Abstract
Histamine (HA), a biogenic amine with a broad spectrum of activities in both physiological and pathological settings, plays a key regulatory role in experimental allergic encephalomyelitis, the autoimmune model of multiple sclerosis. HA exerts its effect through four G protein-coupled receptors designated HA receptor H1, H2, H3, and H4. We report here that, compared with wild-type animals, mice with a disrupted HA H3 receptor (H3RKO), the expression of which is normally confined to cells of the nervous system, develop more severe disease and neuroinflammation. We show that this effect is associated with dysregulation of blood–brain barrier permeability and increased expression of MIP-2, IP-10, and CXCR3 by peripheral T cells. Our data suggest that pharmacological targeting of the H3R may be useful in preventing the development and formation of new lesions in multiple sclerosis, thereby significantly limiting the progression of the disease.
Keywords: blood–brain barrier, experimental allergic encephalomyelitis, multiple sclerosis
Histamine [2-(4-imidazole) ethylamine] (HA) is a ubiquitous mediator of diverse physiological processes including neurotransmission, secretion of pituitary hormones, and regulation of the gastrointestinal and circulatory systems (1). HA is a potent mediator of inflammation and a regulator of innate and adaptive immunity (2). HA exerts its effect through four G protein-coupled receptors [H1, H2, H3, and H4 receptor, designated according to the chronological order of their discovery (1)].
HA is implicated in the pathophysiology of multiple sclerosis (MS) and its animal models, collectively termed experimental allergic encephalomyelitis (EAE). HA and agents causing the release of HA from mast cells, the primary source of HA in the body (3), alter blood–brain barrier (BBB) permeability. Tissue levels of HA correlate with the onset of EAE (4–6). Inhibitors of mast cell degranulation and H1R and H2R antagonists modify EAE severity (7–12). Epidemiological data indicate that use of sedating H1R antagonists is associated with decreased MS risk (13). In a small pilot study, patients with relapsing–remitting or relapsing–progressive MS given the H1R antagonist hydroxyzine remained stable or improved neurologically (14). Microarray analysis revealed that the H1R was overexpressed in the chronic plaques of MS patients (15). Moreover, mouse genetic studies have shown that HA, H1R, and H2R play an important role in regulating encephalitogenic T cell responses and susceptibility to EAE (16–18). The role of H3R and H4R in EAE and MS has not been studied.
H3R is not normally expressed by hematopoietic cells; rather, it is expressed presynaptically where it is an inhibitory autoreceptor (inhibits release of HA from histaminergic neurons) and heteroreceptor (inhibits release of other neurotransmitters such as acetylcholine, noradrenaline, dopamine, and 5-HT from nonhistaminergic neurons) (19). Absence of presynaptic inhibition results in failure to limit neurotransmitter release, increased postsynaptic activity, and neurotransmitter spillover (20). To assess the role of central H3R signaling in regulating autoimmune inflammatory disease of the CNS, we studied MOG35–55 (myelin oligodendrocyte glycoprotein peptide 35–55)-induced EAE in B6.129P2-Hrh3tm1Twl (H3RKO) mice (21). We report here that, compared with B6 mice, H3RKO mice develop more severe acute-early phase disease and neuroinflammation. H3RKO mice did not exhibit a significant increase in either their humoral or cell-mediated anti-MOG35–55 responses, and ex vivo cytokine production of primed macrophages was not different from that of B6 macrophages. However, compared with B6 mice, H3RKO mice exhibited a significant increase in BBB permeability, and their T cells selectively expressed more MIP-2, IP-10, and CXCR3. CXCR3 marks T cells that are competent to migrate across the BBB (22).
Results
Clinical EAE in H3RKO mice.
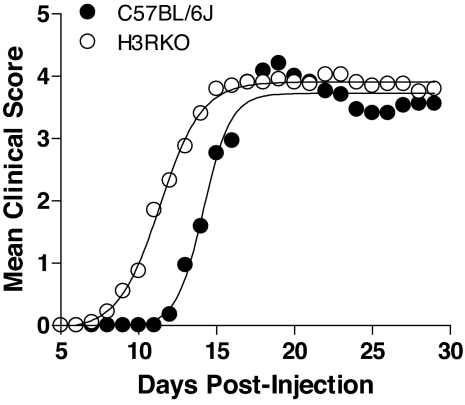
Male and female C57BL/6J (B6) and H3RKO mice were studied for susceptibility to MOG35–55-induced EAE (16–18). No difference was seen between male and female mice within each group [see supporting information (SI) Fig. 7], and the overall disease incidence did not differ significantly between B6 (17/17) and H3RKO (19/20) mice. Consequently, male and female mice were combined. Compared with B6 controls, H3RKO mice develop more severe EAE (Fig. 1). This is especially apparent in the acute-early phase of clinical disease. The mean day of disease onset in H3RKO mice was 11.2 ± 0.7 compared with 14.4 ± 0.5 in B6 controls (P = 0.0002). When the clinical disease parameters were evaluated during the acute-early [day 7 (D7) through D18] and chronic-late (D19 through D30) phases (23), the acute-early phase cumulative disease scores and number of days affected were found to be significantly greater in H3RKO mice compared with B6 animals [27.0 ± 2.6 vs. 16.5 ± 2.2 (P = 0.002) and 8.2 ± 0.6 vs. 4.5 ± 0.5 (P = 1e-05]; in contrast, the chronic-late phase cumulative disease scores and number of days affected were not significantly different [42.8 ± 7.1 vs. 40.5 ± 11.5 (P > 0.05) and 11.95 ± 0.2 vs. 12 ± 0 (P > 0.05)].
