Abstract
Arginine contains the guanidinium group and thus has structural similarity to ligands of imidazoline and α-2 adrenoceptors (α-2 AR). Therefore, we investigated the possibility that exogenous arginine may act as a ligand for these receptors in human umbilical vein endothelial cells and activate intracellular nitric oxide (NO) synthesis. Idazoxan, a mixed antagonist of imidazoline and α-2 adrenoceptors, partly inhibited l-arginine-initiated NO formation as measured by a Griess reaction. Rauwolscine, a highly specific antagonist of α-2 AR, at very low concentrations completely inhibited NO formation. Like l-arginine, agmatine (decarboxylated arginine) also activated NO synthesis, however, at much lower concentrations. We found that dexmedetomidine, a specific agonist of α-2 AR was very potent in activating cellular NO, thus indicating a possible role for α-2 AR in l-arginine-mediated NO synthesis. d-arginine also activated NO production and could be inhibited by imidazoline and α-2 AR antagonists, thus indicating nonsubstrate actions of arginine. Pertussis toxin, an inhibitor of G proteins, attenuated l-arginine-mediated NO synthesis, thus indicating mediation via G proteins. l-type Ca2+ channel blocker nifedipine and phospholipase C inhibitor U73122 inhibited NO formation and thus implicated participation of a second messenger pathway. Finally, in isolated rat gracilis vessels, rauwolscine completely inhibited the l-arginine-initiated vessel relaxation. Taken together, these data provide evidence for binding of arginine to membrane receptor(s), leading to the activation of endothelial NO synthase (eNOS) NO production through a second messenger pathway. These findings provide a previously unrecognized mechanistic explanation for the beneficial effects of l-arginine in the cardiovascular system and thus provide new potential avenues for therapeutic development.
Keywords: agmatine, rauwolscine, calcium
Arginine is critical to normal growth and multiple physiological processes. It serves as a precursor for the synthesis not only of proteins but also of NO, urea, polyamines, and agmatine. The unequivocal demonstration that NO is the product of NO synthase (NOS)-catalyzed oxidation of l-arginine led to widespread interest in the actions of l-arginine. The Km of l-arginine for endothelial NOS (eNOS) is determined to be 2.9 μM (1), and the intracellular l-arginine concentrations are in the range of 0.8–2.0 mM. In other words, cells maintain saturating levels of l-arginine as a substrate for NO synthases. However, an external supply of l-arginine is still required for the cellular production of NO (2). This requirement of exogenous arginine for the cellular NO production is termed “arginine paradox.” A number of mechanisms have been proposed to address this phenomenon, including endogenous NOS inhibitors and compartmentalization of intracellular l-arginine. Some have proposed that endogenous NOS inhibitors [e.g., asymmetric di methyl arginine (ADMA)] modulate NO levels by antagonizing intracellular l-arginine (3). An alternative view hypothesizes that l-arginine is compartmentalized within the cell, and part of the cellular pool of l-arginine is not readily available for eNOS. As part of this hypothesis, it has been proposed that NO signaling occurs through caveolae, a subcompartment of the plasma membrane (4, 5). Because of this compartmentalization, NO synthesis in certain cells might require extracellular l-arginine (6), and thus suggesting a preferential channeling of extracellular arginine to the site of eNOS location (7).
There is ample evidence documenting the beneficial effects of exogenous arginine both in animal and clinical studies. Acute and long-term oral administration of l-arginine has been associated with a significant improvement in NO-dependent vasodilation in cholesterol-fed rabbits (8, 9). Benefits of exogenous arginine are realized both in healthy humans and in disease conditions. For example, in healthy human subjects, i.v. infusion and oral administration of l-arginine induced vasodilation (10). Oral arginine also improved endothelial dysfunction in patients with essential hypertension (11). Plasma and intracellular levels of arginine are altered in certain disease states, hypertension, hypercholesterolemia, and diabetes (12–14). As part of the treatment regimen, l-arginine may serve as a safer and more easily administered alternative to inhaled NO gas or NO donors.
Three important metabolic end products of l-arginine, namely NO, glutamate, and agmatine, are cell-signaling molecules. The decarboxylation of l-arginine, through the action of arginine decarboxylase (ADC), forms agmatine and CO2. The finding that agmatine is present in serum (15) raises the issue of whether the amine participates in vascular function. Agmatine was shown to stimulate nitrite formation as well as Ca2+ uptake in endothelial cells (16). Most recently, a new member of the C family of G protein-binding receptors, GPRC6A, has been shown to bind a structurally wide range of l-α-amino acid analogues of arginine, lysine, and ornithine as agonists (17). However, its physiological role is unknown. Li et al. (18) and Reis et al. (19) have suggested agmatine as an endogenous ligand for α-2 adrenoreceptor (α-2 AR) and imidazoline receptor (I-receptor). Most imidazolines and structurally related ligands (Fig. 1) bind to both I-receptor and α-2 AR. Therefore, these two receptors are always studied together with respect to their mediation in cellular signal transduction mechanisms. Endothelial cells have been shown to express a number of receptors: adrenoceptors (20, 21), imidazoline (22), bradykinin (23, 24), purinoceptor (25, 26), and adenosine A2 receptor (27). I-receptors are a class of nonadrenergic receptors, and α-2 AR belongs to the class of G protein-coupled receptors (GPCR). These receptors are shown to mediate cellular NO formation and relaxation. Liao and Homcy (23), using an α-2 AR agonist, have documented the formation of NO in bovine aortic endothelial cells, which was inhibited by G protein inhibitor pertussis toxin. Also, α-2 AR agonists clonidine and UK14304 are found to mediate endothelium-dependent relaxation in rat aorta (28). The activation of G proteins results in elevated phospholipase C activity, leading to hydrolysis of phosphatidylinositol-4,5-biphosphate, which yields second messengers, inositol-1,4,5-triphosphate (IP3) and diacylglycerol. IP3 subsequently mediates release of Ca2+ from ER. Cytosolic Ca2+ may be the most widely used second messenger in biology. In endothelial cells, ER accounts for ≈75% of the total [Ca2+]i and IP3, and ryanodine receptors mediate [Ca2+]i release from ER. Most functions in these cells depend to various extents on changes in the [Ca2+]i, including the process of eNOS activation.
