Abstract
Tactile sensory information is first channeled from the primary somatosensory cortex on the postcentral gyrus to the parietal opercular region (i.e., the secondary somatosensory cortex) and the rostral inferior parietal lobule and, from there, to the prefrontal cortex, with which bidirectional connections exist. Although we know that tactile memory signals can be found in the prefrontal cortex, the contribution of the different prefrontal areas to tactile memory remains unclear. The present functional MRI study shows that a specific part of the prefrontal cortex in the human brain, namely the midventrolateral prefrontal region (cytoarchitectonic areas 47/12 and 45), is involved in active controlled retrieval processing necessary for the disambiguation of vibrotactile information in short-term memory. Furthermore, we demonstrate that this particular part of the prefrontal cortex interacts functionally with the secondary somatosensory areas in the parietal operculum and the rostral inferior parietal lobule during controlled processing for the retrieval of specific tactile information.
Keywords: functional MRI, retrieval, working memory
Anatomical, physiological, and lesion/behavior studies show that tactile sensory information necessary to interpret texture, shape, and vibration is channeled from the primary somatosensory cortex (SI) on the postcentral gyrus to the frontoparietal opercular region [i.e., the secondary somatosensory cortex (SII)] and the rostral inferior parietal lobule (i.e., area PF of Economo or area 40 of Brodmann) for further processing (1–8). The secondary somatosensory areas in the frontoparietal opercular region and the rostral inferior parietal lobule, unlike the SI, are bidirectionally connected with the prefrontal cortex (9–11), which is responsible for certain aspects of control in working memory (12–15). Research on non-human primates using the tactile flutter discrimination task, which requires the comparison of two frequencies separated by a short delay, suggests that the prefrontal cortex is part of a network of areas involved in tactile working memory (7, 16) and, in human subjects, some neuroimaging studies have observed prefrontal activity during the performance of somatosensory discrimination tasks (17–21). Although we know from the work of Romo et al. (16) that tactile working memory signals can be found in the ventrolateral prefrontal region of the monkey, where neuronal activity was recorded, activity in the polar prefrontal cortex (area 10), the orbital prefrontal cortex (areas 14, 13, 11), the middorsolateral prefrontal cortex (areas 9 and 46), the dorsal posterior prefrontal cortex (area 8), and the medial prefrontal cortex (areas 9, 10, 8, and 14) was not examined in these studies, except in one monkey in which a limited sample of dorsolateral prefrontal cortex was explored (16) and in which (interestingly) no short-term tactile memory neurons could be found. Despite the fact that the clearest delay-related neuronal activity in the tactile flutter discrimination task was observed in the ventrolateral prefrontal cortex (7, 16), delay-related activity in this task also has been observed in parietal SII (7, 22), and delay-related activity may even exist within the SI (23, 24).
What is the contribution of the ventrolateral prefrontal cortex to tactile memory? There is some evidence, based on studies with visuospatial stimuli (25, 26) that, in situations in which there are no strong stimulus–stimulus associations or contextual cues that would be sufficient to guide retrieval, the midventrolateral prefrontal cortex (areas 45 and 47/12) is specifically engaged to disambiguate information in memory and lead to the retrieval of the required information. When retrieving information from memory, one can often rely on familiarity (e.g., “I recognize this person”), strong stimulus–stimulus associations (e.g., “I often see this person together with Jim”), or strong contextual cues (e.g., “This person was at my brother's wedding”). In these situations, there is no ambiguity in the information to be retrieved. What happens, however, when there is ambiguity in the information to be retrieved and retrieval cannot be driven by familiarity and/or strong stimulus–stimulus or stimulus–context associations? For instance, the same stimuli may be related to each other in multiple ways over time and we might be required to isolate one aspect of this information that occurred at a particular point in time. Retrieval in such a situation cannot automatically be driven by familiarity or strong associations with other stimuli and specific contexts but would require the active controlled selection of the required aspect of this information. We distinguish between “active controlled retrieval,” which requires control processing to disambiguate memory information by enhancing relevant dimensions and suppressing irrelevant dimensions of information held in memory, and “automatic retrieval,” which does not require such control processing (14).
The present functional MRI (fMRI) experiment was designed to test the role of the midventrolateral prefrontal cortex and the posterior association regions in such active controlled retrieval from tactile short-term memory. We know from anatomical studies that the midventrolateral prefrontal cortex is linked with posterior cortical association areas, such as the SII and the rostral inferior parietal lobule, which are known to process somatosensory information (9–11). The present fMRI study tested the hypothesis that the midventrolateral prefrontal cortex (cytoarchitectonic areas 47/12 and 45) is specifically involved in the active controlled retrieval required for the disambiguation of somatosensory information within short-term memory.
