Abstract
In addition to classical expression patterns in pituitary and placenta and functions in growth and reproduction, members of the small family of hormones that includes prolactin (PRL), growth hormone (GH), and placental lactogen are expressed by endothelia and have angiogenic effects. In contrast, 16- to 17-kDa proteolytic fragments of these hormones have antiangiogenic effects. Here we show that PRL and GH are bound and processed by members of the bone morphogenetic protein 1 (BMP1) subgroup of extracellular metalloproteinases, previously shown to play key roles in forming extracellular matrix and in activating certain TGFβ superfamily members. BMP1 has previously been suggested to play roles in angiogenesis, as high throughput screens have found its mRNA to be one of those induced to highest levels in tumor-associated endothelia compared with resting endothelia. PRL and GH cleavage is shown to occur in each hormone at a single site typical of sites previously characterized in known substrates of BMP1-like proteinases, and the ≈17-kDa PRL N-terminal fragment so produced is demonstrated to have potent antiangiogenic activity. Mouse embryo fibroblasts are shown to produce both PRL and GH and to process them to ≈17-kDa forms, whereas GH and PRL processing activity is lost in mouse embryo fibroblasts doubly null for two genes encoding BMP1-like proteinases.
Keywords: growth hormone, Tolloid, angiogenesis, phage display, mouse embryo fibroblasts
Prolactin, growth hormone (GH), and placental lactogen (PL) are prototypic members of a small family of ≈22- to 23-kDa hormones related in sequence and domain structure (1). This relatedness arises from gene duplication, because PRL and GH genes diverged from a single ancestor gene ≈4 × 108 years ago, whereas primate and nonprimate PL genes subsequently diverged from GH or PRL genes, respectively (2). In humans, GH and PL have 85% sequence identity, whereas PRL has ≈25% similarity to the other two hormones. Although initially associated with inducing lactation, >300 functions are now ascribed to PRL, including roles in reproduction, growth, metabolism, immunity, osmoregulation, and behavior (3–5). Similarly, GH, initially associated with stimulating growth, is now also associated with modulating metabolism, reproductive and immune functions, and other activities (6–8). PL acts on mammary development, corpus luteum maintenance, and progesterone production (9). Receptors for these hormones, related to the class 1 superfamily of cytokine receptors, activate the Jak/Stat pathway upon ligand binding (10).
PRL and GH are secreted by the anterior pituitary, and PL is secreted by the placenta, although these hormones are also secreted by other tissues. In particular, all three are secreted by endothelia, upon which they and their cleavage products have paracrine/autocrine effects (11). Specifically, each full-length hormone has been shown by various assays to have angiogenic effects, whereas N-terminal ≈16-kDa cleavage products play opposite roles as antiangiogenic factors (11, 12). Some studies have suggested cleavage of PRL to a 16-kDa fragment to be mediated by cathepsin D (13–15) or matrix metalloproteinases (MMPs) (16).
Bone morphogenetic protein 1 (BMP1) is the prototype of a subgroup of structurally similar metalloproteinases that perform various morphogenetic roles in a wide range of species (17, 18). There are four mammalian BMP1-like proteinases: BMP1, mTLD (mammalian Tolloid), and mTLL (mammalian Tolloid-like) 1 and 2 (18). BMP1 and mTLD are encoded by alternatively spliced mRNAs of the same gene, whereas mTLL1 and mTLL2 are genetically distinct. BMP1-like proteinases are key to forming extracellular matrix (ECM) via biosynthetic processing of precursors into functional ECM components. Substrates include procollagens I-III, V, VII, and XI; prolysyl oxidase (zymogen of an enzyme necessary to forming covalent cross-links in collagen and elastic fibers); prolaminin 5; precursors of the proteoglycans biglycan and osteoglycin; and the precursor of dentin matrix protein 1 (associated with initiating ECM mineralization in bones and teeth) (18). In addition to roles in forming ECM, BMP1-like proteinases activate latent complexes of certain TGFβ superfamily members, including BMP2, BMP4, GDF8, GDF11, and TGFβ1 (18, 19). BMP1-like proteinases also cleave, in vitro and in vivo, the C-terminal portion of the basement membrane proteoglycan perlecan to produce a highly potent 26-kDa antiangiogenic factor (20).
Here we demonstrate that BMP1-like proteinases process PRL and GH, in vitro and in vivo, to produce ≈17-kDa N-terminal fragments with antiangiogenic activity. Such cleavage is shown to occur in each hormone at a single site typical of cleavage sites previously characterized in known substrates of BMP1-like proteinases. Processing of substrates by BMP1-like proteinases to produce antiangiogenic factors is of particular interest in the context of findings that BMP1 mRNA is among those transcripts induced to highest levels in tumor-associated endothelia compared with resting endothelia (21). Implications of the data are discussed.
Results
BMP1 Binds PRL Hormone Family Members.
A form of BMP1 designed to bind, but not cleave or release, substrates was constructed for use as “bait” in a phage display screen for novel substrates. Toward this end, site-directed mutagenesis produced BMP1 in which the protease domain zinc-binding site His-Glu-Leu-Gly was changed to His-Ala-Leu-Gly via an E214A substitution. This approach was taken because previous studies showed the BMP1-related Drosophila proteinase Tolloid containing mutations that inactivate the protease domain, but in which substrate binding domains are intact, to trap substrates in a nonproteolytic complex (22, 23).