Fig. 1.
Clinical EAE course in B6 (n = 17) and H3RKO (n = 20) mice elicited by immunization with MOG35–55 plus CFA plus PTX. Regression analysis (23) revealed that the disease course in both strains fits a variable slope sigmoidal curve and is significantly different between the two strains (F = 32.5; P < 0.0001).
EAE Pathology.
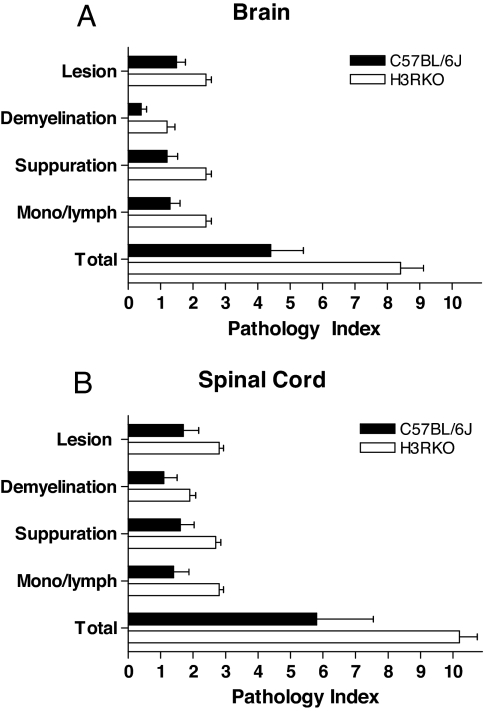
Assessment of the previously defined trait variables associated with EAE pathology (24, 25) (see SI Fig. 8) was performed at D12, the time point equivalent to half-maximal disease severity in H3RKO mice (Fig. 1), and at D30. At D12 H3RKO mice exhibited significantly greater EAE pathology in both the brain (Fig. 2A) and spinal cord (SC) (Fig. 2B) compared with B6. In contrast, at D30 there was not a significant difference in the overall severity of the lesions in either the brain [B6 = 2.2 ± 0.2 vs. H3RKO = 2.3 ± 0.2 (P > 0.05)] or the SC [B6 = 11.0 ± 0.8 vs. H3RKO = 11.0 ± 0.7 (P > 0.05)]. These results suggest that H3R signaling negatively regulates CNS autoimmune inflammatory disease and that the increased clinical disease seen during the acute-early phase in H3RKO mice is associated with earlier and more severe inflammatory infiltrates.
Fig. 2.
Quantification of lesion severity in B6 and H3RKO mice. Lesions in the brains (A) and SC (B) of H3RKO mice are more severe on D12 compared with B6 controls (P < 0.05 for all trait variables studied).
Changes in EAE-Associated Immune Components of H3RKO Mice.
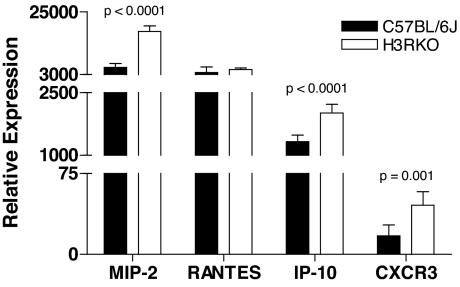
H3R is not detected on hematopoietically derived cells (19). RT-PCR analysis failed to detect H3R expression in normal unmanipulated mouse spleen and lymph node (data not shown). Nevertheless, we compared a variety of immune parameters between B6 and H3RKO mice after sensitization with MOG35–55 plus complete Freund's adjuvant (CFA) plus pertussis toxin (PTX). We hypothesized that, given the lack of expression of H3R on hematopoietically derived cells, we would not observe differences in their innate or adaptive immune functions. True to this expectation, there was no difference in the anti-MOG35–55 antibody response between B6 and H3RKO mice (see SI Fig. 9), and H3RKO mice generate MOG35–55-specific CD4 effector T cells equivalent in encephalitogenic activity to those elicited in B6 mice (see SI Fig. 10). Again as expected, in vitro proliferative responses of splenic and draining lymph node T cells did not differ markedly between B6 and H3RKO mice (see SI Fig. 11), and there was no significant difference in the secretion and/or expression of T helper 1, 2, or 17 cytokines, or Foxp3, after ex vivo restimulation with MOG35–55 (see SI Fig. 12). As expected, cytokine, chemokine, and growth factor production by peritoneal exudate macrophages from immunized B6 and H3RKO mice did not differ significantly in relative abundance (see SI Fig. 13). However, unexpectedly, MIP-2, IP-10, and CXCR3 (a receptor for IP-10), but not RANTES, expression was significantly greater in H3RKO T cells compared with B6 controls (Fig. 3).
Fig. 3.
MIP-2, RANTES, IP-10, and CXCR3 expression was determined by RT-PCR after ex vivo restimulation of B6 and H3RKO cells with MOG35–55. Samples were run in triplicate, and relative expression levels were determined by normalizing to L32. Significance of differences in expression between B6 and H3RKO T cells was determined by using Student's t test with a P value <0.05 as the significance threshold.
Changes in BBB Permeability in H3RKO Mice.