The experimental design involved a task in which, across trials, one dimension of tactile information (vibration frequency of 10 or 20 Hz) would be related to another dimension (duration of 0.5 or 1 s) in multiple and equiprobable ways so that no strong relations could be formed between particular frequencies and particular durations. The vibrotactile stimulation was applied to the index finger. Each trial started with an encoding period during which a stimulus combination (i.e., one of the frequencies together with one of the durations) was presented (Fig. 1). The specific frequency and duration used in the encoding period of each trial were selected pseudorandomly, with equal probability across trials. After a short delay, a test period followed during which a visual cue was presented on the screen while a second vibrotactile stimulus was delivered. The subjects had to make a memory decision based on the visual cue. In the control recognition memory trials, the cue was a white square indicating that the subjects were required to recognize whether the current test stimulus was the same as the one they had encoded and were keeping in short-term memory. Simple comparison of the current test stimulus with the stimulus in short-term memory was sufficient to decide whether it was (or was not) the stimulus previously experienced. By contrast, in the experimental active retrieval trials, the memory decision could not be based on the simple matching of current information with information in short-term memory but required focusing on a specific dimension of both the test stimulus and the stimulus held in short term memory. A red cue instructed the subjects to retrieve the frequency of the encoded stimulus and compare it with the frequency of the test stimulus to decide whether they were the same or not, whereas a blue cue instructed retrieval of the duration of the encoded stimulus. For example, if the encoded stimulus (which is now in short-term memory) was a vibration of 10 Hz with a duration of 0.5 s and the test stimulus was a vibration of 10 Hz with a duration of 1 s, the subjects would have to make different decisions depending on the color of the cue: if the cue was red, the correct decision would be that the frequency of the encoded and the test stimuli was the same, whereas if the cue was blue, the correct decision would be that the duration of the encoded and test stimuli was different (Fig. 1). Clearly, for these active retrieval trials, simple comparison of the test stimulus with the memory trace of the encoded stimulus could not lead to the correct decision. The subjects were required to isolate the relevant dimension of the stimulus in memory and compare it with the relevant dimension of the current information. This active selection and comparison of information in memory is our operational definition of active memory retrieval (14).
Fig. 1.
Schematic diagram of the experimental design. All trials started with the appearance of a fixation cross, followed by the encoding period, during which the vibrotactile stimulation was administered; a delay period; and the test period. The trials differed only in the test period. The duration of the various periods was the same for the active retrieval and control recognition trials and is indicated below the diagram.
Results
Before addressing the key question in the present experiment, we wished to confirm that the classical somatosensory areas of the cerebral cortex were involved during the processing of the vibrotactile stimulation. For this purpose, we compared the blood oxygenation level-dependent (BOLD) signal during the encoding period of all of the trials with that of a baseline period (the last 2 s of the intertrial interval). As expected, we observed increased activity in cortical regions known to be important for the sensory processing and perception of somatosensory stimulation (1–8), such as the somatosensory and motor region around the central sulcus, the frontoparietal opercular cortex (SII), and the rostral inferior parietal lobule (area PF) [see supporting information (SI) Table 1 and SI Text for a complete presentation]. We should note here that the application of a nonlinear registration algorithm offered improved registration of the functional data between subjects and a decrease in the effect of intersubject anatomical variability on the results.