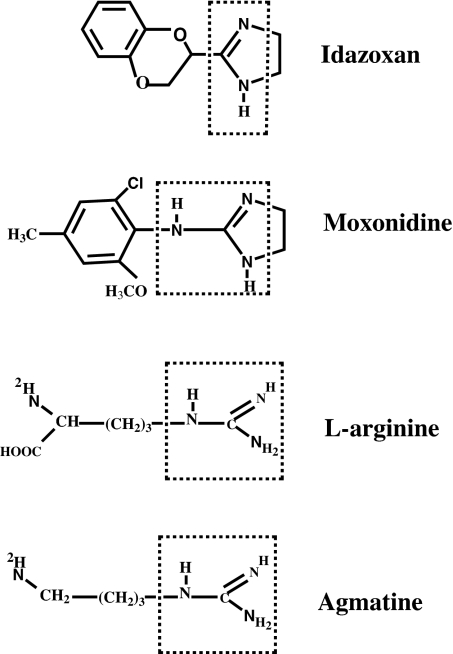
Fig. 1.
Structures of guanidinium-containing compounds. Idazoxan and moxonidine are the ligands for I-receptor and α-2 AR. The guanidinium groups are marked with a dotted box.
Tsukahara et al. (29) have observed that when human umbilical vein endothelial cells (HUVECs) were supplied with l-arginine, there was a transient stimulation in NO production, which returned to baseline levels within a few minutes. If exogenous l-arginine were acting as a substrate, then one would expect a continuous generation of NO, because cells have significant levels of l-arginine. This observation prompted us to speculate that the stimulatory action of exogenous l-arginine may not be due to its actions as a substrate. Instead, l-arginine may bind to the same or similar receptor(s) as agmatine, activating intracellular NOS via signal transduction mechanisms involving second messenger systems and store-operated Ca2+ channels. There is no evidence in the literature about the receptor-mediated actions of l-arginine to activate intracellular NO synthesis. Understanding of receptor-mediated l-arginine actions will have wide implications in vascular function with respect to the biological actions of l-arginine and NO. This system will permit further elucidation of the potential importance of l-arginine-receptor transduction mechanisms in regulating NO production and will potentially provide new avenues for therapeutic development. Here we have investigated the membrane receptor binding of exogenous l-arginine, which resulted in activation of NO in HUVECs. The findings show that both l-and d-arginine receptor-dependently activate cellular NO independent of substrate effects.
Results
l-N G-Nitroarginine Methyl Ester (l-NAME) and Antagonists of I-Receptor and α-2 AR Block l-Arginine-Initiated NO.
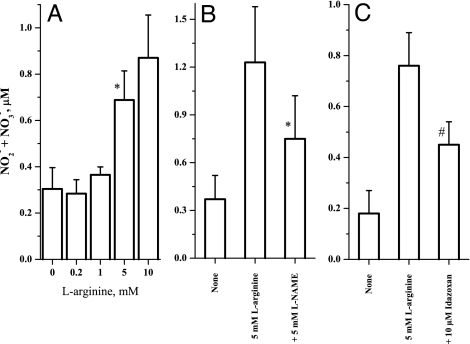
We first conducted dose–response experiments with increasing concentrations of exogenous l-arginine and measured NO2−/NO3− formation as stable end products of cellular NO synthesis. l-arginine, at levels present in normal plasma (0.2 mM), did not activate NO synthesis (Fig. 2A). However, NO was activated at higher millimolar concentrations. Then the effect of antagonists on NO formation from l-arginine was studied. The eNOS inhibitor, l-NAME, reversed the l-arginine-mediated NO2−/NO3− formation (Fig. 2B), thus showing that NO2−/NO3− formation is the result of NO metabolism. The cell cultures were also treated with the hybrid antagonist of I-receptor and α-2 AR, idazoxan, in the presence and absence of l-arginine. Idazoxan partially inhibited l-arginine-initiated NO formation (Fig. 2C). However, the specific α-2 AR antagonist, rauwolscine, completely blocked NO formation at very low concentrations (Fig. 3A). These results show that l-arginine activates cellular NO production possibly through receptor mediation.
Fig. 2.
Arginine dose–response and the effect of NOS inhibitor and receptor antagonist. (A) Dose-dependent activation of cellular NO synthesis by l-arginine (∗, P < 0.05 vs. 0; n = 3). (B) Effect of eNOS inhibition with 5 mM l-NG-nitroarginine methyl ester (l-NAME) on l-arginine (5 mM) -initiated NO2−/NO3− formation. (∗, P = 0.08 vs. 5 mM l-arginine; n = 3). (C) Effect of idazoxan (10 μM) on l-arginine (5 mM) -mediated NO2−/NO3− formation in HUVEC cultures (#, P = 0.033 vs. 5 mM l-arginine; n = 3). The experiments were carried out in 12-well plates at 120,000 cells per well in 95% air/5% CO2 incubator at 37°C for 30 min. The NO2−/NO3− levels were measured by a modified Griess reaction that can detect NO2−/NO3− levels in the lower nanomolar range.
Fig. 3.