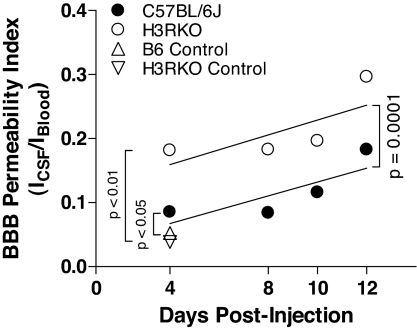
The significant difference in clinical signs and EAE pathology between B6 and H3RKO mice during the acute-early phase of the disease, despite the absence of detectable differences in encephalitogenic T cell activity and proinflammatory cytokine production, pointed to a potential role of H3R at the interface between the circulation and the brain. We hypothesized that H3R signaling may play a role in neurogenic control of cerebrovascular tone and BBB permeability (26, 27). We therefore compared the integrity of the BBB in B6 and H3RKO mice at various time points after injection with MOG35–55 plus CFA plus PTX (Fig. 4). Compared with unmanipulated B6 and H3RKO mice, immunized B6 and H3RKO mice exhibited significantly greater BBB permeability indices. Moreover, the BBB permeability indices were significantly greater in H3RKO mice compared with B6 mice (F = 4.8; P = 0.002). The rate of change was linear in both B6 and H3RKO mice (P > 0.05) and was not significantly different between them (F = 0.01; P = 0.93).
Fig. 4.
H3R signaling regulates BBB permeability. Compared with unmanipulated controls, immunized B6 and H3RKO mice have significantly greater BBB permeability indices, with BBB permeability being greater in H3RKO mice compared with B6 mice (F = 4.8; P = 0.002). The rate of change is linear in both strains (P > 0.05 for both curves) and is not significantly different between them (F = 0.01; P = 0.93).
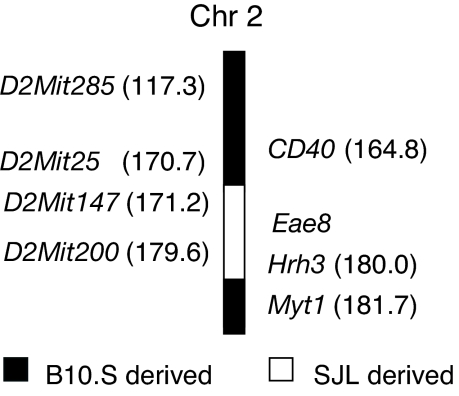
Hrh3 Is a Candidate Gene for Eae8.
Hrh3 is located at 180.03 megabases on telomeric chromosome 2 (www.genome.ucsc.edu) and is linked to Eae8, an EAE-modifying locus associated with disease susceptibility and weight loss (24, 28–30). We immunized congenic mice bearing the Eae8 genetic interval from SJL/J EAE-susceptible mice on the EAE-resistant B10.S background (B10.S.SJL-Eae8) and control mice. We have validated that Hrh3 is in the Eae8 congenic interval (Fig. 5). The incidence of EAE in B10.S.SJL-Eae8 mice was significantly greater than that observed in B10.S control mice (10/19 vs. 0/9; χ2 = 7.4; P = 0.007). The mean day of disease onset in B10.S.SJL-Eae8 mice was 13.9 ± 1.0 with a mean peak score of 1.1 ± 0.1. This study and the fact that H3R is known to regulate body weight (31) make Hrh3 an attractive candidate for Eae8.
Fig. 5.
Hrh3 is linked to Eae8. B10.S.SJL-Eae8 mice were generated by marker-assisted selection using D2Mit147 and D2Mit200. Animals were backcrossed for 10 generations, at which time they were intercrossed and subsequently fixed as a homozygous interval-specific congenic line. The Hrh3 allele was determined by SNP-based genotyping and sequencing. Mice were immunized with PLP139–151 plus CFA plus PTX and monitored for 30 days. The incidence of EAE in B10.S.SJL-Eae8 mice is significantly greater compared with B10.S/SgMcdJ.
Discussion
MS research is largely driven by two hypotheses (32). The immune-initiated hypothesis contends that autoreactive T cells generated in the periphery gain entry to the CNS where they elicit an inflammatory cascade that results in injury to previously normal neural tissues. In contrast, the neural-initiated disease hypothesis proposes that events within the CNS initiate the MS disease process and that autoimmune responses are secondary. EAE is often used to model aspects of these essentially conflicting hypotheses. We have investigated the role of H3R in EAE, and we find that this locus might serve to unite the dueling theories.
Induction of EAE by active immunization with MOG35–55 and adjuvants obviously involves a peripheral initiation phase. Two components of this immunization are CFA and PTX, both of which are known to increase BBB permeability (33–36) and cytokine and inflammatory mediator levels within the CNS rapidly after injection. Specifically, either CFA or PTX can elicit IL-1β, TNF-α, and/or IL-6 production in the CNS within 2 h after their injection (37–42). The increase in IL-1β expression is associated with widespread changes in neuronal activity, including Cox-2 expression in CNS neurons leading to elevated prostaglandin E2 levels in the CSF (43). In this setting, H3R, which regulates neurotransmitter release, may serve as a link between peripheral inflammatory stimuli and neural control of disease. Our data support the role of an H3R-mediated central component that negatively regulates susceptibility to neuroinflammatory reactions leading to EAE.