To address the key question of the present experiment, namely which prefrontal cortical region is involved in the disambiguation of somatosensory information in memory, we compared the test period of the active retrieval trials with that of the control recognition trials. All significant activity increases during the active retrieval test period relative to the control test period within the lateral frontal cortex were located within the midventrolateral prefrontal cortex (Fig. 2). There were three significant peaks of increased activity within the midventrolateral prefrontal cortex in the left hemisphere. One peak was situated anterior and ventral to the rostral tip of the horizontal ramus, where cytoarchitectonic studies (11) have shown that the rostral part of area 47/12 is located, i.e., just in front of the most anterior part of the pars triangularis [Montreal Neurological Institute coordinates (x, y, z) −46, 46, −4; t = 5.8] (Fig. 2a). A second peak of activity increase was found within the complex formed by the horizontal ramus of the Sylvian fissure [(x, y, z) −32, 24, −4; t = 6.3] (Fig. 2b) and where cytoarchitectonic analysis shows that caudal area 47/12 lies (11). A third peak of activity was observed in the caudal part of the pars triangularis of the inferior frontal gyrus in area 45 according to cytoarchitectonic studies (11, 27) [(x, y, z) −50, 24, 22; t = 6.2] (Fig. 2b). In the right hemisphere, there were corresponding increases in activity. For the group average, activity differences in the right hemisphere reached significance in the caudal part of area 47/12 within the complex formed by the horizontal ramus of the Sylvian fissure [(x, y, z) 36, 20, 0; t = 5.3] (Fig. 2b). Although not significant at the group level, in the right hemisphere, activity increases were also observed in the rostral part of area 47/12 in six of 12 subjects [(x, y, z) 42, 42, −4; t = 3.4] and in area 45 in eight of 12 subjects [(x, y, z) 46, 30, 18; t = 2.9].
Fig. 2.
Results from the experimental active retrieval minus control recognition comparison. Cortical surface rendering of the nonlinear registration of the MRIs of the 12 subjects. The t statistical map of the comparison of the experimental test period with the control test period has been superimposed on the cortical surface. The vertical lines indicate the rostrocaudal level (y) in Montreal Neurological Institute space of the coronal sections illustrated in a, b, c, and d, respectively. (a) Coronal section at y = 44 to illustrate the focus of activity increase in rostral area 47/12 within the midventrolateral prefrontal cortex, bilaterally. (b) Coronal section at y = 24 to illustrate the focus of increased activity in caudal area 47/12 bilaterally and in area 45 in the left hemisphere. Note the absence of activity increases outside the midventrolateral prefrontal cortex. (c and d) Point of functional interaction (during active tactile retrieval) of the midventrolateral prefrontal cortex (area 47/12) with the SII in the parietal operculum (c) and with the rostral inferior parietal lobule (Brodmann area 40 or Economo area PF) (d). (e) Schematic diagram of the brain depicting the functional interaction (red arrows) demonstrated in this study between the midventrolateral prefrontal cortex and the SII and area PF in the rostral inferior parietal lobule during the active retrieval of tactile information from short-term memory. Note that SII and area PF (which constitute higher stages in the processing of somatosensory information) are the recipients of tactile sensory input (black arrows) from the SI on the postcentral gyrus. The temporal lobe has been pulled ventrally so that the frontoparietal operculum (where SII lies) and the insula can be seen. AR, ascending ramus; CS; central sulcus; HR, horizontal ramus; IFS, inferior frontal sulcus; IPS, intraparietal sulcus; L, left hemisphere; MI, primary motor cortex; MFS, middle frontal sulcus; PF; area PF of Economo; POS, postcentral sulcus; PRS, precentral sulcus; SF, Sylvian fissure; SFS, superior frontal sulcus; STS, superior temporal sulcus; R, right hemisphere; TS, transverse sulcus.
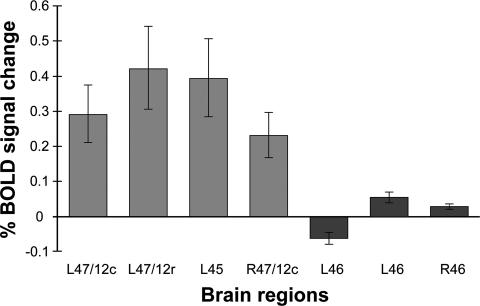
It is important to note that the comparison of the test periods of the experimental and control trials did not reveal increases in activity in any prefrontal areas outside the midventrolateral prefrontal cortex (i.e., areas 47/12 and 45). This can be demonstrated in various ways. First, all voxels within the dorsolateral prefrontal cortex and the frontopolar cortex had t values of <2, reflecting nonsignificant activity differences. Second, we compared the percent BOLD signal change for the peaks within the midventrolateral prefrontal cortex with values obtained from locations within the middorsolateral prefrontal cortex that previous studies (28, 29) had shown to be foci of activity increases related to the monitoring of information in memory [(x, y, z) −35, 42, 22; (x, y, z) −40, 32, 30; (x, y, z) 35, 32, 21] (Fig. 3). Inspection of Fig. 3 clearly shows that there was no significant signal change in the middorsolateral prefrontal cortex, and the difference of the signal change between the middorsolateral prefrontal cortex and the midventrolateral prefrontal cortex was significant [F(6, 77) = 11.517; P < 0.0001, ANOVA]). Outside the prefrontal cortex, activity increases were observed in the paracingulate cortex (area 32) [(x, y, z) 0, 22, 50; t = 6.0], a region that has often been linked to tasks that require faster and more complicated stimulus–response associations (30) and in the septal area [(x, y, z) −8, 4, 0; t = 4.7].