Effect of α-2 AR antagonist on the l-arginine and A23187-mediated NO formation. (A and B) The cell cultures (120,000 cells per well) were treated with either 5 mM l-arginine (A) or 1 μM A23187 (B) in the presence and absence of 0.2 nM rauwolscine for 30 min at 37°C in a 5% CO2/95% air incubator. The supernatants were assayed for NO2−/NO3− by modified a Griess reaction. (A) n = 4; ∗, P < 0.05 vs. 5 mM l-arginine. (B) n = 4; ∗, P < 0.05 vs. none. (C) Dose–response of α-2 AR agonist dexmedetomidine. The cells (180,000 cells per well) were treated with increasing concentrations of dexmedetomidine for 60 min. The culture supernatants were used for a NO2−/NO3− assay by a Griess reaction (n = 4; ∗, P < 0.05 vs. 0.001 nM dexmedetomidine). (D) The cultures (120,000 cells per well) were treated with 5 mM d-arginine in the presence and absence of 0.2 nM rauwolscine and 50 μM idazoxan for 30 min in Krebs solution at 37°C in 5% CO2/95% air incubator. The cell supernatants were assayed for NO2−/NO3− by a modified Griess reaction (n = 4; ∗, P = 0.009 vs. 5 mM d-arginine; #, P = 0.007 vs. 5 mM d-arginine; $, P = 0.031 vs. 5 mM d-arginine).
It is also possible that rauwolscine is blocking NO production in a nonspecific manner at a site downstream from the receptor. To rule out this possibility, we treated cells with calcium ionophore A23187 in the presence and absence of rauwolscine. As shown in Fig. 3B, rauwolscine failed to block NO formation from A23187, thus showing that inhibition in Fig. 3A is due to the specific effect of antagonist on receptor(s).
Agonist of α-2 AR Activates NO.
If l-arginine is activating NO production by binding to α-2 AR on endothelial cell membranes, then a known agonist of α-2 AR should activate NO synthesis as well. To explore this possibility, we used dexmedetomidine, a new specific agonist of α-2 AR. Dexmedetomidine dose-dependently activated NO production at very low concentrations (Fig. 3C). These data show that endothelial cells can produce NO through activation of α-2 AR.
d-Arginine Activated NO and Its Inhibition with Antagonists of I-Receptor and α-2 AR.
The rationale for this experiment is that d-arginine is not a substrate for eNOS reaction to form NO and l-citrulline. However, if d-arginine activates NO, then the NO formation must be due to its nonsubstrate effects. Accordingly, the cells were treated with d-arginine in the presence and absence of antagonists of I-receptor and α-2 AR. The results demonstrate that d-arginine activated cellular NO production, and this NO synthesis could be inhibited by both idazoxan and rauwolscine (Fig. 3D). This finding strongly indicated that arginine may be acting as a ligand on membrane receptor(s). d-arginine was shown to act as a vasodilator in human forearm when locally infused (30, 31).
Pertussis Toxin Attenuates l-Arginine-Mediated NO Formation.
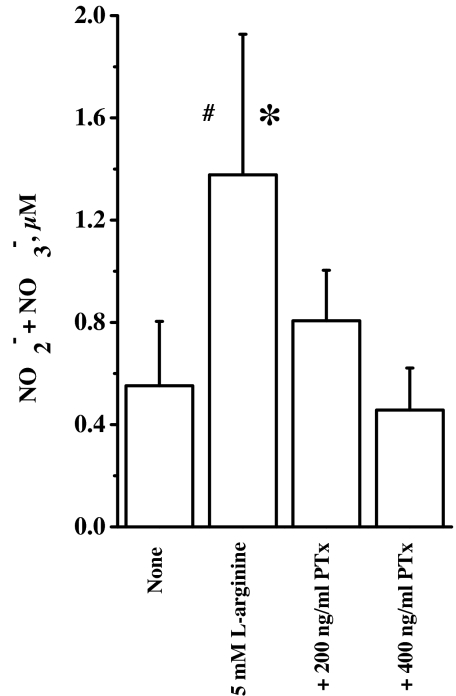
Because α-2 AR is a GPCR, it is possible that l-arginine activates G proteins, leading to the formation of cellular NO. Pertussis toxin is a specific inhibitor of heterotrimeric G protein of the type, Gi/o (32, 33). It does so by ADP-ribosylating the α subunit of G protein. Therefore, we pretreated the cell cultures with pertussis toxin and then treated with l-arginine in the presence and absence of indicated doses of pertussis toxin. We observed that pertussis toxin dose-dependently inhibited NO formation from l-arginine (Fig. 4). These results indicate participation of G proteins in the actions of extracellular l-arginine.
Fig. 4.
Effect of pertussis toxin (PTx) on NO synthesis. The cell cultures (120,000 cells per well) were pretreated with pertussis toxin at 200 and 400 ng/ml for 2 h, followed by l-arginine (5 mM) in the presence and absence of pertussis toxin for 30 min at 37°C in a 5% CO2/95% air incubator. The culture supernatants were assayed for NO2−/NO3− by modified Griess reaction (n = 3; ∗, P < 0.05 vs. + 400 ng/ml PTx; #, P = 0.077 vs. none).
Exogenous l-Arginine Instigates Formation of Second Messengers.
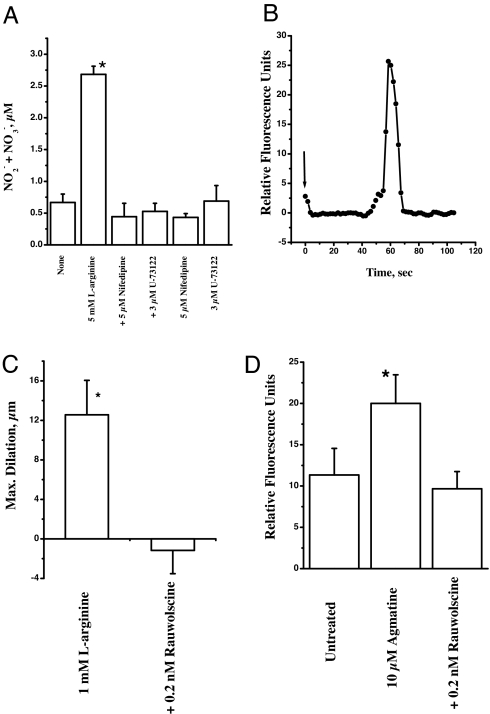
Because l-arginine treatment activates GPCR and G proteins, it is imperative to know the rest of the signal transduction pathway. The activation of G proteins may in turn activate phospholipase C, yielding IP3 and diacylglycerol. As shown in Fig. 5A, treatment of cells with phospholipase C inhibitor U73122 in the presence and absence of l-arginine resulted in complete inhibition of NO formation, thus implying a role for phospholipase C reaction products.