The H3R-deficient animals showed early exacerbation of EAE clinical signs. These results are consistent with the finding that susceptibility to EAE in mice with a disrupted histidine decarboxylase gene exhibit more severe disease compared with B6 mice (16). Because signaling due to HA can occur through each of the four HA receptors, the study of individual receptor knockout mice can reveal the contribution of each receptor to disease pathogenesis. Moreover, the absence of HA itself (in the histidine decarboxylase knockout mice) would be expected to reflect the coordinate action and integration of all of the EAE-relevant HA-induced signals. Thus, the absence of the H1R and H2R by themselves leads to a significant reduction in EAE severity (17, 18), indicating a largely proinflammatory or disease-promoting role for these two receptors; in contrast, the absence of the H3R leads to a significant early exacerbation of clinical signs, suggesting that H3R, when present, is largely CNS-protective. This protective effect has recently been demonstrated in a rat SC injury model, where the administration of H3R selective agonist α-methylhistamine ameliorated signs of injury including edema formation and blood SC barrier permeability (44). The EAE-protective nature of H3R, and the mapping of an EAE-modifying locus controlling disease severity and associated weight loss (Eae8) to the genomic interval containing Hrh3 (24, 28–30), combined with the known role of H3R in weight control (31), all provide evidence that Hrh3 is a good candidate gene for Eae8.
H3R effects on EAE appear to be related in part to neurogenic control of cerebrovascular tone and in part to unexpected changes on immune cells of the hematopoietic lineage that do not themselves express H3R (Fig. 6). We found significant differences very early in the disease process (D4) in BBB integrity of MOG-immunized B6 vs. H3R-deficient animals. In the periphery we saw changes in T cell chemokine/chemokine receptor expression by D10. These differences are both congruent with the earlier, more severe EAE and inflammatory infiltrates in H3RKO mice. The mechanism whereby the absence of H3R-mediated presynaptic inhibition of neurotransmitter release leads to increased EAE severity is unknown but is presumably a function of increased postsynaptic activity and neurotransmitter spillover (20).
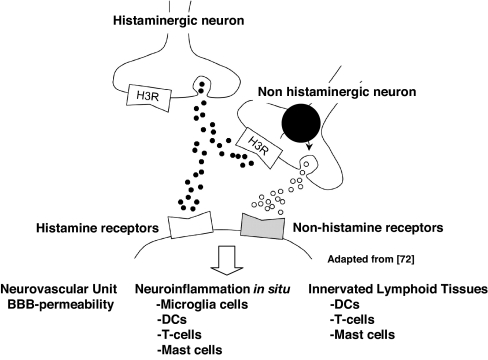
Fig. 6.
The H3R is located on the presynaptic membranes and cell soma of histaminergic and nonhistaminergic neurons, where it negatively regulates the synthesis and release of HA and other neurotransmitters (adapted from ref. 72). Inhibition of negative regulation of presynaptic H3R activity leads to earlier and more severe acute EAE associated with increased BBB permeability and expression of MIP-2, IP-10, and CXCR3 by peripheral CD4 T cells.
The neurogenically controlled changes related to cerebrovascular tone, particularly at the level of the neurovascular unit (26, 27), are consistent with an increasing body of evidence in both EAE (45, 46) and MS (47, 48) that subtle, progressive alterations in BBB integrity precede the formation of inflammatory lesions. These changes are detected in all MS disease subtypes, suggesting that a common abnormality in BBB function exists in the normal-appearing white matter of MS patients and may be a predisposing factor in initiating and propagating new inflammatory foci. Importantly, in our studies the H3R-related BBB effects do not likely derive from T cell activity, because they appear at D4 after immunization, significantly earlier than the appearance of inflammatory cells in the CNS. This supports a role for events predicted by the neural initiated disease hypothesis in the absence of any known infectious process.
A second level of neurogenic control, related to increased postsynaptic activity and neurotransmitter spillover in H3RKO mice, may be related to activation of resident hematopoietic-derived cells that are located in the brain, such as microglial cells. There are elaborate interactions between the brain and the immune systems, and one has only to recall that the hallmark inflammatory responses include “rubor, calor, and dolor” (erythema, heat, and pain) (49). We thus propose that the H3R pathway is a key intermediate in the intersection between brain and immune interactions.
The role of H3R has both antigen-independent and neuroantigen-specific components that influence brain inflammation. It has been shown that the increased levels of IL-1β, TNFα, and IL-6 found in the CNS after exposure to CFA and Bordetella pertussis (37–42) occurs as the result of stimulation of peripheral nocisponsive nerves (50). These axons transmit signals to their presynaptic terminals in the SC dorsal horns, and neurotransmitters released here bind to and activate postsynaptic receptors on pain transmission neurons. In turn, the axons of pain transmission neurons ascend to the brain and carry information about the noxious stimulus to higher centers. The increase in the aforementioned cytokines plays a role in contributing to neurogenic and inflammatory pain. Recently, it has been shown that purinergic transmitter stimulation of microglial cells may also contribute to neuropathic and inflammatory pain (51), which can be a major component of most forms of MS including benign disease (52). With respect to neuroinflammatory responses, purinergic receptors on microglial cells have been shown to regulate their activation, chemotaxis, and phagocytic activity (53, 54). The lack of presynaptic inhibition of neurotransmitter release by H3R-bearing neurons in the knockout may thus impact these antigen-independent pathways, thereby leading to more severe early-acute phase disease. Again, this phase of the disease process does not depend on effector T cell activity, because it occurs earlier than activated encephalitogenic T cells are found in the CNS. However, increased BBB permeability and earlier activation of microglial cells would presumably lead to earlier and more numerous T cell-dependent inflammatory foci within the CNS, which is what we find.