Fig. 3.
Percent BOLD signal change in the midventrolateral prefrontal cortex and the middorsolateral prefrontal cortex. Error bars represent the standard error. The numbers on the x axis (brain regions) refer to the architectonic areas 45, 47/12, and 46. c; caudal; DLPFC, dorsolateral prefrontal cortex; L, left hemisphere; R, right hemisphere; r, rostral; VLPFC, ventrolateral prefrontal cortex.
The findings reported above demonstrate that the midventrolateral prefrontal cortical region (areas 47/12 and 45) is involved in memory decisions that require the disambiguation of somatosensory information in short-term memory. It is hypothesized that during these active memory decisions this region of the prefrontal cortex is interacting functionally with specific posterior association cortical regions, such as SII and the rostral inferior parietal lobule, which are involved in the processing of somatosensory information and which are strongly connected with it. To test this hypothesis of functional interaction, we examined whether there was a difference in the functional connectivity between the midventrolateral prefrontal cortex and these posterior somatosensory areas during the test period of the active retrieval versus the control recognition memory trials by using a procedure described by K. Worsley (www.math.mcgill.ca/keith/fmristat). We observed a significant functional interaction between the midventrolateral prefrontal cortex and the SII in the frontoparietal opercular region (Fig. 2 c and e) and the rostral part of the inferior parietal lobule (Fig. 2 d and e). Activity peaks both in the right and left caudal area 47/12 interacted significantly with SII [left caudal area 47/12: (x, y, z) −40, −20, 18; t = 2.8; right caudal area 47/12: (x, y, z) 58, −16, 20; t = 3.4 and (x, y, z) 50, −36, 20; t = 4.4] and the rostral inferior parietal lobule [left caudal area 47/12: (x, y, z) −64, −30, 40; t = 3.0; right caudal area 47/12: (x, y, z) 49, −20, 44; t = 3.1] (Fig. 2 c and d). The schematic diagram in Fig. 2e, illustrates this functional interaction (red arrows) between the midventrolateral prefrontal cortex and the SII region in the frontoparietal operculum (SII) and area PF (area 40) of the rostral inferior parietal lobule during the test period of the active retrieval trials compared with the test period of the control recognition trials.
Discussion
At the cortical level, tactile sensory information is initially processed in the SI on the postcentral gyrus and is then channeled to the SII and the rostral inferior parietal lobule for further processing so that tactile perception can be achieved (1–8). These SII regions are bidirectionally connected with the prefrontal cortex (9–11). The present fMRI study showed that, within the prefrontal cortex, the midventrolateral prefrontal cortex (i.e., cytoarchitectonic areas 47/12 and 45) shows increased activity when tactile memories require disambiguation (i.e., active controlled retrieval). Importantly, during tactile active controlled retrieval, the midventrolateral prefrontal cortex interacts functionally with the secondary somatosensory regions in the parietal operculum and the rostral inferior parietal lobule.
In the present task, for the experimental active retrieval trials, the subjects were required to compare a particular dimension (frequency or duration) of the currently presented vibrotactile stimulation with vibrotactile stimulation held in short-term memory to decide whether the particular dimension was the same or not. For example, the subjects could be asked whether the currently presented vibrotactile stimulus had the same frequency as the one in short-term memory, regardless of the duration of the stimuli. In this case, the subjects had to make a decision by isolating the relevant dimension from the information in memory, i.e., by facilitating the particular instructed dimension and suppressing the irrelevant one. Interspersed with these trials were control recognition memory trials in which the subjects were instructed to compare the current vibrotactile stimulation with the vibrotactile stimulation held in short-term memory and decide whether the two stimuli were the same or different. Clearly, these control trials did not require any disambiguation of information in memory; i.e., a simple matching/nonmatching decision between the current test information and the information in memory could lead to the correct solution. This paradigm was created to simulate, in an experimental setting, automatic and active memory retrieval processes. Automatic retrieval can be used when there is no ambiguity in the relationships of stimuli to other stimuli/contexts and, therefore, when familiarity, strong stimulus–stimulus associations, or unambiguous contextual cues can lead to successful retrieval. In these cases, there is no need for control processing from the frontal lobes. In situations where information is embedded in ambiguous relations, however, retrieval requires additional control processing necessary for the disambiguation of the different features that comprise these memories. Anatomical studies in the monkey have shown that the midventrolateral prefrontal cortex has connections with posterior association cortical areas that are important for the perception and interpretation of sensory stimuli. The midventrolateral prefrontal cortex is, therefore, in a key position to interact with these posterior association cortical areas for the active controlled retrieval of information from memory. We propose that, for somatosensory memories, the role of the midventrolateral prefrontal cortex lies in its functional interaction with the SII and the rostral inferior parietal cortex for the disambiguation of tactile information in memory (see Fig. 2e).