Fig. 5.
Ca2+ dependence of l-arginine activity and agmatine-mediated cellular NO synthesis. (A) Effect of Ca2+ channel blocker and phospholipase C inhibitor on NO formation. The cell cultures were treated with 5 mM l-arginine in the presence and absence of 5 μM nifedipine and 3 μM U73122. The experiments were conducted at 37°C for 30 min in a 5% CO2/95% air incubator. The culture supernatants were assayed for NO2−/NO3− by using a modified Griess reaction (n = 3; ∗, P < 0.05 vs. none). (B) Fluorescence recording of fluo-4-loaded cells (1 μM in a six-well plate; 150,000 cells per well) with excitation at 488 nm and emission at 526 nm. A 10 mM aliquot of l-arginine was added (at arrow), and a time course of Ca2+ fluorescence was recorded using a Leica deconvolution microscope with a Xenon light source. Digitized images were captured with Slidebook software, and this software is used for time lapse and excitation of the fluorophore probe. The data are representative of three similar recordings. (C) Effect of α-2 AR antagonist on l-arginine-mediated dilation. The gracilis anticus muscle segments of the first-order gracilis muscle arterioles were isolated as described in Materials and Methods. The individual arteriolar segments were cannulated at both ends in a water-jacketed vessel chamber. The distal micropipette was connected to a stopcock, and the proximal micropipette was connected to a reservoir, the height of which was adjusted to achieve 80-mmHg intraluminal pressure. Test compounds (l-arginine and rauwolscine) were included in the superfusion buffer (∗, P < 0.05 vs. + 0.2 nM rauwolscine; n = 3). (D) Effect of α-2 AR antagonist on the agmatine-mediated NO formation. The 90% confluent cells preloaded with 5 μM DAF-FM diacetate were treated with 10 μM agmatine in the presence and absence of 0.2 nM rauwolscine for 10 min as described in Materials and Methods. The fluorescence as a measure of NO synthesis was measured using a UV confocal microscope (n = 3; ∗, P < 0.05 vs. untreated).
Ca2+ Influx Precedes NO Formation.
The cellular NO formation depends on the influx of Ca2+. To test this possibility, we tested the effect of the Ca2+ channel blocker nifedipine. The treatment of cell cultures with nifedipine in the presence and absence of l-arginine resulted in complete inhibition of NO formation (Fig. 5A). We also analyzed Ca2+ influx after treatment with exogenous l-arginine by fluorescence microscopy. The cells were loaded with fluorescence Ca2+ indicator fluo 4 and then treated with l-arginine. As shown in Fig. 5B, there was a Ca2+ spike that quickly dissipated with time. These data demonstrate that l-arginine mediates influx of Ca2+ through Ca2+ channels, thus leading to NO synthesis.
α-2 AR Antagonist Attenuates l-Arginine-Initiated Gracilis Vessel Relaxation.
We wanted to extend the observations made in cell culture experiments to isolated vessel system to demonstrate the vascular relevance of receptor-mediated actions of l-arginine. We isolated rat gracilis vessels and treated them with 1 mM l-arginine in a vessel chamber superfused with a nonrecirculating system (described in Materials and Methods). This treatment led to significant dilation of spontaneously constricted vessels (Fig. 5C). The same experiment carried in the presence of very low concentrations of α-2 AR antagonist rauwolscine (0.2 nM) resulted in complete attenuation of vessel dilation (Fig. 5C). These data indicate that receptor(s) may mediate l-arginine-initiated vessel relaxation.
Agmatine Activated NO Synthesis and Its Inhibition with α-2 AR Antagonist.
Millimolar levels of arginine are required to activate cellular NO synthesis. It is very unlikely that arginine will act as a direct receptor agonist at this high concentration. However, it is possible that arginine undergoes decarboxylation via arginine decarboxylase (ADC) to form agmatine. This agmatine in turn will bind to the membrane as agonist and activate NO synthesis. We tested this hypothesis by exposing the cell culture to exogenous agmatine and measured NO synthesis by using fluorescent dye, DAF-FM diacetate. As shown in Fig. 5D, agmatine activated NO at low concentrations (10 μM, 10 min) and this could be attenuated with rauwolscine (0.2 nM).
In summary, our data demonstrates that exogenous l-arginine and agmatine activate cellular NO formation through receptor binding. This involves the second messenger pathway of activated phospholipase C and Ca2+ influx.
Discussion
The idea that the substrate of an enzymatic reaction also acts as an exogenous ligand for a receptor and initiates signal transduction across the cell membrane seems quite intriguing. However, a closer look at the structures of l-arginine and its decarboxylated product, agmatine, show that both have a guanidinium group and thus partly resemble the structures of imidazolines (Fig. 1). The compounds with imidazoline/guanidinium group are known to act as ligands for both I-receptors and α-2 AR. The activation of these receptors initiates cascade of cellular processes with varying functions, including vasodilation, renal sodium excretion, and modulation of ocular pressure. Agmatine was demonstrated more than a decade ago to be a ligand for I-receptors and α-2 AR in the brain, and it was proposed to be an endogenous ligand for I-receptor (18, 19). Similarly, many biological actions of l-arginine may involve its binding as ligand on membrane receptor(s) and affect the release of NO through signal transduction mechanisms.
