Abstract
Transposable elements (TEs) are proposed as a basis for developing drive systems to spread pathogen resistance genes through vector mosquito populations. The use of transcriptional and translational control DNA elements from genes expressed specifically in the insect germ line to mediate transposition offers possibilities for mitigating some of the concerns about transgene behavior in the target vector species and eliminating effects on nontarget organisms. Here, we describe the successful use of the promoter and untranslated regions from the nanos (nos) orthologous gene of the yellow fever mosquito, Aedes aegypti, to control sex- and tissue-specific expression of exogenously derived mariner MosI transposase-encoding DNA. Transgenic mosquitoes expressed transposase mRNA in abundance near or equal to the endogenous nos transcript and exclusively in the female germ cells. In addition, MosI mRNA was deposited in developing oocytes and localized and maintained at the posterior pole during early embryonic development. Importantly, four of five transgenic lines examined were capable of mobilizing a second MosI transgene into the mosquito genome, indicating that functional transposase was being produced. Thus, the nos control sequences show promise as part of a TE-based gene drive system.
Keywords: dengue, gene drive, transposon
Aedes aegypti, the primary vector of dengue viruses, is indigenous throughout the tropics (1). Estimates for the worldwide dengue fever burden vary from 50 to 100 million cases per year (2), with many more people at risk. Control strategies for dengue fever are based on managed care for those already infected, and the elimination of potential mosquito breeding sites to prevent or limit the size of the vector populations required for outbreaks. However, these options are expensive and labor-intensive and require a sustainable healthcare infrastructure. Many people in areas affected by dengue are poor and overcrowded and have little or no access to such services (3). Thus, there has been a significant increase in dengue incidence and prevalence in the past 30 years (3, 4). These increases encourage researchers to develop novel technologies, such as genetic control, to ease the disease burden on the developing world.
Genetic control strategies include those that propose the insertion of exogenous DNA into the vector mosquito with the aim of either reducing the vector population, or limiting or eliminating their ability to transmit pathogens to humans (population replacement) (5, 6). These strategies benefit from the availability of transgenesis technologies, and stable integration of engineered genes into A. aegypti, using transposable elements (TEs) (7, 8) is now routine (9). Population replacement strategies also depend on the development of effector genes to interfere with and prevent pathogen transmission. RNAi-based effector genes reduce significantly dengue 2 viral titers in transgenic mosquitoes (10). Finally, population replacement strategies require a system for gene drive to spread resistance genes into wild populations, and TEs have been proposed as a mechanistic basis for the development of such a system (6, 11). Releasing transgenic mosquitoes has technical, social, and ethical considerations, and criteria for meeting these have been identified (12). One of the technical challenges involves restricting the activity of the gene drive system to where it will be needed, namely, in the germ line of the target species. This challenge can be met partially by putting the gene drive agent under control of DNA derived from developmentally regulated genes to direct sex-, tissue-, and stage-specific transposition.
Several genes involved in early embryonic development, including vasa, oskar, and nanos (13–18), express products that are localized to the developing germ cells, and therefore are candidates for donating control sequences to a gene drive system. nanos (nos) genes have been characterized in a number of insects including Drosophila species (16–18) and the mosquitoes Anopheles gambiae, Anopheles stephensi, and A. aegypti (14). nos RNA is maternally derived and localized to the pole plasm in embryos (19). Translation of nos mRNA is repressed throughout oogenesis, and unlocalized transcripts in embryos are translationally repressed as well (16–18). The control DNA sequences for localization and translational repression are located in the 3′-end UTR of the nos mRNA (16, 17).
A. aegypti nos promoter and UTR sequences were used to direct the expression of the mariner MosI (7) transposase with the dual purpose of testing the function of the cis-acting control elements and determining whether expression of the transposase in the female germ line results in transposition. The data described here support the conclusion that we have identified functional mosquito DNA fragments that control nos expression and localization. These sequences could be an essential part of a TE-based, gene-drive system for the larger goal of genetic control of transmission of dengue viruses.
Results
The nos-MosI donor plasmids, AenMn and AenMn2 (Fig. 1), were injected in separate experiments into 598 and 964 embryos, respectively. A total of 78 (13%) AenMn and 110 (11%) AenMn2 adult survivors were obtained, and four transformed lines (AenMn 2, 3, 4, and 5; AenMn2 1, 9, 18, and P1) were recovered for each construct for approximate transformation frequencies of 7.7 and 7.2%, respectively. Six lines were selected for Southern blot analyses, and four (lines 3, 4, 18, and P1) had hybridization signals consistent with single insertions. Lines 1 and 9 had insertions of two and four copies, respectively (data not shown). These data indicate that AenMn and AenMn2 transgenes integrate equally well into the mosquito genome.