Note that the activity related to such active decisions on tactile information in memory was observed specifically within the midventrolateral prefrontal cortex. Other prefrontal cortical regions, such as the frontopolar cortex (area 10), the orbital prefrontal cortex (areas 14, 13, 11), the middorsolateral prefrontal cortex (areas 9 and 46), the dorsal posterior prefrontal cortex (area 8), and the medial prefrontal cortex (areas 9, 10, 8, and 14), did not show involvement during the active controlled retrieval of tactile information. It is therefore of considerable interest that Romo et al. (7, 16) found robust tactile delay-related signals within the inferior convexity of the macaque monkey prefrontal cortex during a tactile flutter discrimination task in which a short delay separated two sequentially presented frequencies that had to be compared (16, 31). This prefrontal region in the monkey corresponds architectonically to the human midventrolateral prefrontal cortical areas 45 and 47/12 (11), where we report specific increases in activity for the active retrieval of vibrotactile information. The single-cell recording studies performed by Romo et al. (7, 16) provide essential information about how neurons in this region encode and compare vibrotactile stimuli during memory tasks. The agreement between the fMRI results in human subjects and the single cell recording data from non-human primates is of considerable importance for the understanding of prefrontal function. Romo et al. (22) also reported that, within the SII, during the presentation of the second stimulus, the response of a certain class of neurons depended on both the first and the second stimulus. Thus, clearly, both the inferior convexity of the prefrontal cortex and SII participate in short-term tactile memory decisions. The results obtained in the present study are consistent with the single-cell recording data. What these elegant studies did not address, however, because the recording was restricted to the ventrolateral prefrontal cortex, was whether other prefrontal areas participate in tactile memory and in what capacity. The fMRI data showed that (i) the midventrolateral prefrontal cortical region (areas 45 and 47/12) is specifically involved during the application of a certain type of executive control processing on tactile memory and that (ii), in doing so, the midventrolateral prefrontal cortex increases its functional interaction with the posterior somatosensory areas in the parietal operculum and the rostral inferior parietal lobule.
Although the present study does not examine the delay period between the presentation of the two vibrotactile stimuli, based on the data obtained in the monkey (6, 7, 22), we can postulate that short-term memory depends on reentry of nerve impulses in reverberating circuits (e.g., ref. 32) that involve the midventrolateral prefrontal cortex and parietal cortical association areas for tactile short-term memory. The significant functional interaction demonstrated in the present fMRI study between the midventrolateral prefrontal cortex and the posterior cortical somatosensory areas emphasizes the existence of a critical coupling of information processing in this specific frontoparietal circuit specifically during the active controlled retrieval of information in memory. Although no information exists at the single-neuron level in the tactile domain, in the visual domain there is neurophysiological evidence that the prefrontal cortex exerts an influence on posterior visual association areas by way of its bidirectional connections with these areas (33).
It should be noted that the specific role of the midventrolateral prefrontal cortex in tactile short-term memory is also expressed with other types of sensory information, although in interaction with completely separate posterior cortical association areas (26). In the present experiment, the peaks of activity related to the active retrieval of tactile short-term memories within areas 47/12 and 45 of the midventrolateral prefrontal cortex were in the same general region that was shown to be responsible for the active retrieval of visual spatial and visual nonspatial stimuli (25, 26). However, whereas this particular prefrontal region interacts functionally with the SII and the rostral inferior parietal lobule during the active selection and comparison of somatosensory information in memory, in previous studies increased activity in posterior cortical regions was observed in the fusiform gyrus for the active retrieval of faces (26).