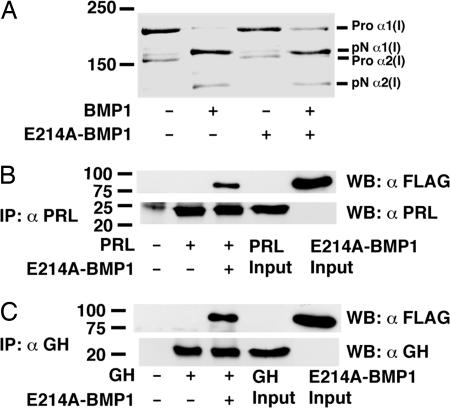
One function of BMP1 is cleavage of the C-propeptides of procollagens I–III (24). Toward characterizing the mutant BMP1 bait protein, it was incubated with type I procollagen in the absence or presence of wild-type BMP1. The E214A mutant BMP1 not only failed to cleave procollagen, it also partially blocked procollagen cleavage by wild-type BMP1, when equimolar amounts of the two proteases were added simultaneously to the procollagen sample (Fig. 1A). When E214A BMP1 was preincubated with procollagen, it completely blocked cleavage when wild-type BMP1 was subsequently added [supporting information (SI) Fig. 6]. Because BMP1 does not form oligomers, such results suggest that E214A BMP1 blocks wild-type BMP1 by binding substrate, thus accounting for the apparent dominant negative effect. E214A mutant BMP1 was thus deemed appropriate bait for a phage display screen for novel BMP1 substrates.
Fig. 1.
BMP1 binds and cleaves PRL and GH. (A) Type I procollagen, metabolically radiolabeled with [3H]proline, was incubated in the presence (+) or absence (−) of wild-type BMP1 and/or E214A BMP1 and then subjected to SDS/PAGE and autofluorography. Flag-tagged E214A BMP1 was incubated with PRL (B) or GH (C), and proteins were immunoprecipitated with antibody against the relevant hormone followed by Western blotting with anti-Flag antibody (αFLAG) or antibody to the relevant hormone (αPRL or αGH).
Screening of 109 individual clones of a human placenta phage cDNA expression library resulted in isolation of ≈250 clones that bound E214A BMP1. Sequence analysis of these clones identified coding sequences only for human PL and for one additional protein not germane to the present study. Although there is extensive literature regarding proteolytic cleavage of the related hormones PRL and GH to produce biologically active ≈16-kDa N-terminal fragments (11), evidence is lacking for in vivo processing of PL to yield an ≈16-kDa form. To characterize the ability of BMP1 to bind members of this hormone family, a Flag-tagged version of E214A BMP1 was separately incubated with PL, PRL, and GH followed by immunoprecipitation with anti-PL, anti-PRL, or anti-GH antibodies, respectively, and Western blotting to determine whether the mutant BMP1 was coprecipitated by binding any of the hormones. Surprisingly, PL did not pull down BMP1 under conditions of the assay (data not shown), but PRL and GH both readily bound BMP1 (Fig. 1 B and C). Despite such binding, it is not surprising that PRL and GH clones were not picked up via phage display, because PRL is not expressed in human placenta and placental GH mRNA is ≈4,000-fold less abundant than that of PL (25, 26).
PRL Family Members Are Cleaved by BMP1-Like Proteinases.
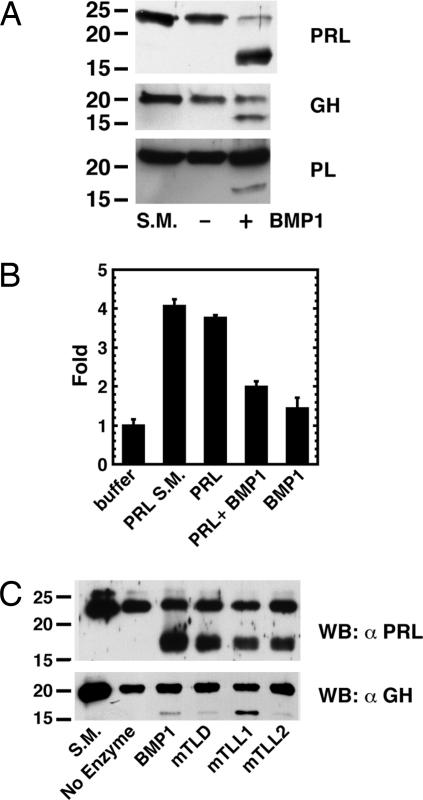
We next sought to determine the ability of BMP1 to cleave PRL, GH, and/or PL. Incubation of the same amount of wild-type BMP1 with equal amounts of the three hormones resulted in cleavage of all three to produce fragments of ≈17 kDa (Fig. 2A), similar in size to PRL and GH cleavage fragments thought to be of physiological importance (11). Products of no other sizes were observed, nor was there evidence of nonspecific proteolytic degradation. The effect of cleavage by BMP1 on function was examined for PRL in a reporter gene assay in 293 cells cotransfected with an expression vector for the long form of the PRL receptor and a reporter gene containing three copies of the consensus Stat5 binding sequence upstream of a luciferase reporter (27, 28). BMP1 cleavage of 23-kDa PRL to the 17-kDa form appeared to destroy ability to signal via the Jak/Stat pathway (Fig. 2B).