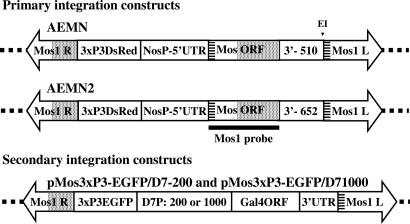
Fig. 1.
Schematic representations of the primary and secondary transformation constructs. All constructs were flanked by the mariner MosI right- (MosI R) and left-hand (MosI L) inverted repeat sequences. The dotted lines represent A. aegypti genomic DNA into which the constructs are transposed. Primary integration constructs carry the marker gene (3xP3DsRed), which confers red eye-specific fluorescence, and the MosI transposase ORF (MosORF) flanked by the nos promoter and 5′- and 3′-UTRs (NosP + 5′-UTR). AEMN has a 510-bp 3′-UTR (3′- 510) and an internal EcoRI site (E1). AEMN2 has a 652-bp 3′-UTR (3′- 652) and lacks the restriction endonuclease site. The striped and dotted boxes indicate regions of identity derived from the MosI left- and right-hand repeat regions, respectively. The thick line represents the extent of probes (MosI probe) used to verify the presence of the MosI ORF-encoding DNA or mRNA in Southern and Northern blot analyses, respectively, and in hybridizations in situ. Secondary integration constructs carry the marker gene (3xP3EGFP), which confers green eye-specific fluorescence, one of two versions of the D7 gene promoter (42) 200 or 1,000 nucleotides in length (D7P: 200 or 1,000), and 3′-UTR flanking the Gal4 ORF (Gal4ORF).
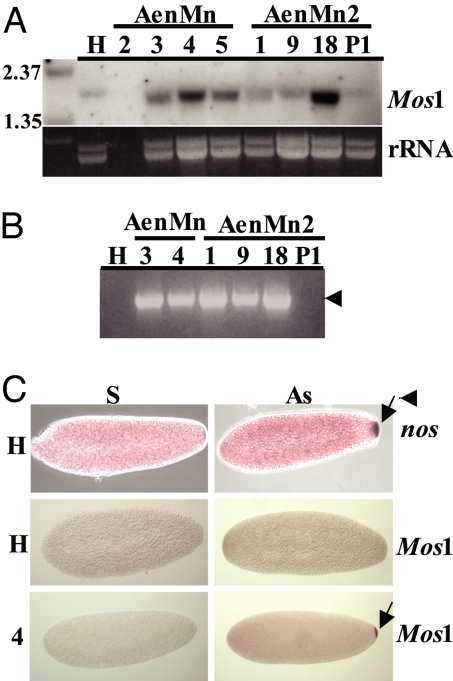
The presence of MosI transposase mRNA was used to assay expression in transgenic mosquitoes. Total RNA isolated from fully developed ovaries 3 days after a bloodmeal was subject to Northern blot analyses to determine whether MosI mRNA accumulated in the transgenic lines (Fig. 2A). Hybridization with a MosI-specific probe (Fig. 1) produced a strong signal of the expected size (≈1.6 kb) in the three lines, 1, 3, and 18, selected for this analysis, but not in untransformed H mosquitoes. A duplicate membrane hybridized with a nos-specific probe showed strong signals in all tested samples. Although variations in the size and specific activity of the labeled MosI and nos probes prevents rigorous quantization, these results are consistent with the interpretation that the MosI mRNA in some transgenic lines accumulates to levels equivalent to the endogenous nos transcription products.
Fig. 2.
Northern blot analyses of adult AenMn and AenMn2 transgenic mosquitoes. (A) Northern blot analysis conducted by using 10 μg of total RNA isolated from fully developed ovaries from untransformed Higgs strain mosquitoes (H), AenMn transgenic line 3 or AenMn2 transgenic lines 1 and 18. Duplicate membranes were hybridized with a probe specific for either the MosI ORF (MosI) or the A. aegypti nanos ORF (nos). The RNA size marker lane is indicated (M). Ethidium bromide-stained rRNA loading control of samples is shown below. (B) Northern blot analysis conducted by using 10 μg of total RNA isolated from fully developed ovaries (O), dissected carcasses (C), or whole males (♂) from AenMn and AenMn2 transgenic lines or from untransformed Higgs strain mosquitoes (H). Membranes were hybridized with a radiolabeled probe specific for the MosI ORF (MosI). rRNA loading control of samples is shown below. RNA size markers (M) are shown on the left.