There is by now considerable evidence that the midventrolateral prefrontal cortex is involved in a number of memory tasks that require retrieval under challenging situations. For instance, in the left hemisphere, on the basis of studies that examined semantic retrieval, it has been suggested that the midventrolateral prefrontal cortex is involved in controlled semantic retrieval (27, 34–37), as well as the control of proactive interference (38, 39). Activity increases have also been reported within the midventrolateral prefrontal cortex during challenging spatial and other nonverbal memory tasks in which stimuli across trials are similar to each other and retrieval cannot be automatic (25, 26, 40–42). These results have led to the notion that the midventrolateral prefrontal cortex is involved in challenging retrieval situations requiring the application of control mechanisms. What is the source of difficulty in these challenging retrieval situations and what are the control processes that emanate from the midventrolateral prefrontal cortex? It has proved difficult to specify the aspect of difficulty that requires the involvement of the midventrolateral prefrontal cortex. In the present study, the source of retrieval difficulty is known precisely because it was experimentally created: It stems from the fact that, across trials, stimuli appear in multiple and equiprobable combinations. All vibrotactile frequencies are presented with all durations without any strong or stable associations being formed between them. On the test period of each trial, the subjects have to disambiguate within memory the two aspects of the memory trace; i.e., they must actively select and compare the relevant one while at the same time suppressing the irrelevant one.
Although it is not possible to know the exact source of retrieval difficulty in many of the fMRI studies that examined memory retrieval and observed activity increases within the midventrolateral prefrontal cortex, close examination of the paradigms used reveals that, in many of these studies, retrieval cannot be automatic because memory traces overlap and there are no direct and unambiguous links in memory between the particular stimuli that must be retrieved and other stimuli/contexts associated with them. For instance, verbal fluency tasks that reveal increases in activity within the midventrolateral prefrontal cortex require the production of words from a specific category (27, 43). In this task, retrieval requires the active selection of the appropriate words from among many other similar words in memory that overlap in their links to various contexts. The present study provides specification of one source of difficulty in retrieval tasks and identifies the midventrolateral prefrontal cortex as a critical structure during such memory retrieval. The proposed operational definition for the role of the midventrolateral prefrontal cortex in tactile memory retrieval can generalize to many other retrieval situations in a number of other sensory modalities, including verbal memory.
Methods
Subjects.
Twelve right-handed healthy volunteers (five males; mean age ± SD, 23.5 ± 5.0) participated in this study after providing informed, written consent. All subjects were trained outside the scanner 1 day before the scanning session.
Data Acquisition and Analysis.
Scanning was performed on a 1.5-T Sonata MRI Scanner (Siemens, Erlangen, Germany). After a high-resolution T1 anatomical scan (whole head, 1-mm3 isotropic resolution), six runs of 165 images each (38 oblique T2* gradient echo planar EPI images; 3.4 × 3.4 × 3.4 mm; time to repeat = 3.5 s; time to echo = 45 ms) sensitive to the BOLD signal were acquired. Each run consisted of 30 trials and lasted ≈10 min. We excluded from the analysis the first two frames of each run and the trials in which the subjects made an error. Images were realigned by using the AFNI image registration software (44), blurred, and nonlinearly registered in the Montreal Neurological Institute standardized stereotaxic space (45–47). Statistical analysis of the functional data were performed with fMRISTAT (48). A detailed description of the methods is provided in SI Text.
Experimental Design.
The subjects received vibrotactile stimulation on the index finger of one hand and responded with the index and middle finger of the opposite hand. Half of the subjects started with the vibrostimulator positioned underneath the index finger of the right hand and the other half started with the vibrostimulator positioned underneath the index finger of the left hand. The position of the computer mouse and the vibrostimulator was switched half way through the scanning session. Thus, half of the subjects received the vibrotactile stimulation on the right hand for the first three runs and then on the left hand for the last three runs, and the other half received the vibrotactile stimulation on the left hand for the first three runs and then on the right hand for the last three runs. On the day of scanning, all subjects received a short training session involving 10 trials at the beginning of the scanning session and after the switch.