Fig. 2.
BMP1-like proteinases cleave PRL, GH, and PL to ≈17-kDa fragments. (A) Hormones were incubated for 6 h in the presence (+) or absence (−) of wild-type BMP1 and then subjected to SDS/PAGE and Western blotting with antibodies to the relevant hormone. S.M., starting material before incubation. (B) 293 cells cotransfected with a PRL receptor construct and a reporter gene containing three copies of the consensus Stat5 binding sequence upstream of the luciferase gene were incubated with buffer, PRL starting material (S.M.), BMP1 alone, or PRL incubated for 24 h in the absence or presence of BMP1. Levels of luciferase activity were normalized to levels of β-galactosidase activity from a cotransfected β-galactosidase expression construct to control for transfection efficiency. Levels of luciferase activity are in arbitrary units normalized to a value of 1 for buffer alone. Data are given as means ± SD (n = 3). A Student t test, performed with SigmaPlot, showed a significant difference between levels of luciferase activity for the PRL and PRL plus BMP1 samples (P < 0.003). (C) PRL and GH were incubated for 6 h alone (No Enzyme) or in the presence of BMP1, mTLD, mTLL1, or mTLL2 and then subjected to SDS/PAGE and Western blot analysis with antibodies to the relevant hormone.
To determine the sites at which BMP1 cleaved the three hormones, scaled-up cleavage reactions were performed by using 3 μg each of PRL, GH, and PL. Cleavage reactions were then run on SDS/PAGE gels under nonreducing conditions, such that N- and C-terminal cleavage fragments of each protein remained disulfide bonded. In this way, the small C-terminal fragments were not lost out of the bottom of gels, but instead comigrated with N-terminal fragments as 23-kDa (PRL), 20-kDa (GH), and 21-kDa (PL) complexes (data not shown) whereas intact PRL, GH, and PL migrated as 18-kDa, 15-kDa, and 17-kDa bands under the same conditions. Coomassie blue staining (data not shown) revealed ≈70% and 50% of PRL and GH, respectively, but only 5–10% of PL, to migrate as the slower migrating complexes, consistent with the results of Western blots (Fig. 2) showing PL to be less efficiently cleaved by BMP1 than are the other two hormones. Complexes were isolated and subjected to Edman degradation for determination of N-terminal amino acid sequences. Sequences corresponded to the N termini of mature PRL, GH, and PL. In the case of PRL and GH these, at least in part, represented the N termini of 17-kDa N-terminal fragments, because Edman degradation of the same bands also produced sequences DEESRLSAYYN (PRL) and DDALLKNYGL (GH), representing the N termini of the C-terminal cleavage products. No additional sequences were obtained for PL, consistent with the conclusion that insufficient cleavage had occurred for detection of cleavage product N-terminal sequences.
N-terminal sequences for PRL and GH C-terminal fragments place the BMP1 cleavage sites between Ala-159 and Asp-160 (PRL) and between Asn-152 and Asp-153 (GH). Alignment (SI Fig. 7) shows PRL and GH BMP1 cleavage sites to have similarities with cleavage sites in other reported BMP substrates. In particular, the PRL and GH sites have an Asp in the P1′ position of the scissile bone, an almost invariant feature of cleavage sites of reported substrates of BMP1-like proteinases (18). They also have a Met residue (PRL) or residues with aromatic side chains (GH) upstream of the scissile bone, also common features of cleavage sites in substrates of BMP1-like proteinases (18). The predicted human PL cleavage site, based on full-length sequence alignments of human PL with human GH and human PRL, is also shown (SI Fig. 7).
We next sought to characterize potential cleavage of PRL, GH, and/or PL by the other mammalian BMP1-like proteinases, mTLD, mTLL1, and mTLL2. All three cleaved both PRL and GH to produce fragments that comigrated on SDS/PAGE with the 17-kDa fragments produced by BMP1 cleavage (Fig. 2C). BMP1 had the most robust activity in cleaving PRL, whereas mTLL1 was the most efficient at cleaving GH. Cleavage of PL by the other proteinases was even less pronounced than cleavage by BMP1 (data not shown).
Cells Employ BMP1-Like Proteinases to Cleave PRL and GH.
Mice homozygous null for the Bmp1 gene, which encodes alternatively spliced RNAs for BMP1 and mTLD (29), are perinatal lethal (30), whereas mice homozygous null for Tll1, which encodes mTLL1, are embryonic lethal (31). However, Bmp1+/− to Tll1+/− intercrosses produce Bmp1+/−;Tll1+/− heterozygotes that can be crossed to produce Bmp1;Tll1 doubly homozyogous null embryos (32, 33). Although such embryos are also embryonic lethal (32, 33), derived Bmp1;Tll1 doubly null mouse embryo fibroblasts (MEFs), lacking BMP1, mTLD, and mTLL1, have markedly decreased processing of substrates normally cleaved by BMP1-like proteinases in vivo (32–36), because removal of the three functionally overlapping proteinases leaves little residual activity. MEFs are fairly heterogeneous populations of cells (37), and we sought to determine whether such populations might produce detectable levels of PRL and/or GH and, if so, whether levels of PRL and GH proteolytic processing differed in Bmp1;Tll1 doubly null and wild-type cultures.