Northern blot analyses were performed on total RNA isolated from fully developed ovaries, female carcasses after ovary dissection, and whole sibling males to determine sex- and tissue-specificity of nos-directed MosI RNA expression (Fig. 2B). Strong MosI-specific hybridization signals were visible in RNA samples from ovaries of transgenic lines 1, 3, 4, 5, and 18, whereas weak hybridization was visible in ovary RNA from lines 2 and 9. No specific signal was seen in line P1. What appears to be a weak hybridization signal was detected in some samples derived from carcasses and males, but the presence of a similar signal in untransformed mosquitoes is consistent with nonspecific binding of the probe with the rRNA resolving at ≈1.8 kb (see Fig. 3). This interpretation is supported by RT-PCR analyses when MosI-specific primers amplified a product from ovary and not carcass or male RNA (data not shown). These results support the conclusion that the putative nos control DNA directs tissue- and sex-specific expression and accumulation of MosI mRNA in ovaries with a pattern consistent with that of endogenous nos expression.
Fig. 3.
RNA analysis of AenMn and AenMn2 transgenic embryos. (A) Northern blot analysis was conducted by using 10 μg of total RNA isolated from 0- to 5-h-old embryos collected from untransformed Higgs strain mosquitoes (H), AenMn lines 2, 3, 4, and 5, and AenMn2 lines 1, 9, 18, and P1. Membranes were hybridized with a radiolabeled probe specific for the MosI ORF (MosI), with rRNA loading control of samples shown below. The RNA size marker lane is indicated (M). (B) One-Step RT-PCR of 0- to 5-h-old embryos RNA collected from untransformed (H) or transgenic mosquitoes (1, 3, 4, 9, 18, and P1), using the MosI-specific forward primer MLF2 and the A. aegypti nos-specific reverse primer nosUTRR2. The arrow indicates the expected transcript size. (C) In situ hybridization of MosI in AenMn transgenic embryos. Control (H) and transgenic line (4) embryos hybridized with sense (S) or antisense (AS) nos and MosI probes. The arrow points to specific localization of the mRNA to the posterior pole.
Northern blot analyses were conducted by using total RNA isolated from transgenic embryos 6 h after oviposition to confirm postfertilization maintenance of MosI mRNA (Fig. 3A). Strong MosI-specific hybridization signals were detected in lines 3, 4, 5, and 18, with weaker, more diffuse signals visible in lines 1 and 9. A signal resolving at a slightly larger size is visible in untransformed mosquitoes, but as in the previous experiments (Fig. 2), this is likely the result of nonspecific probe binding to the rRNA. In support of this interpretation, RT-PCR performed on embryo RNA produced an abundant amplicon from lines 1, 3, 4, 9, and 18, but did not amplify untransformed embryo RNA (Fig. 3B). Hybridizations in situ performed on early embryos from transgenic line AenMn 4 demonstrated that nos-generated MosI RNA was localized properly to the pole plasm as occurs with the nos endogenous mRNA (14) (Fig. 3C).
Experiments to test whether MosI mRNA was translated into functional transposase were based on determining whether secondary insertions of donor constructs (pMos3xP3-EGFP/D7–200 or pMos3xP3-EGFP/D71000) (Fig. 1) (Z.N.A., unpublished work) marked with EGFP could integrate without helper plasmids after their injection into transgenic embryos. Thus, the only source of transposase would be the integrated transgene. Four of the five nos-MosI transgenic lines were able to catalyze the mobilization of the EGFP-MosI donor to produce secondary transformed mosquitoes (Table 1). In total, the 1,676 embryos injected resulted in 440 survivors (26%) and an average transformation frequency of 3.6%. These results are similar to those obtained when helper plasmids are used (7, 20). Importantly, no secondary integrations were detected in control animals injected with the EGFP test constructs without helper plasmids.
Table 1.
Integration of a secondary Mos1 plasmid in primary nos-Mos1 strains
| nos-Mos line | No. injected | No. of G0 survivors (%) | TL* | TF,† % |
|---|---|---|---|---|
| AenMn no. 2 | 354 | 100: 61♂, 39♀ (28) | 1 | 2 |
| AenMn no. 3 | 292 | 104: 63♂, 41♀ (35) | 4 | 8 |
| AenMn no. 4 | 288 | 83: 28♂, 55♀ (29) | 0 | <2.4 |
| AenMn no. 5 | 415 | 28: 14♂, 14♀ (6.7) | 2 | 14 |
| AenMn2 no. 18 | 327 | 125: 65♂, 60♀ (38) | 1 | 1.6 |
| Total | 1,676 | 440: 231♂, 209♀ (26) | 8 | 3.6 |
| Higgs white-eye (control) | 992 | 361: 205♂, 156♀ (36) | 0‡ | 0.0 |
*TL, number of independent GFP-marked transformed lines obtained.
†TF, transformation frequency represents the number of independent transformed lines generated per fertile G0 survivor.
‡48,483 G1 progeny screened from 43 male and 20 female pools.