We observed that millimolar levels of exogenous l-arginine were required to produce significant cellular NO production, and l-arginine at concentrations present in normal human plasma failed to activate NO (Fig. 2A). In vivo studies have demonstrated that plasma arginine levels reached 822 ± 59 μM upon i.v. infusion of exogenous l-arginine (6 g). However, this level of arginine had no effect on vasorelaxation. Significant relaxations in humans have only been observed when the plasma arginine levels reach 6.22 ± 0.4 mM (34–36) upon infusion of significantly higher amounts (30 g). Our data show that exogenous l-arginine stimulates NO production in HUVECs, and this could be attenuated by hybrid I-receptor and α-2 AR antagonist idazoxan and specific α-2 AR antagonist rauwolscine. This result indicates that l-arginine may be acting by way of receptor(s). Rauwolscine was more potent that idazoxan in attenuating l-arginine effects. Thus, it appears that α-2 AR is the more likely target for l-arginine binding. These observations from cell cultures were extended to isolated vessels where we find potent antagonistic effects of rauwolscine on l-arginine-initiated dilation (Fig. 5C). The role of α-2 AR in the vascular relaxation was previously demonstrated in isolated rings of rat superior mesenteric arteries (37). In these studies, rauwolscine effectively blocked agonist-mediated relaxation.
The observation that millimolar levels of arginine are required for the activation of cellular NO production raises the possibility that either arginine is binding to a low affinity binding site on cell membrane or it is metabolizing to form a product that in turn acts as a direct ligand on the receptor. Agmatine could be one such product because endothelial cells do possess arginine decarboxylase (ADC) activity (38). We found that agmatine at low micromolar concentrations activated NO synthesis and could be attenuated with rauwolscine (Fig. 5D). Morrisey and Klahr (16) have similarly demonstrated endothelial NO synthesis in response to agmatine exposure. This could be inhibited by idazoxan (16), thus suggesting possible involvement of receptor(s) in the actions of agmatine. But a detailed study of this agmatine binding and the signal transduction pathway that leads to the activation of cellular NO production are lacking. Also, histamine, acetylcholine, adenosine, and clonidine have been demonstrated to enhance endothelium-dependent vasorelaxation via specific membrane receptors (39). Thus, there is precedence for agonist activation of various receptors in endothelial cells resulting in cellular NO synthesis. A nonsubstrate role for arginine was supported by the observation that NO was stimulated by exogenous d-arginine and was attenuated by idazoxan and rauwolscine (Fig. 3C). Several in vivo studies corroborate this observation. d-arginine was found to increase venous diameter in humans and hypotension in rats (30, 40). Rhodes et al. (31) have found that d-isomer of arginine was more potent than l-isomer as vasodilator of human forearm. However, mediation of NO in these studies was not clearly documented, which raises the important question about whether other structural analogs of l-arginine may also exogenously activate endothelial NO.
To further clarify the receptor type, we tested the effect of specific α-2 AR agonist, dexmedetomidine. If arginine is stimulating NO synthesis by binding to α-2 AR as an agonist, then dexmedetomidine must also activate NO. We found that dexmedetomidine dose-dependently stimulated endothelial NO production (Fig. 3C). Other α-2 AR agonists also have been shown to stimulate NO synthesis in endothelial cell cultures (41) and to stimulate NO-dependent vasodilation in animals (28). However, l-arginine targeting receptor(s) other than α-2 AR cannot be discounted, because rauwolscine is also known to act on serotonin [5-hydroxytryptamine (5-HT)] receptors (42, 43).
If l-arginine is binding to the membrane receptor(s) and activating intracellular NO production, then it is imperative to know the signal transduction mechanism involved. Pertussis toxin, which inhibits G proteins by ADP-ribosylation of α subunit, attenuated NO synthesis from l-arginine (Fig. 4). Thus, our data showed mediation of G proteins in the actions of l-arginine, which is consistent with the receptor being a GPCR. The sensitivity to pertussis toxin indicates that the heterotrimeric G protein may be of the type Gi. In endothelial cells, α-2 AR and other receptors are believed to couple selectively to heterotrimeric Gi proteins (23). A similar observation was made with UK14304, an agonist of α-2 AR, wherein pretreatment with pertussis toxin attenuated agonist-initiated NO synthesis in bovine aortic endothelial cells (41) and relaxations in porcine coronary arteries (24). Signaling via G proteins leads to a variety of cellular responses, including activation of phospholipase C, yielding second messengers, diacylglycerol and IP3. Activation of several receptor systems that lead to endothelial NO synthesis has been shown to involve G protein-coupled signaling via phospholipase C activation (44–46). A similar pathway may be responsible for l-arginine-mediated NO signaling. The activation of phospholipase C ultimately results in initiation of Ca2+ signaling that plays important second messenger roles. Our results show that l-arginine treatment yielded a spike in Ca2+ as measured by Ca2+ indicator dye, fluo 4 (Fig. 5B). This observation prompted an important question about the interdependence of l-arginine-mediated NO synthesis and Ca2+ spike. The Ca2+ channel blocker, nifedipine, and the phospholipase C inhibitor, U73122, both attenuated l-arginine-initiated NO production (Fig. 5A), thus showing that NO synthesis depends on intracellular Ca2+ mobilization.