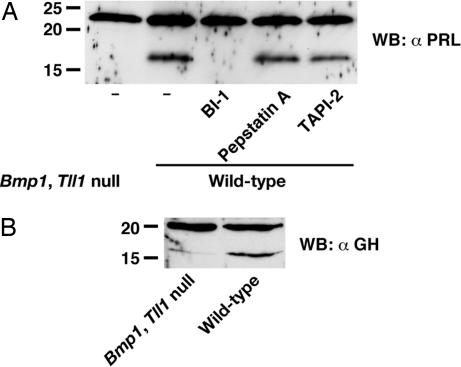
Detectable endogenous GH and PRL are produced by MEFs, and processed 17-kDa cleavage products are clearly detectable in wild-type culture medium (Fig. 3). However, markedly lower levels of 17-kDa GH cleavage products are found, and 17-kDa PRL cleavage products are undetectable, in Bmp1;Tll1 null MEF media. Thus, results are consistent with the interpretation that BMP1-like proteinases are involved in processing of PRL and GH to 17-kDa cleavage products by cells. Also consistent with this possibility is the conservation of potential BMP1-proteinase cleavage sites in murine (and rat) PRL and GH (SI Fig. 7). To further test the possibility that BMP1-like proteinases are involved in cellular processing of PRL and GH, PRL processing levels were compared in conditioned media of wild-type MEFs cultured in the presence or absence of the previously described hydroxamic acid-based inhibitor BI-1, which is highly specific for BMP1/TLD-like proteinases (38, 39). Treatment with the BMP1-like proteinase inhibitor led to markedly decreased processing of PRL to the 17-kDa form (Fig. 3A), again supporting the probability that BMP1-like proteinases are responsible for processing of PRL to a 17-kDa cleavage product by MEFs.
Fig. 3.
MEFs employ BMP1-like proteinases in processing PRL and GH to ≈17-kDa forms. Western blotting using antibodies to PRL (A) and GH (B) was used to monitor processing in cultures of wild-type MEFs or MEFs doubly null for Bmp1 and Tll1. Addition of a highly specific inhibitor of BMP1-like proteinases (BI-1) inhibits PRL processing in wild-type MEFs, but addition of cathepsin D inhibitor pepstatin A or MMP inhibitor TAPI-2 had no effect (A). The percentage of PRL cleaved in wild-type MEF media without inhibitor, or in the presence of pepstatin A or TAPI-2, was indistinguishable at 34%, 37%, and 32%, respectively, as determined via scanning densitometry using NIH Image software.
Although the most straightforward interpretation of the cell culture and in vitro data presented above is that BMP1-like proteinases directly process PRL to its 17-kDa form, the possibility existed that BMP1-like proteinases were indirectly responsible for this cleavage in MEF cultures, via activation of other proteinases. To explore the possibility that BMP1-proteinases might be involved in somehow increasing MEF activity levels of cathepsin D or MMPs, both of which have been implicated in PRL processing in previous reports (13–16), MEFs were cultured in the presence of cathepsin D inhibitor pepstatin A or MMP inhibitor TAPI-2. In contrast to the BMP1 inhibitor BI-1, neither pepstatin A nor TAPI-2 had any discernable effect on PRL processing in MEF cultures (Fig. 3A). Thus, neither cathepsin D nor MMPs are likely to play roles in MEF processing of PRL. However, we cannot formally exclude the possibility that BMP1-like proteinases might activate unidentified proteinases involved in PRL cleavage in MEF cultures.
Truncated Protein Corresponding to the 17-kDa PRL Fragment Produced by BMP1 Cleavage Is Antiangiogenic.
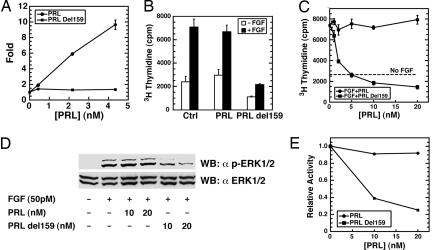
We next sought to determine whether 17-kDa fragments generated via PRL cleavage at the site used by BMP1 have antiangiogenic properties similar to those reported for ≈16-kDa PRL fragments isolated from tissues and cell cultures (11, 13, 15, 40). Toward this end, we produced a 17-kDa truncated form of recombinant PRL terminating at Ala-159, inclusive. As BMP1 cleaves PRL between Ala-159 and Asp-160, the truncated recombinant form (designated PRLdel159) represents the N-terminal BMP1 cleavage product. As can be seen (Fig. 4A), full-length PRL prepared in the same Escherichia coli expression system as PRLdel159 shows clear-cut activity in a standard assay for PRL-induced signaling, whereas PRLdel159, like BMP1-cleaved PRL (Fig. 2B), does not. To determine whether PRLdel159 has antiangiogenic properties, it was tested for the ability to inhibit bFGF-induced endothelial cell proliferation. Although bFGF-induced proliferation of human umbilical vein endothelial cells (HUVECs), denoted by increased [3H]thymidine incorporation, was not significantly reduced by full-length PRL, it was markedly inhibited by PRLdel159 (Fig. 4 B and C). Because it was previously reported that antiangiogenic activity of 16-kDa PRL fragments is associated with reduced MAPK phosphorylation (15, 41), we examined phosphorylation of ERK1/2 in FGF-treated HUVECs in the presence or absence of full-length PRL or PRLdel159. Whereas full-length PRL had no effect on ERK1/2 phosphorylation, exposure to PRLdel159 markedly decreased phosphorylation in a dose-dependent manner (Fig. 4 D and E).