Genetic and Southern blot analyses verified the presence and number of secondary integrations in the primary lines (Fig. 4). Individual animals had both DsRed and EGFP marker genes indicating insertions of both the primary and secondary transgene constructs. Southern blot analyses of genomic DNA, using the EGFP ORF-encoding DNA as a probe revealed that three of the lines (5.1, 3.12, and 3.22 in Fig. 4) have two secondary inserts, whereas three (5.3, 2.4, 18.13) have only one.
Fig. 4.
Characterization of secondary integrations in transgenic mosquitoes. (A) Marker gene expression in eyes of a mosquito containing a primary insertion with the DsRed reporter gene (DsRed) and a secondary insertion with the EGFP reporter gene (EGFP). The image on the right shows the same head under visible light (visible). (B) Southern blot analyses of secondary lines with or without primary integrations. Hybridization patterns of the EGFP-specific probe are shown with both primary and secondary insertions (5.1, 5.3, 2.4, 3.12, and 3.22), and lines derived from these with only the secondary insertions (5.3.1, 2.4.1, 3.22.1, and 18.13.1). Control Higgs (H) also is shown. Numbers on the left refer to the relative positions of molecular-weight makers. (C) Transposon-chromosome junction fragments from a secondary insertion. The nucleotide sequence of genomic DNA adjacent to the right- and left-hand terminal inverted repeats of the 2.4.1 secondary insertion. The bolded GATC and CATG are the endogenous Sau3AI and CviAII restriction endonuclease cleavage sites, respectively, used to recover the junction fragments. The double-headed block arrow represents the location of the secondary insertion DNA. The TA dinucleotides in bold are the duplications of the endogenous target sites. Sequence polymorphisms between the transformed line and the strain from which the genomic sequence was derived are shown in bold italics.
MosI integration into chromosome is accompanied by duplications of the target-site dinucleotide TA (7). To verify further that secondary integrations resulted from MosI-mediated transposition, junction fragments consisting of the MosI right- and left-hand repeat DNA immediately adjacent to the mosquito chromosomal DNA were isolated from a genetically segregated secondary insertion (line 2.4.1) carrying a single insertion, and sequenced (Fig. 4). Both right- and left-hand repeat sequences were contiguous with a TA dinucleotide. The mosquito genomic DNA sequence adjacent to the insertion site was reconstructed by removing the MosI-specific sequences, which was used to search the A. aegypti genomic database. A region consisting of 215 nucleotides was found in a genomic scaffold region (gi|108865749|gb|CH902049|CH902049) that differs only in four bases from the query sequence. The genomic target site has two TA dinucleotides in tandem, whereas the recovered junction sites imply a single TA. This could be a result of allelic variation, or more likely, strain polymorphisms, because we use the Higgs white-eye strain as our recipient, and the sequenced genome originates from the Liverpool (LVP) strain (http://aaegypti.vectorbase.org/SequenceData/). This sequence also was 98% similar to an unknown transcription product (GenBank accession no. AY432981.1) identified in an EST sequencing project of mRNAs isolated from mosquito hemocytes (21). These sequencing data verify that the recovered junction fragments are adjacent in the mosquito genome and are a canonical target for MosI-mediated integration.
In summary, these combined data support the conclusion that the nos control DNA sequences tested here are sufficient to confer germ line-specific transcription and translation of an exogenous coding region. Expressed transcription products of the fused nos-MosI transgenes accumulate in the ovaries of transformed mosquitoes, but not in somatic tissues or males. Furthermore, nos-MosI transcripts are localized to the posterior pole of the developing embryo, where germ cell development will occur. Importantly, functional MosI transposase activity present in embryos of transgenic mosquitoes can catalyze integration of a secondary transgene into the germ line, indicating that the transcripts were translated.
Discussion
The nos gene sequences should help meet some of the criteria for a regulated gene drive system (12). Specificity is required of a gene drive system to mitigate concerns about horizontal transfer and impact on nontarget organisms of mobilized autonomous elements. Future work will determine whether the A. aegypti nos promoter is transcriptionally active in other insects; however, this is unlikely based on work that shows that nos promoters from two Diptera, Musca domestica and Chironomus samoensis, are unable to drive transgene expression in Drosophila (18). Therefore, mobilization, using nos sequences may confer species specificity. Furthermore, mobilization localized specifically to the germ line may mitigate a potential load imposed by somatic transposition.