Neuroantigen-specific responses within the CNS may also be subject to increased postsynaptic activity and neurotransmitter spillover (Fig. 6). This may be directly at the level of the infiltrating T cells or indirectly through CNS resident antigen-presenting cells, such as dendritic cells, which have been proposed to be critical for the development and perpetuation of autoimmunity (55). Pathogenic T cells likely interact with “dendritic cell-like microglia” (56) and/or radiation-sensitive CD11c+ dendritic cells (57, 58) to enter the CNS. Our study of peritoneal exudate macrophages collected 10 days after immunization revealed no changes in their cytokine synthesis, although an exhaustive survey of monocytes, dendritic cells, and glia was not conducted.
The absence of H3R is unexpectedly associated with the expression of a novel set of chemokines/chemokine receptors (MIP-2, IP-10, and CXCR3) on peripheral T cells. The selective increase in T cell expression of MIP-2, IP-10, and CXCR3 in H3RKO mice is concordant with the appearance of earlier and more severe inflammatory infiltrates in these mice. CXCR3 expression, although not absolutely required for entry into the CNS, has been reported to mark T cells that are competent to migrate across the BBB (22).
Moreover, in support of this view, IP-10 is expressed by human CD8 T cells that react to neuroantigens and recruit CD4 T cells in MS patients (59, 60). The role of IP-10 expression in T cells in mouse EAE may mirror that of the human counterpart, given that B6 mice immunized with MOG35–55 generate encephalitogenic CD8 T cell responses (61–63). Interestingly, in other inflammatory conditions like oral lichen planus, IP-10 and its receptor CXCR3 are prominent on infiltrating human CD8 T cells. This suggests that there is a potential self-recruiting mechanism involving activated effector cytotoxic T cells (64). The possibility exists that this pattern of expression is associated with earlier CD8 T cell responses in H3RKO mice compared with B6 mice and that H3R signaling, which is also an inhibitory autoreceptor and heteroreceptor on peripheral neurons (65), clearly plays a role in regulating peripheral neural–immune cell interactions (49).
In this regard, it was recently shown that Idd4.1, a diabetes risk locus in NOD mice, is Trpv1 (transient receptor potential cation channel, subfamily V, member 1). Trpv1 is a receptor for capsaicin on nociceptive neurons in dorsal root and trigeminal ganglia, and capsaicin engagement of Tprv1 expressed on immature dendritic cells leads to their maturation and induction of antigen-presenting function (66). The diabetes-associated allele of Idd4.1 was identified as a hypofunctional mutant of Tprv1 that influences peripheral neuronal control of β cell stress and islet inflammation in NOD mice via a proinflammatory state (67). Importantly, H3R stimulation has been shown to prevent capsaicin-induced substance P release from C-fibers (68, 69). By analogy, in our model H3R (Eae8) presynaptic signaling in the CNS or in the peripheral nervous system could affect T cell gene expression. At the level of innervated secondary lymphoid tissues, increased neurotransmitter release could act directly on the T cell or indirectly via antigen-presenting cells or other members of the innate immune system.
In summary, our studies reveal the existence of an H3R-mediated central component in susceptibility to EAE related to neurogenic control of cerebrovascular tone and expression of chemokines and chemokine receptors by peripheral T cells. The role of H3R expression in response to peripheral inflammatory stimuli, such as the adjuvants used to elicit EAE, suggests a functional link between susceptibility to CNS autoimmune disease and neurogenic control of BBB permeability. This pathway may also be important in MS patients, where subtle changes in BBB integrity precede inflammatory lesions (47, 48). Pharmacologic targeting of the H3R may therefore be useful in preventing the development and formation of new lesions in MS, thereby significantly limiting the progression of the disease.
Materials and Methods
Animals.
C57BL/6J (B6), B10.S/SgMcdJ (B10.S), and SJL/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6.129P2-Hrh3tm1Twl mice (21) (H3RKO), developed at Johnson and Johnson Pharmaceutical Research and Development (San Diego, CA), were maintained at the University of Vermont (Burlington, VT). B10.S.SJL-Eae8 mice were generated by marker-assisted selection using markers spanning the Eae8 interval (24, 28–30). Animals were backcrossed for a minimum of 10 generations, at which point they were intercrossed and subsequently fixed as a homozygous interval-specific congenic line. All animals were maintained under specific pathogen-free conditions on a 12:12 light dark cycle and were fed Purina mouse pellets (Ralston-Purina, St. Louis, MO) and water ad libitum.
Induction and Evaluation of Actively (17, 18, 25) and Passively (70) Induced EAE.
Mice were injected s.c. in the flanks with 0.2 ml of an emulsion containing 200 μg of MOG35–55 or proteolipid protein 139–151 (PLP139–151) in saline and an equal volume of CFA containing 200 μg of Mycobacterium tuberculosis H37RA (Difco, Detroit, MI). On the day of immunization, each mouse received 100 ng of PTX (List Biological Laboratories, Campbell, CA) i.v. For induction of passive disease, draining lymph nodes were harvested 10 days after immunization and the lymphocytes cultured with 30 μg of MOG35–55 and 20 ng of murine rIL-12. After 4 days, the cells were washed and resuspended in PBS for transfer by i.v. injection. Recipients also received 100 ng of PTX immediately after cell transfer and 48 h later. Clinical and histological assessments were made as previously described (24, 25, 28, 29). Briefly, EAE pathology reflects the overall severity of the lesions observed, extent and degree of myelin loss and tissue injury (swollen axon sheathes, swollen axons, and reactive gliosis), severity of the acute inflammatory response (predominantly neutrophils), and the severity of the chronic inflammatory response (lymphocytes/macrophages).