Fig. 4.
Truncated recombinant PRL representing the ≈17-kDa N-terminal BMP1 cleavage product inhibits endothelial cell proliferation and ERK1/2 phosphorylation. (A) In the same type of reporter assay used in Fig. 2B, PRLdel159 is shown to lack the Jak/Stat signaling activity shown by full-length PRL. (B) HUVECs in the presence or absence of 50 pM bFGF were treated with 20 nM full-length PRL or PRL del159 followed by determination of [3H]thymidine incorporation. A Student t test, performed with SigmaPlot, showed PRLdel159 (P < 0.003), but not full-length PRL (P < 0.24), to significantly differ from buffer alone in the ability to inhibit FGF-induced [3H]thymidine incorporation by HUVECs. (C) HUVECs in the presence or absence of 50 pM bFGF were treated with various amounts of full-length and del159 PRL, and thymidine incorporation was determined as in B. Data for A–C are given as means ± SD (n = 3). (D) Extracts of HUVECs treated with PRL or PRLdel159 in the presence (+) or absence (−) of FGF were subjected to SDS/PAGE and then Western blotting with antibody specific for phosphorylated ERK1/2 (α p-ERK1/2) or total ERK1/2 (α ERK1/2). (E) The data of D were quantified and plotted by using scanning densitometry and NIH Image software.
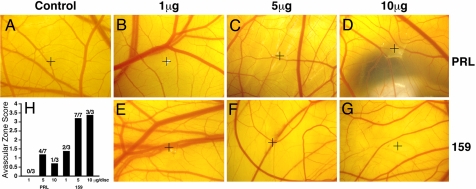
For an in vivo test of the antiangiogenic activity of 17-kDa PRL fragments truncated at the BMP1 cleavage site, we determined the ability of PRLdel159 to inhibit unstimulated angiogenesis in the chick chorioallantoic membrane (CAM) assay. For the assay, CAMs were treated with 1, 5, or 10 μg of full-length PRL (Fig. 5 B–D) or PRLdel159 (Fig. 5 E–G). For full-length PRL, no inhibition was observed at 1 μg, and small zones of apparent clearing confined to the immediate site of the disk were observed at 5 and 10 μg. In contrast, PRLdel159 showed strong dose-dependent antiangiogenic activity, with marked vessel clearance extending considerably beyond the site of the disk at 5 and 10 μg. These results are similar to those previously reported for full-length PRL and ≈16-kDa PRL fragments in CAM assays in which inhibition of neovascularization has been considerable for 16-kDa PRL fragments but in which there has been absence of vessel clearing or small zones of clearance for full-length PRL (12, 42).
Fig. 5.
Truncated recombinant PRL representing the ≈17-kDa N-terminal BMP1 cleavage product is antiangiogenic in the CAM assay. Representative CAMs are shown for BSA control (A) or full-length PRL (PRL) (B–D) or del159 PRL (159) (E–G) in 1-, 5-, and 10-μg doses tested via the CAM assay. Crosshairs mark the site of each disk. (H) A histogram shows the number of CAMs performed, the number of zones of clearing, and the average avascular zone score per dose of PRL or PRLdel159. Avascular zones were scored on a scale of 1–4, with 4 representing zones of maximum inhibition. CAMs in which no inhibition was observed were scored as zero.
Discussion
Angiogenesis occurs primarily in development, or in response to morphogenetic events accompanying female reproductive cycles, or in response to pathological stimuli. Within the adult, in which endothelia are normally quiescent, angiogenesis can play an important positive role in response to tissue damage, or a profoundly negative role, in providing the nourishment for tumor growth (43). Angiogenesis is thought to be controlled via a balance of proangiogenic and antiangiogenic factors (43) that can be skewed in response to tumor growth or tissue damage, such that effects of proangiogenic factors predominate.
There have been numerous reports of in vitro and in vivo antiangiogenic effects of ≈16-kDa PRL fragments (11, 12, 15, 41, 42, 44). However, the mechanism whereby such fragments may be generated in vivo has remained unclear. It was previously shown that ≈16-kDa N-terminal fragments can be cleaved from PRL by the lysosomal degradative protease cathepsin D (13, 15). It is not surprising that cathepsin D can cleave PRL to produce ≈16-kDa fragments, because potential cleavage sites for this degradative protease are located in an exposed surface loop of PRL (15, 40), and cleavage within this loop would yield ≈16-kDa N-terminal fragments. It is also not surprising that cathepsin D, which has an acidic pH optimum, would be highly active in cleaving various substrates when liberated from lysosomes in the acidified tissue and cell extracts used in previous studies (13, 15). Interestingly, it was also once proposed that cathepsin D is involved in biosynthetic processing of the C-propeptides of procollagens I–III based on similar experimental approaches proceeding from initial observations of procollagen C-propeptide cleavage in acidified tissue extracts (45). Biochemical and genetic approaches have subsequently demonstrated that procollagen I–III C-propeptides are cleaved in vivo by BMP1-like proteinases (24, 32, 46). Biochemical and genetic approaches are similarly used in the present report to demonstrate that BMP1-like proteinases process PRL at a single specific site in vitro to produce an ≈17-kDa fragment and that the same proteinases are used by cells to process PRL to an ≈17-kDa form.