Support for the use of TEs in a gene drive system is derived in part from the recent transfer of the P element into Drosophila melanogaster and its subsequent spread into wild populations worldwide within a period of 50 years (22). Experimental evidence shows that P can spread rapidly in cage populations of fruit flies (23, 24). The hobo element also was examined for its ability to spread in cage populations (25), and these experiments, as well as some predictive models (11), support the proposal that TEs may be robust enough to achieve gene drive in mosquitoes. Although P has transferred horizontally among Drosophila species, it is considered a “dead” element in other insects (26). Thus, the choice of TE for donating the terminal inverted repeats and transposase for the gene drive systems here was limited to those demonstrated to mobilize in A. aegypti: MosI (7), piggyBac (27), and Hermes (8). Although our data demonstrate that nos-controlled MosI can mobilize a plasmid-based element, remobilization, movement of a chromosomally integrated transgene, has yet to be observed. However, there is little evidence of remobilization of any of the three TEs once inserted into the mosquito chromosomes. piggyBac can be unstable after integration in some configurations (28), but the reasons are not known. Analyses of integrated Hermes constructs show that they frequently are accompanied by deletions of one of the inverted repeats, making it unlikely that they could ever remobilize (29). A comprehensive analysis of MosI postintegration behavior showed that remobilization in the germ line was infrequent (one event in 14,000 progeny screened), leading the investigators to conclude that this element would not be a good choice on which to base a gene drive system (30). We also had evidence that MosI remobilization did not occur often (31). However, of the three available elements, MosI was the only one to remobilize, and therefore we chose to work with it first. Our expectation was that, with germ line-directed expression of the transposases, we might be able to improve on the mobilization/remobilization rate. In addition, a thorough survey of the A. aegypti genomic (32, 33) failed to identify any potentially active members of the mariner family of TEs. Thus, remobilization we could detect would not be due to endogenous mariner or mariner-like transposase activity. Our results support this in that no integration was ever observed in the absence of a transposase source.
We emphasize that the nos system described in this work is not restricted to a particular TE. As newer, more mobile elements are engineered or discovered, they can be controlled in the same fashion by the regulatory sequences defined here. Analysis of the A. aegypti genome revealed >12 full-length class II transposons potentially active in this species (33). Furthermore, the nos control sequences described here could also be used for non-TE-based genetic control strategies, such as the expression of a female-lethal gene, homing endonucleases (34), or the nuclear-based rescue of cytoplasmic incompatibility in Wolbachia infection (35). A gene drive system inspired by the Tribolium castaneum selfish genetic element, Medea, and using the control DNA from the fruit fly bicoid gene was developed for D. melanogaster (36). The authors comment that insufficient knowledge of mosquito control DNA sequences involved in early egg and embryo development hampers a translation of this system to vectors [bicoid homologous genes have not been found in A. aegypti (Z.N.A., unpublished work) or A. gambiae (37)]. Most recently, a transgenesis system using homologous nos control DNA and the phi-C31 integrase was demonstrated in D. melanogaster (38). The integrase-expressing helper lines catalyzed site-specific integration at high rates. Our data validate the A. aegypti nos control DNA sequences as viable candidates for similar types of helper constructs expressing integrases, recombinases, or other molecules to aid in the study of genes and gene function in this important disease vector.
Materials and Methods
Plasmid Construction.
All amplification fragments used in plasmid construction were generated by using the proofreading DNA polymerase Pfx (Invitrogen, San Diego, CA). Amplification products were subcloned first into the TOPO-Blunt II or TOPO4-PCR Blunt vectors (Invitrogen) and sequenced before incorporation into plasmid constructs. The genome sequencing project for A. aegypti had yet to generate sufficient information at the time this work was initiated to identify a putative nos promoter for potential transformation constructs. Therefore, inverse PCR was used to amplify mosquito genomic DNA adjacent to the 5′- and 3′-ends of the nos ORF. DNA was digested with XbaI, and fragments between 5 and 7 kb in length were extracted (Qiagen, La Jolla, CA) and self-ligated. Ligated DNA was used as a template for amplification, using primers AenosF5 (5′-ttaaagagaagatgaatcgaagccataacgc-3′) and AenosR1 (5′-ttccgcttcacgtttgcaccgctttatgacc-3′) based on the A. aegypti nos cDNA (14). An amplification product was identified that consisted of the genomic sequence from −1340 to +254 (with +1 being the start of transcription) (GenBank accession no. DQ212955). After this, a fragment 1,572 bp in length corresponding to the A. aegypti nos potential promoter/enhancer and 5′-end UTR was amplified from genomic DNA by using primers AenanSacIIF (5′-ccgcggatcactatcaaacccctaaggaca-3′) and AenanXbaR (5′-tctagatttgttcgttgatctcgatcagcca-3′), and cloned into the SacII/XbaI sites of the shuttle vector pSLfa (39). Primers Aenan3UTRXbaF (5′-tctagacgtaatcgaagtgttggacggggaaag-3′) and AenanUTRR1 (5′-ttcgtcataaaatcgtagactccagcatccc-3′) and genomic DNA as template were used to generate an amplification product of 510 bp corresponding to the nos 3′-end UTR, and this was cloned into the XbaI/EcoRI sites of the same pSLfa plasmid, yielding pSLfa/Aenospro-3′-UTR.