Generation of Peritoneal Exudate Macrophages (18).
Peritoneal exudate cells were collected on D10 by peritoneal lavage 3 days after the i.p. injection of 1.5 ml of sterile thioglycolate (3%). The cells were suspended at a concentration of 1 × 106 macrophages per milliliter in media, and the nonadherent cells were removed after 3 h of plating. The adherent peritoneal exudate macrophages (>95%) were subsequently cultured for 24 h in media.
Proliferation Assay (17, 18).
Mice were immunized for the active induction of EAE, and draining lymph nodes and spleens were harvested on D10. Single-cell suspensions were prepared, and 5 × 105 cells per well were plated on standard 96-well flat-bottom tissue culture plates for 72 h at 37°C and 5% CO2 with and without MOG35–55 and in the presence of 1.0 μCi of [3H]thymidine during the last 18 h. Cells were harvested onto glass fiber filters, and thymidine uptake was determined by liquid scintillation.
Cytokine Assays (17, 18).
Mice were immunized for the active induction of EAE, and spleens were harvested on D10. Single-cell suspensions were prepared, and 4 × 106 cells per milliliter were cultured in 24-well culture places in media containing 50 μg/ml MOG35–55. Cell culture supernatants were recovered after 72 h and frozen at −70°C until assayed. IL-12, IL-10, IL-6, TNF-α, MCP-1, and IFNγ were detected by cytometric bead assay (Becton Dickinson Bioscience, San Jose, CA).
For RT-PCR analysis, total RNA was isolated by using the RNeasy minikit protocol (Qiagen, Valencia, CA) and then converted to cDNA by using oligo(dT), random hexamers, and SuperScript RT II enzyme (Invitrogen, Grand Island, NY). Message levels were quantified by using the ABI 7700 Real-Time PCR System (Applied Biosystems, Foster City, CA). Amplification was performed in a total volume of 25 μl for 40 cycles, and products were detected by using SYBR Green I dye (Molecular Probes, Eugene, OR). Samples were run in triplicate, and relative expression level was determined by normalizing to L32 with the results presented as relative abundance. Primer sequences are provided in SI Table 1.
Cytokine secretion by peritoneal exudate macrophages was quantified by using the RayBio Mouse Cytokine Array 1 (RayBiotech, Norcross, GA). Cell culture supernatants were collected and assayed as per the manufacturer's specifications. Complete medium was used as the negative control in all assays. The results were normalized, and the data are presented as the relative abundance with respect to the cytokine/growth factor being produced in the highest concentration (18).
Assessment of Antibody Responses (17, 18).
Anti-MOG35–55 antibody levels were determined by indirect ELISA. Dilutions of mouse sera from MOG35–55-immunized B6 control and H3RKO mice were incubated in MOG35–55-coated wells, and bound antibody was detected spectrophotometrically with peroxidase-conjugated anti-mouse Ig, anti-mouse IgG1, and anti-mouse IgG2a and o-phenylene-diamine as a substrate.
Assessment of BBB Permeability (71).
FITC-labeled BSA (50 μg/g) was administered by i.v. injection. Fifteen minutes later, both cerebrospinal fluid and plasma, prepared by centrifugation of the blood at 2,500 × g for 15 min, were diluted in PBS, and the fluorescence intensity was measured by using a fluorometer with an excitation wavelength of 497 nm and an emission wavelength of 519 nm. The BBB permeability index was determined by expressing the ratio of the fluorescence intensity of the cerebrospinal fluid sample divided by the fluorescence intensity of the plasma.
Supplementary Material
Acknowledgments
We thank Dr. Timothy W. Lovenberg (Johnson and Johnson Pharmaceutical Research and Development) for making the H3RKO mice available and for his helpful discussion, Dr. Vijay Kuchroo (Brigham and Woman's Hospital, Boston, MA) for the B10.S.SJL-Eae8 mice, and Dr. Lawrence Steinman (Stanford University, Stanford, CA) for critical comments on the manuscript. This work was supported by National Institutes of Health Grants NS036526, AI041747, AI058052, and AI045666 and National Multiple Sclerosis Society Grant RG-3575.
Abbreviations
- BBB
blood–brain barrier
- CFA
complete Freund's adjuvant
- EAE
experimental allergic encephalomyelitis
- HA
histamine
- MS
multiple sclerosis
- PTX
pertussis toxin
- SC
spinal cord
- Dn
day n.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0702291104/DC1.