After submission of this article, a role was reported for ≈16-kDa PRL forms in the etiology of postpartum cardiomyopathy in both humans and cardiomyocyte-specific Stat3-null mice (47). Hearts of the latter also had elevated levels of cathepsin D, capable of processing PRL to ≈16-kDa forms when recombinant PRL was added to acidified cardiac extracts (47). However, processing of endogenous PRL by endogenous cathepsin D was not demonstrable in that study, nor has it been demonstrated in other reports. We do not exclude here the possibility that cathepsin D, and perhaps other proteases such as MMPs, may be involved to some extent in processing PRL in vivo. Nevertheless, the genetic and biochemical data provided herein constitute definitive evidence identifying endogenous proteinases used by cells for the processing of endogenous PRL to ≈16/17-kDa forms. The latter findings may take on added importance in light of the findings of a role for ≈16-kDa PRL forms in postpartum cardiomyopathy (47).
The role demonstrated here for BMP1-like proteinases in processing endogenous proangiogenic PRL to produce antiangiogenic forms suggests that these proteinases may be key modulators of the homeostatic balance between proangiogenic and antiangiogenic factors controlling angiogenesis in the adult. This conclusion is bolstered by a high-throughput screen that previously showed BMP1 mRNA to be among those transcripts most highly elevated in tumor-associated, versus resting, endothelia (21). It is also bolstered by the finding that BMP1-like proteinases are responsible for in vivo processing of perlecan, a major structural component of endothelial basement membranes, to produce a potent antiangiogenic factor (20). Manifold roles of BMP1-like proteinases in generating antiangiogenic fragments, activating growth factors such as TGF-β1 (19), and forming ECM (18) demonstrate how such proteinases may serve as key orchestrators of normal and pathological morphogenetic events.
Materials and Methods
Protein Expression and Purification.
See http://www.pnas.org/cgi/content/full/0704179104/DC1SI Materials and Methods.
Phage Display.
A total of 300 ng of E214A BMP1 in 100 μl of 50 mM Tris·HCl (pH 7.5)/150 mM NaCl/5 mM CaCl2/1 mM MgCl2/1 mM ZnCl2 was left overnight in one well of a 96-well tissue culture plate at 4°C. The well was washed three times for 2 min with PBS/0.5% Tween 20 (PBS-T), blocked for 2.5 h with 1% BSA in PBS-T at room temperature, and then washed three times for 2 min with PBS-T. A total of 50 μl of phage in PBS/5 mM MgCl2/5 mM CaCl2 was added to the well and incubated for 2 h at room temperature. The well was then washed with PBS-T 10 times for 2 min, and log phase TG1 strain E. coli was added. Enrichment of E214A BMP1-binding phage and other steps were as described in the directions for the Phage Display Library Screening Kit (Spring Bioscience, Fremont, CA).
In Vitro Enzyme Assays.
A total of 100 ng of human PRL, GH, or PL was incubated for 6 h alone or with 50 ng of Flag-tagged BMP1, mTLD, mTLL1, or mTLL2 in 20 μl of 50 mM Tris·HCl (pH 7.5)/150 mM NaCl/5 mM CaCl2 at 37°C. Reactions were stopped with 5× SDS/PAGE sample buffer/1% 2-mercaptoethanol and boiling. Proteins were separated by SDS/PAGE on 15% acrylamide gels and detected by Western blot. Recombinant human PRL, GH, and PL and corresponding antibodies were purchased from A. F. Parlow (National Hormone and Peptide Program, Torrance, CA).
Collagen processing was in 50 mM Tris·HCl (pH 7.5)/150 mM NaCl/5 mM CaCl2 with 400 ng of 3H-radiolabeled type I procollagen, prepared as described (48). A total of 15 ng of BMP1, E214A mutant BMP1, or both were added to the reaction, which was stopped after 16 h at 37°C with SDS/PAGE sample buffer. Samples were subjected to SDS/PAGE on a 5% acrylamide gel, followed by treatment with EN3HANCE (PerkinElmer, Boston, MA) and autofluorography.
Amino Acid Sequencing.
A total of 3 μg of PRL or GH was cleaved as above. Products resolved by SDS/PAGE on a 12% acrylamide gel under nonreducing conditions were electrotransferred to a Sequi-Blot PVDF membrane (Bio-Rad) and revealed with Coomassie brilliant blue R-250, and 23-kDa PRL and 21-kDa GH cleavage fragments were excised for N-terminal amino acid sequencing at the Harvard Microchemistry Facility.
MEFs.
Wild-type and Bmp1/Tll1 doubly homozygous null MEFs were isolated from embryos 13.5 days after conception and immortalized as described (49). Confluent MEFs were washed twice with PBS, incubated for 15 min in serum-free DMEM at 37°C, and then incubated for 24 h in serum-free DMEM with 40 μg/ml soybean trypsin inhibitor. Conditioned media were harvested, and protease inhibitors were added to final concentrations of 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM N-ethylmaleimide, and 1 mM p-aminobenzonic acid. Media were centrifuged to remove debris, and proteins were ethanol-precipitated and separated by SDS/PAGE on 15% acrylamide gels.
Reporter Gene Assay.
