and clones resistant to both G418 and histidinol were isolated as described (19).
Construction of F-BAC Mutants.
HSV-1 mutants containing single gene replacements of the gD, gE, gB, or gH genes with a kanr cassette were constructed from an HSV-1 BAC as described (23). To construct BAC gB−/gH−, the flp recombinase genes were used to remove the kanr gene from the gB gene in BAC gB−, and then the gH gene was replaced with a kanr cassette using lambda Red recombinase enzymes as described (23). All BACs were sequenced upstream and downstream of the insertions. HSV mutants were produced by transfecting complementing cells with BAC DNA as described (10).
Antibodies.
Chicken anti-UL34 antibodies (25) were used as antiserum diluted 1:25. mAb LP11 (18) and 15βB3 (10) were described. Anti-gD (R#45), anti-gB (R68), and anti-gH (R137) antisera were produced in rabbits by injecting HSV glycoproteins purified from virus-infected cells (37).
Microscopy.
For fluorescence microscopy infected cells were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and stained with primary and secondary antibodies as described (10). EM was as described (10). For immunoelectron microscopy, infected cells were fixed with 4% paraformaldehyde/0.25% glutaraldehyde and then postfixed, dehydrated in acetone, and embedded in LR White resin. Sections were cut and attached onto gold or nickel grids, stained with primary antibodies followed by secondary antibodies conjugated to 6- or 18-nm gold beads, then poststained with uranyl acetate and lead citrate as described (38).
Supplementary Material
Acknowledgments
We thank Catherine Wright for excellent technical assistance with the EM experiments. This work was supported by National Eye Institute Grant EY11245 (to D.C.J.) and National Institute of Allergy and Infectious Diseases Grants AI73996 (to D.C.J.), AI41478 (to R.R.), AI18289 (to G.C.), and AI56045 (to R.E.).
Abbreviations
- NE
nuclear envelope
- NM
nuclear membrane
- BAC
bacterial artificial chromosome
- HSV
herpes simplex virus.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703790104/DC1.
References
- 1.Stuurman N, Heins S, Aebi U. J Struct Biol. 1998;122:42–66. doi: 10.1006/jsbi.1998.3987. [DOI] [PubMed] [Google Scholar]
- 2.Daniels R, Rusan NM, Wilbuer AK, Norkin LC, Wadsworth P, Hebert DN. J Virol. 2006;80:6575–6587. doi: 10.1128/JVI.00347-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WS. J Virol. 1996;70:2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mettenleiter TC. J Virol. 2002;76:1537–1547. doi: 10.1128/JVI.76.4.1537-1547.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wild P, Engels M, Senn C, Tobler K, Ziegler U, Schraner EM, Loepfe E, Ackermann M, Mueller M, Walther P. J Virol. 2005;79:1071–1083. doi: 10.1128/JVI.79.2.1071-1083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mettenleiter TC, Minson T. J Virol. 2006;80:1610–1612. doi: 10.1128/JVI.80.3.1610-1612.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mettenleiter TC, Klupp BG, Granzow H. Curr Opin Microbiol. 2006;9:423–429. doi: 10.1016/j.mib.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume G, Roizman B. J Virol. 2006;80:6716–6719. doi: 10.1128/JVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roizman B, Knipe DM. Herpes Simplex Viruses and Their Replication. Philadelphia: Lippincott, Willams, and Wilkins; 2001. [Google Scholar]
- 10.Farnsworth A, Goldsmith K, Johnson DC. J Virol. 2003;77:8481–8494. doi: 10.1128/JVI.77.15.8481-8494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds AE, Liang L, Baines JD. J Virol. 2004;78:5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson-Holley M, Baines J, Roller R, Knipe DM. J Virol. 2004;78:5591–5600. doi: 10.1128/JVI.78.11.5591-5600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH. Science. 2002;297:854–857. doi: 10.1126/science.1071506. [DOI] [PubMed] [Google Scholar]
- 14.Skepper JN, Whiteley A, Browne H, Minson A. J Virol. 2001;75:5697–5702. doi: 10.1128/JVI.75.12.5697-5702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stannard LM, Himmelhoch S, Wynchank S. Arch Virol. 1996;141:505–524. doi: 10.1007/BF01718314. [DOI] [PubMed] [Google Scholar]
- 16.Torrisi MR, Di Lazzaro C, Pavan A, Pereira L, Campadelli-Fiume G. J Virol. 1992;66:554–561. doi: 10.1128/jvi.66.1.554-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai WZ, Person S, Warner SC, Zhou JH, DeLuca NA. J Virol. 1987;61:714–721. doi: 10.1128/jvi.61.3.714-721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forrester A, Farrell H, Wilkinson G, Kaye J, Davis-Poynter N, Minson T. J Virol. 1992;66:341–348. doi: 10.1128/jvi.66.1.341-348.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ligas MW, Johnson DC. J Virol. 1988;62:1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roop C, Hutchinson L, Johnson DC. J Virol. 1993;67:2285–2297. doi: 10.1128/jvi.67.4.2285-2297.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spear PG, Longnecker R. J Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granzow H, Klupp BG, Fuchs W, Veits J, Osterrieder N, Mettenleiter TC. J Virol. 2001;75:3675–3684. doi: 10.1128/JVI.75.8.3675-3684.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farnsworth A, Johnson DC. J Virol. 2006;80:3167–3179. doi: 10.1128/JVI.80.7.3167-3179.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao M, Matusick-Kumar L, Hurlburt W, DiTusa SF, Newcomb WW, Brown JC, McCann PJ, III, Deckman I, Colonno RJ. J Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. J Virol. 2001;75:8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutchinson L, Johnson DC. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryckman BJ, Roller RJ. J Virol. 2004;78:399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purves FC, Spector D, Roizman B. J Virol. 1992;66:4295–4303. doi: 10.1128/jvi.66.7.4295-4303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrmann H, Aebi U. Annu Rev Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 30.Maraldi NM, Squarzoni S, Sabatelli P, Capanni C, Mattioli E, Ognibene A, Lattanzi G. J Cell Physiol. 2005;203:319–327. doi: 10.1002/jcp.20217. [DOI] [PubMed] [Google Scholar]
- 31.Heldwein EE, Lou H, Bender FC, Cohen GH, Eisenberg RJ, Harrison SC. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 32.Gianni T, Martelli PL, Casadio R, Campadelli-Fiume G. J Virol. 2005;79:2931–2940. doi: 10.1128/JVI.79.5.2931-2940.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian RP, Geraghty RJ. Proc Natl Acad Sci USA. 2007;104:2903–2908. doi: 10.1073/pnas.0608374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papworth MA, Van Dijk AA, Benyon GR, Allen TD, Arrand JR, Mackett M. J Gen Virol. 1997;78:2179–2189. doi: 10.1099/0022-1317-78-9-2179. [DOI] [PubMed] [Google Scholar]
- 35.Lee SK, Longnecker R. J Virol. 1997;71:4092–4097. doi: 10.1128/jvi.71.5.4092-4097.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan HH, Sharma-Walia N, Zeng L, Gao SJ, Chandran B. J Virol. 2005;79:10952–10967. doi: 10.1128/JVI.79.17.10952-10967.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. J Virol. 2005;79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timms BG. Am J Anat. 1986;175:267–275. doi: 10.1002/aja.1001750211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.