In atherosclerosis or hypercholesterolemia, impaired NO synthesis is believed to be a major contributor to endothelial dysfunction (47–49). Interestingly, bradykinin- and A23187-mediated relaxations are not affected, but that relaxation due to acetylcholine is impaired. The prevailing hypothesis is that receptor-mediated relaxation via Gi proteins is affected in this condition (50). Whereas acetylcholine-mediated NO formation is via Gi proteins, that due to bradykinin is not coupled to Gi. Thus, the endothelial dysfunction is not a general phenomenon in atherosclerosis/hypercholesterolemia. In attempts to correct the endothelium dysfunction, i.v. l-arginine injections were able to restore acetylcholine-mediated relaxations (51). Unfortunately, the plasma l-arginine levels in hypercholesterolemia were not determined. However, we hypothesize that exogenous l-arginine restored the depleted substrate levels occurring during the development of hypercholesterolemia. If this argument were true, then bradykinin- and A23187-mediated relaxation should also be affected in hypercholesterolemia. Therefore, the availability of l-arginine as substrate for eNOS may not be the limiting factor. The l-arginine reversal of hypercholesterolemic dysfunction may, however, be due to l-arginine acting as a receptor agonist and activating eNOS (and thus relaxation) through signal transduction, as proposed here.
In conclusion, this investigation using endothelial cells shows that exogenous arginine or agmatine can act as an agonist for membrane receptor(s). This binding of arginine activated a cascade of signal transduction involving G proteins, phospholipase C, and mobilization of intracellular Ca2+, resulting in activation of eNOS to produce NO. These findings could alter many fundamental aspects of cardiovascular physiology by revealing that various effects of exogenous arginine are due to its actions on receptor(s), either directly as agonist or via catalytic conversion to agmatine. This finding may lead to the development of new areas of therapeutic strategies in a variety of cardiovascular diseases.
Materials and Methods
Cell Culture and Reagents.
HUVECs were obtained from American Type Culture Collection (Manassas, VA). The cells were cultured in media consisting of modified Kaigan's F-12 (American Type Culture Collection) supplemented with 10% FBS (American Type Culture Collection), 0.1 mg/ml heparin (Sigma Chemical, St. Louis, MO), 0.03 mg/ml endothelial cell growth supplement (Upstate, Lake Placid, NY), 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified chamber at 37°C in a 5% CO2/95% air atmosphere. The cells were cultured in T75 flasks and 12-well tissue culture plates. For these studies, the cells were used for up to eight passages.
The experiments were conducted by preincubating cultures in Krebs solution for 30 min. The cells were then treated with arginine and/or various compounds for another 15–30 min in a humidified chamber at 37°C in a 5% CO2/95% air atmosphere. Both l-arginine and d-arginine (Sigma Chemical) were in free acid form and prepared in Hepes buffer, pH 7.4. l-arginine had undetectable levels of d-isomer as contaminant, and vice versa with d-arginine.
Nitrate/Nitrite Determination.
At the end of incubations, the culture supernatants were collected by centrifugation (10,000 × g for 10 min) in a Centra MP4R centrifuge (International Equipment, Needham Heights, MA). The supernatants were used for determination of nitrate/nitrite by a modification of Griess reaction (52). Briefly, nitrate in the samples was first converted into nitrite with nitrate reductase. After an incubation of 45 min, the samples were mixed with a solution consisting of equal volumes of 14 mM dapsone (4, 4′-diamino-diphenylsulpfone) and 4 mM N-(1-Naphthyl)ethylenediamine. The color was allowed to develop for 5 min, and absorbance was read at 550 nm in a Bio-Rad (Hercules, CA) plate reader. The nitrate/nitrite was also determined using a chemiluminescence technique (53). The nitrate in the samples was converted to nitrite with nitrate reductase and then analyzed by chemiluminescence. The nitrite was determined as the difference between in the presence and absence of 0.5% sulfanilamide in 0.1 M HCl.
NO Measurements with DAF-FM Diacetate.
The fluorescence measurements were made according to published procedures (54). In brief, the cells grown on a chambered borosilicate coverglass system were incubated with serum-free and phenol red-free M 199 medium for 60 min. These cells were then loaded with 5 μM DAF-FM diacetate (Invitrogen, Carlsbad, CA) for 30 min at 37°C. After loading, the cells were washed with the media and then treated with agmatine for 10 min in the presence and absence of rauwolscine at room temperature. The cellular fluorescence was measured using a Leica (Exton, PA) DMIRBE inverted epifluorescence /Nomarski microscope outfitted with Leica TCS-NT/SP1 laser confocal optics and appropriate filters for fluorescence microscopy.
Ca2+ Measurements.
The fluorescence recording of fluo-4-loaded cells (1 μM in a 35 × 10-mm plate; 150,000 cells per plate) was monitored with excitation at 488 nm and emission at 526 nm. A 10 mM aliquot of l-arginine was added, and a time course of Ca2+ fluorescence was recorded using a Leica deconvolution microscope with a Xenon light source. Digitized images were captured with Slidebook software, and this software was used for time-lapse and excitation of the fluorophore probe (55).
Isolated Rat Gracilis Vessel Studies.
The gracilis anticus muscles were removed from rats (male, Sprague–Dawley), and segments of the first-order gracilis muscle arterioles were isolated as described in ref. 56. The individual arteriolar segments were cannulated at both ends in a water-jacketed vessel chamber. The distal micropipette was connected to a stopcock, and the proximal micropipette was connected to a reservoir, the height of which was adjusted to 108.8 cm to achieve 80 mmHg intraluminal pressure. The vessel chamber was superfused continuously via a nonrecirculating system with oxygenated (14% O2/5% CO2-balance N2) modified Krebs buffer (5 ml/min) at 37°C. For internal diameter measurements, the vessel chamber was mounted on the stage of a microscope that was fitted with a video camera leading to a video caliper. Mounted vessels were allowed to stabilize for 60 min before initiation of dilation experiments. During the stabilization period, the internal diameter of the vessels decreased spontaneously from 193.5 ± 22.92 μm to 135.92 ± 10.38 μm (n = 4). Only those vessels that developed an active tone during the stabilization period were used for the studies. Test compounds (l-arginine and rauwolscine) were included in the superfusion buffer.