By using Lipofectamine (Invitrogen) 293 cells were cotransfected with the long-form PRL receptor construct pcDNA3/PRLR (28) and the PRL-responsive PRE3-luciferase reporter construct, containing three copies of consensus Stat5 binding sequence TTCTTGGAA (27). Both constructs were gifts from Linda Schuler (University of Wisconsin, Madison, WI). The pSV40/β-galactosidase expression construct was cotransfected for normalizing transfection efficiencies. After transfection, cells were treated 24 h at 37°C with 50 ng/ml human PRL in serum-free DMEM containing 1 mg/ml BSA in a 24-well culture plate (Becton Dickinson). Luciferase and β-galactosidase activity levels were measured by using the manufacturer's protocols (Promega).
MAP Kinase Assay.
HUVECs maintained in M200 medium with low serum growth supplement were used at passages 3–5. Approximately 80% confluent HUVECs were serum-starved overnight and treated with or without 250 pM bFGF in the presence or absence of 10 or 20 nM full-length PRL or PRL del159 for 15 min at 37°C. Cells were then washed with ice-cold PBS and scraped into hot SDS/PAGE sample buffer. Twenty micrograms of total protein was separated on SDS/PAGE 10% acrylamide gels, transferred to nitrocellulose membranes, and probed with anti-phospho-ERK1/2 antibodies (Biosource). The blot was stripped and reprobed with antibody to total ERK1/2 (Upstate).
Endothelial Cell Proliferation Assay.
A total of 1 × 104 HUVECs were plated on 24-well plates and treated for 4 days with M200/2% FBS with or without 50 pM bFGF in the presence or absence of indicated amounts of full-length PRL or PRLdel159. Medium was changed every 2 days. Five hours before harvest, 0.5 μCi of [3H]methyl-thymidine was added to each well. Harvested cells were washed with ice-cold PBS followed by an ice-cold 5% trichloroacetic acid wash and solubilization in 0.25 N NaOH.
CAM Assay.
See http://www.pnas.org/cgi/content/full/0704179104/DC1SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Linda Schuler for helpful conversations and provision of PRL receptor and reporter constructs. This work was supported by National Institutes of Health Grant R01 GM71679 (to D.S.G.).
Abbreviations
- PRL
prolactin
- GH
growth hormone
- PL
placental lactogen
- BMP
bone morphogenetic protein
- MEF
mouse embryo fibroblast
- HUVEC
human umbilical vein endothelial cell
- CAM
chick chorioallantoic membrane
- MMP
matrix metalloproteinase
- ECM
extracellular matrix.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0704179104/DC1.
References
- 1.Nicoll CS, Mayer GL, Russell SM. Endocr Rev. 1986;7:169–203. doi: 10.1210/edrv-7-2-169. [DOI] [PubMed] [Google Scholar]
- 2.Goffin V, Shiverick KT, Kelly PA, Martial JA. Endocr Rev. 1996;17:385–410. doi: 10.1210/edrv-17-4-385. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Jonathan N, Mershon JL, Allen DL, Steinmetz RW. Endocr Rev. 1996;17:639–669. doi: 10.1210/edrv-17-6-639. [DOI] [PubMed] [Google Scholar]
- 4.Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- 5.Freeman ME, Kanyicska B, Lerant A, Nagy G. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 6.Hull KL, Harvey S. J Endocrinol. 2001;168:1–23. doi: 10.1677/joe.0.1680001. [DOI] [PubMed] [Google Scholar]
- 7.Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Endocr Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 8.Waters MJ, Shang CA, Behncken SN, Tam SP, Li H, Shen B, Lobie PE. Clin Exp Pharmacol Physiol. 1999;26:760–764. doi: 10.1046/j.1440-1681.1999.03129.x. [DOI] [PubMed] [Google Scholar]
- 9.Talamantes F, Ogren L. In: The Physiology of Reproduction. Knobil E, Neill JD, editors. New York: Raven; 1988. pp. 2093–2144. [Google Scholar]
- 10.Ihle JN, Kerr IM. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 11.Corbacho AM, Martinez De La Escalera G, Clapp C. J Endocrinol. 2002;173:219–238. doi: 10.1677/joe.0.1730219. [DOI] [PubMed] [Google Scholar]
- 12.Struman I, Bentzien F, Lee H, Mainfroid V, D'Angelo G, Goffin V, Weiner RI, Martial JA. Proc Natl Acad Sci USA. 1999;96:1246–1251. doi: 10.1073/pnas.96.4.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldocchi RA, Tan L, King DS, Nicoll CS. Endocrinology. 1993;133:935–938. doi: 10.1210/endo.133.2.8344226. [DOI] [PubMed] [Google Scholar]
- 14.Piwnica D, Fernandez I, Binart N, Touraine P, Kelly PA, Goffin V. Mol Endocrinol. 2006;20:3263–3278. doi: 10.1210/me.2006-0044. [DOI] [PubMed] [Google Scholar]
- 15.Piwnica D, Touraine P, Struman I, Tabruyn S, Bolbach G, Clapp C, Martial JA, Kelly PA, Goffin V. Mol Endocrinol. 2004;18:2522–2542. doi: 10.1210/me.2004-0200. [DOI] [PubMed] [Google Scholar]