During scanning, the experimental active retrieval and control recognition trials were pseudorandomly intermixed (Fig. 1). Both trial types started with the same encoding period and differed only in the type of memory decision that they required during the test period that followed. All trials started with the presentation of a fixation cross in the middle of the screen for 1 s. A vibrotactile stimulus was then presented on the index finger of the subjects. The vibrotactile stimulus had a specific duration (0.5 or 1 s) and a specific frequency (10 or 25 Hz). After a delay of 4–6.5 s (in steps of 0.5 s and pseudorandomly ordered), the test period started with the delivery of a second vibrotactile stimulus (which again had a specific frequency and duration) together with one of three visual cues that appeared on the screen. The subjects had 3 s to respond and, during these 3 s, the visual cue remained on the screen. If the cue was red, the subjects were instructed to make a memory decision based on the frequency of the stimuli and indicate, by pressing the appropriate mouse key, whether the test stimulus had the same frequency as the encoded vibrotactile stimulus, regardless of the duration. If the cue was blue, the subjects were instructed to make a memory decision about the duration of the stimuli and indicate whether the test stimulus had the same duration as the encoded vibrotactile stimulus, regardless of the frequency (Fig. 1). Because the subjects had experienced the two different frequencies and the two different durations of the vibrotactile stimuli in all possible combinations (e.g., high frequency with short duration, high frequency with long duration, etc.) there were no strong associations between any one of the frequencies and any one of the durations. For that reason, during the experimental trials, subjects could not rely on simple stimulus- or context-driven retrieval but were required to isolate a particular aspect of the encoded vibrotactile stimulation and compare it with the relevant aspect of the test stimulus. In the test period of the control recognition trials, a white cue instructed the subjects to compare the test stimulus with the encoded stimulus in short-term memory to decide whether these two vibrotactile stimuli were the same or different and indicate their decision by pressing the appropriate mouse key. In these control trials, the vibrotactile test stimulus would be either the same as the one presented during the encoding period of the trial or a vibrotactile stimulus with the frequency of 75 Hz and durations of either 0.5 or 1 s. On the basis of pilot studies showing that subjects could not discriminate frequencies with durations of <0.5 s and that they could not discriminate 0.75 or 1.25 from 1 s, we decided to keep the durations of the alternative control stimuli the same as those of the experimental stimuli. Based on the debriefing of the subjects at the end of the scanning session, it was clear that the subjects relied on recognizing the test stimulus as being the same as the one that they had just encoded based on familiarity and did not pay attention to the alternative stimulus. This is in fact how we instructed the subjects to perform the control trials.
Supplementary Material
Acknowledgments
We thank M. Chakravarty, L. Collins, K. Worsley, G. Duncan, and B. Pike for assistance. This work was supported by Canadian Institutes of Health Research Grant MOP-37753 (to M.P.) and Canadian Institutes of Health Research and Fonds de la Recherche en Santé du Québec scholarships (to P.K.).
Abbreviations
- BOLD
blood oxygenation level-dependent
- fMRI
functional magnetic resonance imaging
- SI
primary somatosensory cortex
- SII
secondary somatosensory cortex.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700253104/DC1.
References
- 1.Burton H, Videen TO, Raichle ME. Somatosens Mot Res. 1993;10:297–308. doi: 10.3109/08990229309028839. [DOI] [PubMed] [Google Scholar]
- 2.Burton H, Sinclair RJ. J Clin Neurophysiol. 2000;17:575–591. doi: 10.1097/00004691-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Johnson KO, Hsiao SS. Annu Rev Neurosci. 1992;15:227–250. doi: 10.1146/annurev.ne.15.030192.001303. [DOI] [PubMed] [Google Scholar]
- 4.Krubitzer L, Clarey J, Tweedale R, Elston G, Calford M. J Neurosci. 1995;15:3821–3839. doi: 10.1523/JNEUROSCI.15-05-03821.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray EA, Mishkin M. Behav Brain Res. 1984;11:67–83. doi: 10.1016/0166-4328(84)90009-3. [DOI] [PubMed] [Google Scholar]
- 6.Ridley RM, Ettlinger G. Brain Res. 1976;109:656–660. doi: 10.1016/0006-8993(76)90048-2. [DOI] [PubMed] [Google Scholar]
- 7.Romo R, Salinas E. Nat Rev Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 8.Schneider RJ, Friedman DP, Mishkin M. Brain Res. 1993;621:116–120. doi: 10.1016/0006-8993(93)90305-7. [DOI] [PubMed] [Google Scholar]