References
- 1.Parsons ME, Ganellin CR. Br J Pharmacol. 2006;147:S127–S135. doi: 10.1038/sj.bjp.0706440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis CA, Simons FE. Eur J Pharmacol. 2006;533:69–76. doi: 10.1016/j.ejphar.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 3.Metcalfe DD, Baram D, Mekori YA. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 4.Orr EL, Stanley NC. J Neurochem. 1989;53:111–118. doi: 10.1111/j.1471-4159.1989.tb07301.x. [DOI] [PubMed] [Google Scholar]
- 5.Stanley NC, Jackson FL, Orr EL. J Neuroimmunol. 1990;29:223–228. doi: 10.1016/0165-5728(90)90165-j. [DOI] [PubMed] [Google Scholar]
- 6.Ichigi J. J Mol Neurosci. 1999;13:93–99. doi: 10.1385/JMN:13:1-2:93. [DOI] [PubMed] [Google Scholar]
- 7.Babington RG, Wedeking PW. J Pharmacol Exp Ther. 1971;177:454–460. [PubMed] [Google Scholar]
- 8.Linthicum DS, Munoz JJ, Blaskett A. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 9.Linthicum DS. Immunobiology. 1982;162:211–220. doi: 10.1016/S0171-2985(11)80001-X. [DOI] [PubMed] [Google Scholar]
- 10.Dimitriadou V, Pang X, Theoharides TC. Int J Immunopharmacol. 2000;22:673–684. doi: 10.1016/s0192-0561(00)00029-1. [DOI] [PubMed] [Google Scholar]
- 11.Emerson MR, Orentas DM, Lynch SG, LeVine SM. NeuroReport. 2002;13:1407–1410. doi: 10.1097/00001756-200208070-00012. [DOI] [PubMed] [Google Scholar]
- 12.Pedotti R, DeVoss JJ, Youssef S, Mitchell D, Wedemeyer J, Madanat R, Garren H, Fontoura P, Tsai M, Galli SJ, et al. Proc Natl Acad Sci USA. 2003;100:1867–1872. doi: 10.1073/pnas.252777399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso A, Jick SS, Hernan MA. Neurology. 2006;66:572–575. doi: 10.1212/01.wnl.0000198507.13597.45. [DOI] [PubMed] [Google Scholar]
- 14.Logothetis L, Mylonas IA, Baloyannis S, Pashalidou M, Orologas A, Zafeiropoulos A, Kosta V, Theoharides TC. Int J Immunopathol Pharmacol. 2005;18:771–778. doi: 10.1177/039463200501800421. [DOI] [PubMed] [Google Scholar]
- 15.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 16.Musio S, Gallo B, Scabeni S, Lapilla M, Poliani PL, Matarese G, Ohtsu H, Galli SJ, Mantegazza R, Steinman L, et al. J Immunol. 2006;176:17–26. doi: 10.4049/jimmunol.176.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Ma RZ, Gao J, Meeker ND, Fillmore PD, Tung KS, Watanabe T, Zachary JF, Offner H, Blankenhorn EP, Teuscher C. Science. 2002;297:620–623. doi: 10.1126/science.1072810. [DOI] [PubMed] [Google Scholar]
- 18.Teuscher C, Poynter ME, Offner H, Zamora A, Watanabe T, Fillmore PD, Zachary JF, Blankenhorn EP. Am J Pathol. 2004;164:883–892. doi: 10.1016/S0002-9440(10)63176-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- 20.MacDermott AB, Role LW, Siegelbaum SA. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 21.Toyota H, Dugovic C, Koehl M, Laposky AD, Weber C, Ngo K, Wu Y, Lee DH, Yanai K, Sakurai E, et al. Mol Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- 22.Callahan MK, Williams KA, Kivisakk P, Pearce D, Stins MF, Ransohoff RM. J Neuroimmunol. 2004;153:150–157. doi: 10.1016/j.jneuroim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Polanczyk M, Yellayi S, Zamora A, Subramanian S, Tovey M, Vandenbark AA, Offner H, Zachary JF, Fillmore PD, Blankenhorn EP, et al. Am J Pathol. 2004;164:1915–1924. doi: 10.1016/s0002-9440(10)63752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Teuscher C. Am J Pathol. 2000;157:637–645. doi: 10.1016/S0002-9440(10)64574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Proc Natl Acad Sci USA. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raichle ME. Acta Neuropathol Suppl (Berlin) 1983;8:75–79. doi: 10.1007/978-3-642-68970-3_6. [DOI] [PubMed] [Google Scholar]
- 27.Hamel E. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 28.Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 29.Butterfield RJ, Blankenhorn EP, Roper RJ, Zachary JF, Doerge RW, Sudweeks J, Rose J, Teuscher C. J Immunol. 1999;162:3096–3102. [PubMed] [Google Scholar]
- 30.Encinas JA, Lees MB, Sobel RA, Symonowicz C, Weiner HL, Seidman CE, Seidman JG, Kuchroo VK. Int Immunol. 2001;13:257–264. doi: 10.1093/intimm/13.3.257. [DOI] [PubMed] [Google Scholar]
- 31.Hancock AA, Brune ME. Expert Opin Invest Drugs. 2006;14:223–241. doi: 10.1517/13543784.14.3.223. [DOI] [PubMed] [Google Scholar]
- 32.Prat A, Antel J. Curr Opin Neurol. 2005;18:225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 33.Brooks TA, Hawkins BT, Huber JD, Egleton RD, Davis TP. Am J Physiol. 2005;289:738–743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brooks TA, Ocheltree SM, Seelbach MJ, Charles RA, Nametz N, Egleton RD, Davis TP. Brain Res. 2006;1120:172–182. doi: 10.1016/j.brainres.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruckener KE, el Baya A, Galla HJ, Schmidt MA. J Cell Sci. 2003;116:1837–1846. doi: 10.1242/jcs.00378. [DOI] [PubMed] [Google Scholar]