Statistical Analysis.
Data are expressed as mean ± SD values for the number of culture preparations indicated in the figure legends. Statistical significance was evaluated by one-way ANOVA, and P < 0.05 is considered significant. The n values represent the number of independent experiments.
Acknowledgments
We thank Dr. Daniel Kim-Shapiro for providing laboratory space and facilities to carry out part of this work. This work was supported in part by National Institutes of Health Grants HL074391 and HL71189 (to J.R.L.).
Abbreviations
- α-2 AR
α-2 adrenoceptor
- eNOS
endothelial NOS
- HUVEC
human umbilical vein endothelial cell
- I-receptor
imidazoline receptor
- IP3
inositol-1,4,5-triphosphate
- NOS
NO synthase.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Proc Natl Acad Sci USA. 1991;88:10480–10484. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forstermann U, Closs EI, Pollock JS, Nakane M, Schwarz P, Gath I, Kleinert H. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.hyp.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 3.Tsikas D, Boger RH, Sandmann J, Bode-Boger SM, Frolich JC. FEBS Lett. 2000;478:1–3. doi: 10.1016/s0014-5793(00)01686-0. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Cardena G, Martasek P, Masters BS, Skidd PM, Couet J, Li S, Lisanti MP, Sessa WC. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 6.Hardy TA, May JM. Free Rad Biol Med. 2002;32:122–131. doi: 10.1016/s0891-5849(01)00781-x. [DOI] [PubMed] [Google Scholar]
- 7.McDonald KK, Zharikov S, Block ER, Kilberg MS. J Biol Chem. 1997;272:31213–31216. doi: 10.1074/jbc.272.50.31213. [DOI] [PubMed] [Google Scholar]
- 8.Girerd XJ, Hirsch AT, Cooke JP, Dzau VJ, Creager MA. Circ Res. 1990;67:1301–1308. doi: 10.1161/01.res.67.6.1301. [DOI] [PubMed] [Google Scholar]
- 9.Cooke JP, Andon NA, Girerd XJ, Hirsch AT, Creager MA. Circulation. 1991;83:1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- 10.Bode-Boger SM, Boger RH, Galland A, Tsikas D, Frolich JC. Br J Clin Pharmacol. 1998;46:489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lekakis JP, Papathanassiou S, Papaioannou TG, Papamichael CM, Zakopoulos N, Kotsis V, Dagre AG, Stamatelopoulos K, Protogerou A, Stamatelopoulos SF. Int J Cardiol. 2002;86:317–323. doi: 10.1016/s0167-5273(02)00413-8. [DOI] [PubMed] [Google Scholar]
- 12.Boger RH, Bode-Boger SM, Kienke S, Stan AC, Nafe R, Frolich JC. Atherosclerosis. 1998;136:67–77. doi: 10.1016/s0021-9150(97)00183-4. [DOI] [PubMed] [Google Scholar]
- 13.Roth-Isigkeit A, Hasselbach L, Ocklitz E, Bruckner S, Ros A, Gehring H, Schmucker P, Rink L, Seyfarth M. Clin Exp Immunol. 2001;125:80–88. doi: 10.1046/j.1365-2249.2001.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takagawa Y, Berger ME, Tuck ML, Golub MS. Hypertens Res. 2002;25:197–202. doi: 10.1291/hypres.25.197. [DOI] [PubMed] [Google Scholar]
- 15.Raasch W, Regunathan S, Li G, Reis DJ. Life Sci. 1995;56:2319–2330. doi: 10.1016/0024-3205(95)00226-v. [DOI] [PubMed] [Google Scholar]
- 16.Morrissey JJ, Klahr S. Proc Assoc Am Phys. 1997;109:51–57. [PubMed] [Google Scholar]
- 17.Christiansen B, Wellendorph P, Brauner-Osborne H. Br J Pharmacol. 2006;147:855–863. doi: 10.1038/sj.bjp.0706682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 19.Reis DJ, Li G, Regunathan S. Ann NY Acad Sci. 1995;763:295–313. doi: 10.1111/j.1749-6632.1995.tb32416.x. [DOI] [PubMed] [Google Scholar]
- 20.Angus JA, Cocks TM, Satoh K. Fed Proc. 1986;45:2355–2359. [PubMed] [Google Scholar]
- 21.Steinberg SF, Jaffe EA, Bilezikian JP. Naunyn Schmiedebergs Arch Pharmacol. 1984;325:310–313. doi: 10.1007/BF00504374. [DOI] [PubMed] [Google Scholar]
- 22.Regunathan S, Youngson C, Wang H, Reis DJ. Ann NY Acad Sci. 1995;763:580–590. doi: 10.1111/j.1749-6632.1995.tb32453.x. [DOI] [PubMed] [Google Scholar]
- 23.Liao JK, Homcy CJ. Circ Res. 1992;70:1018–1026. doi: 10.1161/01.res.70.5.1018. [DOI] [PubMed] [Google Scholar]
- 24.Flavahan NA, Shimokawa H, Vanhoutte PM. J Physiol. 1989;408:549–560. doi: 10.1113/jphysiol.1989.sp017475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Needham L, Cusack NJ, Pearson JD, Gordon JL. Eur J Pharmacol. 1987;134:199–209. doi: 10.1016/0014-2999(87)90166-x. [DOI] [PubMed] [Google Scholar]