- 9.Carmichael ST, Price JL. J Comp Neurol. 1995;363:642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 10.Cipolloni PB, Pandya DN. J Comp Neurol. 1999;403:431–458. [PubMed] [Google Scholar]
- 11.Petrides M, Pandya DN. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 12.Buckner RL. J Neurosci. 2003;23:3999–4004. doi: 10.1523/JNEUROSCI.23-10-03999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrides M. In: Handbook of Neuropsychology. Boller F, Grafman J, editors. Amsterdam: Elsevier; 1994. pp. 59–82. [Google Scholar]
- 14.Petrides M. Philos Trans R Soc B. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Postle BR. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romo R, Brody CD, Hernandez A, Lemus L. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 17.Deibert E, Kraut M, Kremen S, Hart J. Neurology. 1999;52:1413–1417. doi: 10.1212/wnl.52.7.1413. [DOI] [PubMed] [Google Scholar]
- 18.Hagen MC, Zald DH, Thornton TA, Pardo JV. J Neurophysiol. 2002;88:1400–1406. doi: 10.1152/jn.2002.88.3.1400. [DOI] [PubMed] [Google Scholar]
- 19.Kitada R, Hashimoto T, Kochiyama T, Kito T, Okada T, Matsumura M, Lederman SJ, Sadato N. NeuroImage. 2005;25:90–100. doi: 10.1016/j.neuroimage.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Reed CL, Klatzky R, Halgren E. NeuroImage. 2005;25:718–726. doi: 10.1016/j.neuroimage.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 21.Stoeckel MC, Wedes B, Binkofski F, Buccino G, Shah NJ, Seitz RJ. NeuroImage. 2003;19:1103–1114. doi: 10.1016/s1053-8119(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 22.Romo R, Hernandez A, Zainos A, Lemus L, Brody CD. Nat Neurosci. 2002;5:1217–1225. doi: 10.1038/nn950. [DOI] [PubMed] [Google Scholar]
- 23.Harris JA, Miniussi C, Harris IM, Diamond ME. J Neurosci. 2002;22:8720–8725. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou YD, Fuster JM. Proc Natl Acad Sci USA. 1996;93:10533–10537. doi: 10.1073/pnas.93.19.10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadoret G, Pike GB, Petrides M. Eur J Neurosci. 2001;14:1164–1170. doi: 10.1046/j.0953-816x.2001.01737.x. [DOI] [PubMed] [Google Scholar]
- 26.Kostopoulos P, Petrides M. Eur J Neurosci. 2003;17:1489–1497. doi: 10.1046/j.1460-9568.2003.02574.x. [DOI] [PubMed] [Google Scholar]
- 27.Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eichkhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K. NeuroImage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 28.Petrides M, Alivisatos B, Evans AC, Meyer E. Proc Natl Acad Sci USA. 1993a;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrides M, Alivisatos B, Meyer E, Evans AC. Proc Natl Acad Sci USA. 1993b;90:878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paus T, Koski L, Caramanos Z, Westbury C. NeuroReport. 1998;9:R37–40. doi: 10.1097/00001756-199806220-00001. [DOI] [PubMed] [Google Scholar]
- 31.Machens CK, Romo R, Brody CD. Science. 2005;306:1121–1124. doi: 10.1126/science.1104171. [DOI] [PubMed] [Google Scholar]
- 32.Zipser D, Kehoe B, Littlewort G, Fuster J. J Neurosci. 1993;13:3406–3420. doi: 10.1523/JNEUROSCI.13-08-03406.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y. Nature. 1999;401:699–703. doi: 10.1038/44372. [DOI] [PubMed] [Google Scholar]
- 34.Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Klein D, Olivier A, Milner B, Zatorre RJ, Johnsrude I, Meyer E, Evans AC. NeuroReport. 1997;8:3275–3279. doi: 10.1097/00001756-199710200-00017. [DOI] [PubMed] [Google Scholar]
- 36.Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. NeuroImage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- 37.Wagner AD, Pare-Blagoev EJ, Clark J, Poldrack RA. Neuron. 2001;31:329–338. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- 38.Badre D, Wagner AD. Cereb Cortex. 2005;15:2003–2012. doi: 10.1093/cercor/bhi075. [DOI] [PubMed] [Google Scholar]
- 39.Jonides J, Nee DE. Neuroscience. 2006;139:181–193. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 40.Owen AM, Evans AC, Petrides M. Cereb Cortex. 1996;6:31–38. doi: 10.1093/cercor/6.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Nature. 1993;363:623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 42.Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Proc Natl Acad Sci USA. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Nature. 1988;331:585–589. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- 44.Cox RW, Jesmanowicz A. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain: 3-Dimentional Proportional System: An Approach to Cerebral Imaging. Stuttgart, Germany: Thieme; 1988. [Google Scholar]
- 46.Collins DL, Neelin P, Peters TM, Evans AC. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 47.Collins DL, Holmes CJ, Peters TM, Evans AC. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 48.Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. NeuroImage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.