- 36.Kugler S, Bocker K, Heusipp G, Greune L, Kim KS, Schmidt MA. Cell Microbiol. 2007;9:619–632. doi: 10.1111/j.1462-5822.2006.00813.x. [DOI] [PubMed] [Google Scholar]
- 37.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong ME, Loscher CE, Lynch MA, Mills KH. J Neuroimmunol. 2003;136:25–33. doi: 10.1016/s0165-5728(02)00468-x. [DOI] [PubMed] [Google Scholar]
- 39.Raghavendra V, Tanga FY, DeLeo JA. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 40.Loscher CE, Donnelly S, Lynch MA, Mills KH. J Neuroimmunol. 2000;102:172–181. doi: 10.1016/s0165-5728(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 41.Loscher CE, Donnelly S, Mills KH, Lynch MA. J Neuroimmunol. 2000;111:68–76. doi: 10.1016/s0165-5728(00)00366-0. [DOI] [PubMed] [Google Scholar]
- 42.Donnelly S, Loscher CE, Lynch MA, Mills KH. Infect Immun. 2001;69:4217–4223. doi: 10.1128/IAI.69.7.4217-4223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ek M, Engblom D, Saha S, Blomqvist A, Jakobsson PJ, Ericsson-Dahlstrand A. Nature. 2001;410:430–431. doi: 10.1038/35068632. [DOI] [PubMed] [Google Scholar]
- 44.Sharma HS, Vannemreddy P, Patnaik R, Patnaik S, Mohanty S. Acta Neurochir Suppl. 2006;96:316–321. doi: 10.1007/3-211-30714-1_67. [DOI] [PubMed] [Google Scholar]
- 45.Tonra JR, Reiseter BS, Kolbeck R, Nagashima K, Robertson R, Keyt B, Lindsay RM. J Comp Neurol. 2001;430:131–144. [PubMed] [Google Scholar]
- 46.Tonra JR. Cerebellum. 2002;1:57–68. doi: 10.1080/147342202753203096. [DOI] [PubMed] [Google Scholar]
- 47.Matthews PM, Arnold DL. Curr Opin Neurol. 2001;14:279–287. doi: 10.1097/00019052-200106000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Minagar A, Hy W, Jimenez JJ, Alexander JS. Neurol Res. 2006;28:230–235. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 49.Steinman L. Nat Immunol. 2004;5:571–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 50.Watkins LR, Maier SF. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 51.Inoue K. Novartis Found Symp. 2006;276:263–272. [PubMed] [Google Scholar]
- 52.Glads S, Nyland H, Myhr KM. Acta Neurol Scand Suppl. 2006;183:55–57. doi: 10.1111/j.1600-0404.2006.00617.x. [DOI] [PubMed] [Google Scholar]
- 53.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 54.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miller SD, McMahon EJ, Schreiner B, Bailey SL. Ann NY Acad Sci. 2007 Mar 21; doi: 10.1196/annals.1394.023.. [DOI] [PubMed] [Google Scholar]
- 56.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, et al. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 57.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 58.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 59.Biddison WE, Taub DD, Cruikshank WW, Center DM, Connor EW, Honma K. J Immunol. 1997;158:3046–3053. [PubMed] [Google Scholar]
- 60.Biddison WE, Cruikshank WW, Center DM, Pelfrey CM, Taub DD, Turner RV. J Immunol. 1998;160:444–448. [PubMed] [Google Scholar]
- 61.Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 62.Ford ML, Evavold BD. Eur J Immunol. 2005;35:76–85. doi: 10.1002/eji.200425660. [DOI] [PubMed] [Google Scholar]
- 63.Ford ML, Evavold BD. Cell Immunol. 2006;240:53–61. doi: 10.1016/j.cellimm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Iijima W, Ohtani H, Nakayama T, Sugawara Y, Sato E, Nagura H, Yoshie O, Sasano T. Am J Pathol. 2003;163:261–268. doi: 10.1016/S0002-9440(10)63649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bertaccini G, Coruzzi G, Poli E. Aliment Pharmacol Ther. 1991;5:585–591. doi: 10.1111/j.1365-2036.1991.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 66.Basu S, Srivastava P. Proc Natl Acad Sci USA. 2005;102:5120–5125. doi: 10.1073/pnas.0407780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P, et al. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 68.Nemmar A, Delaunois A, Beckers JF, Sulon J, Bolden S, Gustin P. Eur J Pharmacol. 1999;371:23–30. doi: 10.1016/s0014-2999(99)00176-4. [DOI] [PubMed] [Google Scholar]
- 69.Hossen MA, Inoue T, Shinmei Y, Fujii Y, Watanabe T, Kamei C. J Pharmacol Sci. 2006;100:297–302. doi: 10.1254/jphs.fpj05028x. [DOI] [PubMed] [Google Scholar]
- 70.Ito A, Matejuk A, Drought H, Dwyer J, Subramanian S, Vandenbark AA, Offner H. J Immunol. 2003;170:4802–4809. doi: 10.4049/jimmunol.170.9.4802. [DOI] [PubMed] [Google Scholar]
- 71.Tang T, Frenette PS, Hynes RO, Wagner DD, Mayadas TN. J Clin Invest. 1996;97:2485–2490. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto R, Miyamoto Y, Shimamura K, Ishihara A, Takahashi K, Kotani H, Chen AS, Chen HY, Macneil DJ, Kanatani A, Tokita S. Proc Natl Acad Sci USA. 2006;103:13866–13871. doi: 10.1073/pnas.0506104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.