An amplification product corresponding to the MosI transposase ORF was generated by using the helper plasmid pKhsp82M (40) as template and the primers MosORF-F, 5′-tctagaatgtcgagtttcgtgccg-3′, and MosORF-R, 5′-tctagattattcaaagtatttgccgtcgc-3′. The MosI-ORF was inserted into the XbaI site of pSLfa/Aenospro-3′-UTR, generating pSLfa/AenMn. The nos-MosI-nos gene cassette was excised with AscI and inserted into the AscI site of pMos3xP3-DsRED1 yielding pMos3xP3-DsRED/AenMn. The construction of donor plasmid pMos3xP3-DsRED/AenMn2 was identical with that of pMos3xP3-DsRED/AenMn, except that before the insertion of the MosI ORF into pSLfa/Aenospro-3′-UTR, the 3′-end UTR was excised with XbaI and replaced by a larger genomic XbaI fragment, containing an additional 142 bp of genomic sequence at the 3′-end. This fragment was obtained by digesting a genomic amplification product obtained with the primers Aenan3UTRXbaF and AenanR1 (5′-accagacatccgtttaagctgaaccac-3′). Plasmids pMos3xP3-EGFP1 and pMos3xP3-DsRED1 are modified versions of plasmids pMos3xP3-EGFP and pMos3xP3-DsRED, respectively, digested with BsaAI and self-ligated. This reduces the overall plasmid size to 5.5 kb as a result of the excision of extraneous Drosophila mauritiana genomic DNA.
Mosquito Rearing and Transformation.
A. aegypti were reared as described (8). Donor plasmid constructs for primary transformation experiments were injected following published procedures (8) along with the MosI helper plasmid pKhsp82M (40) into embryos of the white-eyed Higgs (H) strain of A. aegypti (41). Surviving adult G0 males were mated individually to 10–15 H females, whereas G0 females were batch-mated to H males. G1 progeny were screened as larvae for eye-specific fluorescence (EGFP or DsRED). Potential transformants were reared to adults and mated to H mosquitoes. Fluorescent progeny were analyzed by Southern blot hybridization with DNA isolated from 10 male or 6 female adult mosquitoes to confirm the presence of the transgene. Digestion of genomic DNA, electrophoresis, blotting, and hybridization, using 32P-labeled random primed probes were performed as described (28). Secondary transformation experiments were conducted with similar protocols except that primary transformed lines served as recipient strains, and no helper plasmids were included in the injection mixes. Secondary lines are designated by a number system as follows: the first number is the primary line from which it is derived. The second number is the line carrying both the primary and secondary insertions. The third number is a line carrying only the secondary insertion derived by segregation of the secondary insertion from the primary by crossing.
Tissue Dissection and RNA Isolation.
Ovaries were dissected in PBS from adult female mosquitoes 3 days after bloodmeal. Dissected ovaries, carcasses, and whole males were flash-frozen in liquid N2 and stored at −80°C until used. Carcasses refer here to the head and thorax from the same females from which ovaries were dissected. The abdomen was discarded to prevent sample contamination from oocytes ruptured or loosened during dissection. Embryos were collected from gravid females by addition of wet filter paper/cotton to the appropriate cage, and were removed from the filter paper by using a fine paintbrush and transferred to a 1.6-ml tube containing distilled H2O. Embryos were pelleted by low-speed centrifugation (3,000 × g) and all H2O removed before freezing in liquid N2. The average age of the embryos was ≈6 h after oviposition. Total RNA was extracted from frozen samples with TRIzol (Invitrogen) and used directly in Northern blot analysis. For RT-PCR, total RNA was treated for 20 min at 37°C with RQ1 DNase (Promega, Madison, WI). DNase-treated RNA samples were extracted with an equal volume of phenol:chloroform:isoamyl alcohol (25:24:1), followed by an equal volume of chloroform, isopropanol-precipitated, and resuspended in diethyl pyrocarbonate-treated distilled H2O.
Northern Blot Analyses.
Ten micrograms of total RNA per sample were denatured at 65°C in a formaldehyde-sucrose buffer before resolving in a 1.25% agarose/2% formaldehyde/1× Mops gel. Gels were electrophoresed at 70–80 V for 4 h and were rinsed 3 times in H2O before blotting overnight to a nylon membrane (ProBond). The RNA was UV-crosslinked to the membrane and hybridized to a 32P-labeled random-primed DNA probe, using standard conditions (28).
RT-PCR.