- 26.Pearson JD, Slakey LL, Gordon JL. Biochem J. 1983;214:273–276. doi: 10.1042/bj2140273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Des RC, Nees S. Naunyn Schmiedebergs Arch Pharmacol. 1987;336:94–98. doi: 10.1007/BF00177757. [DOI] [PubMed] [Google Scholar]
- 28.Sunano S, Li-Bo Z, Matsuda K, Sekiguchi F, Watanabe H, Shimamura K. J Cardiovasc Pharmacol. 1996;27:733–739. doi: 10.1097/00005344-199605000-00017. [DOI] [PubMed] [Google Scholar]
- 29.Tsukahara H, Gordienko DV, Goligorsky MS. Biochem Biophys Res Commun. 1993;193:722–729. doi: 10.1006/bbrc.1993.1685. [DOI] [PubMed] [Google Scholar]
- 30.Calver A, Collier J, Vallance P. Clin Sci. 1991;81:695–700. doi: 10.1042/cs0810695. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes P, Barr CS, Struthers AD. Eur. J Clin Invest. 1996;26:325–331. doi: 10.1046/j.1365-2362.1996.144277.x. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Longo J, Dufour MN, Guillon G, Lugnier C. Eur J Pharmacol. 1993;247:119–125. doi: 10.1016/0922-4106(93)90068-k. [DOI] [PubMed] [Google Scholar]
- 33.Shibano T, Codina J, Birnbaumer L, Vanhoutte PM. Biochem Biophys Res Commun. 1992;189:324–329. doi: 10.1016/0006-291x(92)91561-4. [DOI] [PubMed] [Google Scholar]
- 34.Bode-Boger SM, Boger RH, Alfke H, Heinzel D, Tsikas D, Creutzig A, Alexander K, Frolich JC. Circulation. 1996;93:85–90. doi: 10.1161/01.cir.93.1.85. [DOI] [PubMed] [Google Scholar]
- 35.Bode-Boger SM, Boger RH, Creutzig A, Tsikas D, Gutzki FM, Alexander K, Frolich JC. Clin Sci (Lond) 1994;87:303–310. doi: 10.1042/cs0870303. [DOI] [PubMed] [Google Scholar]
- 36.Kanno K, Hirata Y, Emori T, Ohta K, Eguchi S, Imai T, Marumo F. Clin Exp Pharmacol Physiol. 1992;19:619–625. doi: 10.1111/j.1440-1681.1992.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 37.Bockman CS, Gonzalez-Cabrera I, Abel PW. J Pharmacol Exp Ther. 1996;278:1235–1243. [PubMed] [Google Scholar]
- 38.Regunathan S, Youngson C, Raasch W, Wang H, Reis DJ. J Pharmacol Exp Ther. 1996;276:1272–1282. [PubMed] [Google Scholar]
- 39.Kysela S, Torok J. Physiol Res. 2000;49:115–122. [PubMed] [Google Scholar]
- 40.Jun T, Wennmalm A. Acta Physiol Scand. 1994;152:385–390. doi: 10.1111/j.1748-1716.1994.tb09820.x. [DOI] [PubMed] [Google Scholar]
- 41.Liao JK, Homey CJ. J Biol Chem. 1993;268:19528–19533. [PubMed] [Google Scholar]
- 42.Feletou M, Dellazuana O, Duhault J. J Pharmacol Exp Ther. 1994;268:124–132. [PubMed] [Google Scholar]
- 43.Hoyer D, Schoeffter P, Waeber C, Palacios JM. Ann NY Acad Sci. 1990;600:168–181. doi: 10.1111/j.1749-6632.1990.tb16880.x. [DOI] [PubMed] [Google Scholar]
- 44.Tang Y, Li GD. Diabetologia. 2004;47:2093–2104. doi: 10.1007/s00125-004-1589-y. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka N, Kawasaki K, Nejime N, Kubota Y, Nakamura K, Kunitomo M, Takahashi K, Hashimoto M, Shinozuka K. J Pharmacol Sci. 2004;95:174–180. doi: 10.1254/jphs.fpj03036x. [DOI] [PubMed] [Google Scholar]
- 46.Waeber C, Blondeau N, Salomone S. Drug News Perspect. 2004;17:365–382. doi: 10.1358/dnp.2004.17.6.829028. [DOI] [PubMed] [Google Scholar]
- 47.Arcaro G, Zenere BM, Travia D, Zenti MG, Covi G, Lechi A, Muggeo M. Atherosclerosis. 1995;114:247–254. doi: 10.1016/0021-9150(94)05489-6. [DOI] [PubMed] [Google Scholar]
- 48.Ross R. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 49.Wennmalm A. J Intern Med. 1994;235:317–327. doi: 10.1111/j.1365-2796.1994.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 50.Shimokawa H, Flavahan NA, Vanhoutte PM. Circulation. 1991;83:652–660. doi: 10.1161/01.cir.83.2.652. [DOI] [PubMed] [Google Scholar]
- 51.Cooke JP, Andon NA, Girerd XJ, Hirsch AT, Creager MA. Circulation. 1991;83:1057–1062. doi: 10.1161/01.cir.83.3.1057. [DOI] [PubMed] [Google Scholar]
- 52.Marzinzig M, Nussler AK, Stadler J, Marzinzig E, Barthlen W, Nussler NC, Beger HG, Morris SM, Jr, Bruckner UB. Nitric Oxide. 1997;1:177–189. doi: 10.1006/niox.1997.0116. [DOI] [PubMed] [Google Scholar]
- 53.Marley R, Feelisch M, Holt S, Moore K. Free Radical Res. 2000;32:1–9. doi: 10.1080/10715760000300011. [DOI] [PubMed] [Google Scholar]
- 54.Montagnani M, Chen H, Barr VA, Quon MJ. J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 55.DeCoster MA, Lambeau G, Lazdunski M, Bazan NG. J Neurosci Res. 2002;67:634–645. doi: 10.1002/jnr.10131. [DOI] [PubMed] [Google Scholar]
- 56.Johnson FK, Johnson RA. Am J Physiol. 2003;285:R536–R541. doi: 10.1152/ajpregu.00624.2002. [DOI] [PubMed] [Google Scholar]