- 16.Macotela Y, Aguilar MB, Guzman-Morales J, Rivera JC, Zermeno C, Lopez-Barrera F, Nava G, Lavalle C, Martinez de la Escalera G, Clapp C. J Cell Sci. 2006;119:1790–1800. doi: 10.1242/jcs.02887. [DOI] [PubMed] [Google Scholar]
- 17.Bond JS, Beynon RJ. Protein Sci. 1995;4:1247–1261. doi: 10.1002/pro.5560040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge G, Greenspan DS. Birth Defects Res C Embryo Today. 2006;78:47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- 19.Ge G, Greenspan DS. J Cell Biol. 2006;175:111–120. doi: 10.1083/jcb.200606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, Greenspan DS, Iozzo RV. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 21.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, et al. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 22.Childs SR, O'Connor MB. Dev Biol. 1994;162:209–220. doi: 10.1006/dbio.1994.1079. [DOI] [PubMed] [Google Scholar]
- 23.Finelli AL, Bossie CA, Xie T, Padgett RW. Development (Cambridge, UK) 1994;120:861–870. doi: 10.1242/dev.120.4.861. [DOI] [PubMed] [Google Scholar]
- 24.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Science. 1996;271:360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 25.Soares MJ, Faria TN, Roby KF, Deb S. Endocr Rev. 1991;12:402–423. doi: 10.1210/edrv-12-4-402. [DOI] [PubMed] [Google Scholar]
- 26.Walker WH, Fitzpatrick SL, Barrera-Saldana HA, Resendez-Perez D, Saunders GF. Endocr Rev. 1991;12:316–328. doi: 10.1210/edrv-12-4-316. [DOI] [PubMed] [Google Scholar]
- 27.Schroeder MD, Symowicz J, Schuler LA. Mol Endocrinol. 2002;16:45–57. doi: 10.1210/mend.16.1.0762. [DOI] [PubMed] [Google Scholar]
- 28.Scott P, Kessler MA, Schuler LA. Mol Cell Endocrinol. 1992;89:47–58. doi: 10.1016/0303-7207(92)90210-w. [DOI] [PubMed] [Google Scholar]
- 29.Takahara K, Lyons GE, Greenspan DS. J Biol Chem. 1994;269:32572–32578. [PubMed] [Google Scholar]
- 30.Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, Hogan BL. Development (Cambridge, UK) 1996;122:3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- 31.Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, Hogan BL, Greenspan DS. Development (Cambridge, UK) 1999;126:2631–2642. doi: 10.1242/dev.126.12.2631. [DOI] [PubMed] [Google Scholar]
- 32.Pappano WN, Steiglitz BM, Scott IC, Keene DR, Greenspan DS. Mol Cell Biol. 2003;23:4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott IC, Imamura Y, Pappano WN, Troedel JM, Recklies AD, Roughley PJ, Greenspan DS. J Biol Chem. 2000;275:30504–30511. doi: 10.1074/jbc.M004846200. [DOI] [PubMed] [Google Scholar]
- 34.Ge G, Seo NS, Liang X, Hopkins DR, Höök M, Greenspan DS. J Biol Chem. 2004;279:41626–41633. doi: 10.1074/jbc.M406630200. [DOI] [PubMed] [Google Scholar]
- 35.Unsöld C, Pappano WN, Imamura Y, Steiglitz BM, Greenspan DS. J Biol Chem. 2002;277:5596–5602. doi: 10.1074/jbc.M110003200. [DOI] [PubMed] [Google Scholar]
- 36.Uzel MI, Scott IC, Babakhanlou-Chase H, Palamakumbura AH, Pappano WN, Hong HH, Greenspan DS, Trackman PC. J Biol Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 37.Hogan BLM, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1994. [Google Scholar]
- 38.Ge G, Hopkins DR, Ho WB, Greenspan DS. Mol Cell Biol. 2005;25:5846–5858. doi: 10.1128/MCB.25.14.5846-5858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veitch DP, Nokelainen P, McGowan KA, Nguyen TT, Nguyen NE, Stephenson R, Pappano WN, Keene DR, Spong SM, Greenspan DS, et al. J Biol Chem. 2003;278:15661–15668. doi: 10.1074/jbc.M210588200. [DOI] [PubMed] [Google Scholar]
- 40.Andries M, Tilemans D, Denef C. Biochem J. 1992;281:393–400. doi: 10.1042/bj2810393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Angelo G, Struman I, Martial J, Weiner RI. Proc Natl Acad Sci USA. 1995;92:6374–6378. doi: 10.1073/pnas.92.14.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clapp C, Martial JA, Guzman RC, Rentier-Delure F, Weiner RI. Endocrinology. 1993;133:1292–1299. doi: 10.1210/endo.133.3.7689950. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 44.Martini JF, Piot C, Humeau LM, Struman I, Martial JA, Weiner RI. Mol Endocrinol. 2000;14:1536–1549. doi: 10.1210/mend.14.10.0543. [DOI] [PubMed] [Google Scholar]
- 45.Helseth DL, Jr, Veis A. Proc Natl Acad Sci USA. 1984;81:3302–3306. doi: 10.1073/pnas.81.11.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li SW, Sieron AL, Fertala A, Hojima Y, Arnold WV, Prockop DJ. Proc Natl Acad Sci USA. 1996;93:5127–5130. doi: 10.1073/pnas.93.10.5127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, et al. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 48.Scott IC, Blitz IL, Pappano WN, Imamura Y, Clark TG, Steiglitz BM, Thomas CL, Maas SA, Takahara K, Cho KW, et al. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 49.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.