The Qiagen One-Step RT-PCR kit was used for all cDNA amplifications. Primer pairs used for the amplification of MosI-nos-3′-UTR transgene mRNA were as follows: MLF2 (5′-tcgtgccgaataaagagcaaacgcggacag-3′) and nosUTRR2 (5′-tgtacgttcgaacatacaatcatcactatgg-3′); and for A. aegypti nos mRNA, nosF0.5 (5′-taattgatgtgaacggaatggtgagg-3′) and nosUTRR2.
Embryo Fixation and Hybridization in Situ.
Embryos were prepared and stored before use as described (15, 36). Hybridization of nucleic acid probes was performed by using digoxygenin-labeled (DIG) sense and antisense MosI and nos RNA (15).
Analysis of Transposon–Chromosome Junctions.
Transposon–chromosome junction sites were recovered from transgenic lines by using inverse PCR (7). Genomic DNA from six transgenic females was digested to completion with Sau3AI or CviAII, and ligated under conditions of dilute DNA concentration with excess T4 DNA ligase. Gene amplification was performed with the following oligonucleotide primers complementary to the MosI DNA sequence: MLF1, 5′-TTGTTTACTCTCAGTGCAGTCAACATGTCG-3′; MLR1, 5′-TTCGACAGTCAAGGTTGACACTTCACAAGG-3′; MRF1, 5′-AAGACGATGAGTTCTACTGGCGTGGAATCC-3′; MRR1, 5′-CTTGCCGTATGTGATGGAGCGTTGTCATGG-3′. Amplification reactions were performed under the following conditions: 1 cycle of 95°C for 5 min, 25 cycles of 95°C for 45 s, 65°C for 45 s, 72°C for 1 min, and 1 cycle of 72°C for 5 min. Amplification products corresponding to putative MosI right- and left-hand repeat junctions with the chromosomal DNA were cloned into the TOPO4-PCR plasmid (Invitrogen), and the DNA sequences were determined by using the MLR1 and MLF1 primers. The A. aegypti (version 1.0; http://www.vectorbase.org/index.php) and GenBank databases were searched for sequences corresponding to the junction fragments and insertion sites.
Acknowledgments
We thank Dr. Osvaldo Marinotti for discussion and Lynn Olson for help in typing the manuscript. This work was supported by National Institutes of Health Grant AI44238.
Abbreviations
- TE
transposable element
- nos
nanos.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Calisher CH. Emerg Infect Dis. 2005;11:738–739. doi: 10.3201/eid1105.050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ. Comp Immunol Microbiol Infect Dis. 2004;27:319–330. doi: 10.1016/j.cimid.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Gubler DJ. Nat Med. 2004;10:129–130. doi: 10.1038/nm0204-129. [DOI] [PubMed] [Google Scholar]
- 4.Greenwood B, Mutabingwa T. Nature. 2002;415:670–672. doi: 10.1038/415670a. [DOI] [PubMed] [Google Scholar]
- 5.Curtis CF, Graves PM. J Trop Med Hyg. 1988;91:43–48. [PubMed] [Google Scholar]
- 6.Collins FH, James AA. Sci Med. 1996;3:52–61. [Google Scholar]
- 7.Coates CJ, Jasinskiene N, Miyashiro L, James AA. Proc Natl Acad Sci USA. 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Proc Natl Acad Sci USA. 1998;95:3743–3747. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brochta DA, Atkinson PW. Methods Mol Biol. 2004;260:227–254. doi: 10.1385/1-59259-755-6:227. [DOI] [PubMed] [Google Scholar]
- 10.Franz AWE, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Proc Natl Acad Sci USA. 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro JM, Kidwell MG. J Med Entomol. 1994;31:10–16. doi: 10.1093/jmedent/31.1.10. [DOI] [PubMed] [Google Scholar]
- 12.James AA. Trends Parasitol. 2005;21:64–67. doi: 10.1016/j.pt.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Mahowald AP. Int Rev Cytol. 2001;203:187–213. doi: 10.1016/s0074-7696(01)03007-8. [DOI] [PubMed] [Google Scholar]
- 14.Calvo E, Walter M, Adelman ZN, Jimenez A, Onal S, Marinotti O, James AA. Insect Biochem Mol Biol. 2005;35:789–798. doi: 10.1016/j.ibmb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Juhn J, James AA. Insect Mol Biol. 2006;15:363–372. doi: 10.1111/j.1365-2583.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 16.Gavis ER, Lunsford L, Bergsten SE, Lehmann R. Development (Cambridge, UK) 1996;122:2791–2800. doi: 10.1242/dev.122.9.2791. [DOI] [PubMed] [Google Scholar]
- 17.Gavis ER, Curtis D, Lehmann R. Dev Biol. 1996;176:36–50. doi: 10.1006/dbio.1996.9996. [DOI] [PubMed] [Google Scholar]
- 18.Curtis D, Apfeld J, Lehmann R. Development (Cambridge, UK) 1995;121:1899–1910. doi: 10.1242/dev.121.6.1899. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Lehmann R. Cell. 1991;66:637–647. doi: 10.1016/0092-8674(91)90110-k. [DOI] [PubMed] [Google Scholar]
- 20.Adelman ZN, Jasinskiene N, James AA. Mol Biochem Parasitol. 2002;121:1–10. doi: 10.1016/s0166-6851(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 21.Bartholomay LC, Cho WL, Rocheleau A, Boyle JP, Beck ET, Fuchs JF, Liss P, Rusch M, Butler KM, Wu RCC, et al. Infect Immun. 2004;72:4114–4126. doi: 10.1128/IAI.72.7.4114-4126.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniels SB, Peterson KR, Strausbaugh LD, Kidwell MG, Chovnick A. Genetics. 1990;124:339–355. doi: 10.1093/genetics/124.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meister GA, Grigliatti TA. Genome. 1993;36:1169–1175. doi: 10.1139/g93-155. [DOI] [PubMed] [Google Scholar]
- 24.Carareto CM, Kim W, Wojciechowski MF, O'Grady P, Prokchorova AV, Silva JC, Kidwell MG. Genetica. 1997;101:13–33. doi: 10.1023/a:1018339603370. [DOI] [PubMed] [Google Scholar]
- 25.Galindo MI, Ladeveze V, Lemeunier F, Kalmes R, Periquet G, Pascual L. Mol Biol Evol. 1995;12:723–734. doi: 10.1093/oxfordjournals.molbev.a040251. [DOI] [PubMed] [Google Scholar]
- 26.Atkinson PW, James AA. Adv Genet. 2002;47:49–86. doi: 10.1016/s0065-2660(02)47002-2. [DOI] [PubMed] [Google Scholar]
- 27.Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. Insect Biochem Mol Biol. 2001;31:1137–1143. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- 28.Adelman ZN, Jasinskiene N, Vally KJ, Peek C, Travanty EA, Olson KE, Brown SE, Stephens JL, Knudson DL, Coates CJ, et al. Transgenic Res. 2004;13:411–425. doi: 10.1007/s11248-004-6067-2. [DOI] [PubMed] [Google Scholar]
- 29.Jasinskiene N, Coates CJ, James AA. Insect Mol Biol. 2000;9:11–18. doi: 10.1046/j.1365-2583.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 30.Wilson R, Orsetti J, Klocko AD, Aluvihare C, Peckham E, Atkinson PW, Lehane MJ, O'Brochta DA. Insect Biochem Mol Biol. 2003;33:853–863. doi: 10.1016/s0965-1748(03)00044-4. [DOI] [PubMed] [Google Scholar]
- 31.O'Brochta DA, Sethuraman N, Wilson R, Hice RH, Pinkerton AC, Levesque CS, Bideshi DK, Jasinskiene J, Coates CJ, James AA, et al. Exp Biol. 2003;206:3823–3834. doi: 10.1242/jeb.00638. [DOI] [PubMed] [Google Scholar]
- 32.Tu Z, Coates C. Insect Biochem Mol Biol. 2004;34:631–644. doi: 10.1016/j.ibmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M. Science. 2007 doi: 10.1126/science.1138878. [DOI] [Google Scholar]
- 34.Burt A. Proc R Soc Lond B Biol Sci. 2003;270:921–928. doi: 10.1098/rspb.2002.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinkins SP, Godfray HC. Proc R Soc Lond B Biol Sci. 2004;271:1421–1426. doi: 10.1098/rspb.2004.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- 37.Goltsev Y, Hsiong W, Lanzaro G, Levine M. Dev Biol. 2004;275:435–446. doi: 10.1016/j.ydbio.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Bischof J, Maeda RK, Hediger M, Karch F, Basler K. Proc Natl Acad Sci USA. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horn C, Wimmer EA. Dev Genes Evol. 2000;210:630–637. doi: 10.1007/s004270000110. [DOI] [PubMed] [Google Scholar]
- 40.Coates CJ, Turney CL, Frommer M, O'Brochta DA, Atkinson PW. Mol Gen Genet. 1997;253:728–733. doi: 10.1007/s004380050377. [DOI] [PubMed] [Google Scholar]
- 41.Wendell MD, Wilson TG, Higgs S, Black WC. Insect Mol Biol. 2000;9:119–125. doi: 10.1046/j.1365-2583.2000.00166.x. [DOI] [PubMed] [Google Scholar]
- 42.James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. Mol Biochem Parasitol. 1991;44:245–254. doi: 10.1016/0166-6851(91)90010-4. [DOI] [PubMed] [Google Scholar]