Abstract
Galanin-like peptide (GALP) is a hypothalamic neuropeptide belonging to the galanin family of peptides. The GALP gene is characterized by extensive differential splicing in a variety of murine tissues. One splice variant excludes exon 3 and results in a frame shift leading to a novel peptide sequence and a stop codon after 49 aa. In this peptide, which we termed alarin, the signal sequence of the GALP precursor peptide and the first 5 aa of the mature GALP are followed by 20 aa without homology to any other murine protein. Alarin mRNA was detected in murine brain, thymus, and skin. In accordance with its vascular localization, the peptide exhibited potent and dose-dependent vasoconstrictor and anti-edema activity in the cutaneous microvasculature, as was also observed with other members of the galanin peptide family. However, in contrast to galanin peptides in general, the physiological effects of alarin do not appear to be mediated via the known galanin receptors. Alarin adds another facet to the surprisingly high-functional redundancy of the galanin family of peptides.
Keywords: galanin-like peptide, regulatory peptide, splicing, plasma extravasation, cutaneous microvasculature
Neuropeptides coordinate, integrate, and regulate physiological processes in all animals. Multiple neuropeptides may regulate a specific physiological function, which might represent an evolutionary failsafe series of redundant controls. The existence of neuropeptide families indicates that they were generated through successive gene duplications from a common ancestral peptide (1). An excellent example of neuropeptide evolution can be seen in the galanin family of peptides. Galanin, initially isolated from porcine intestine in 1983, is a 29-aa peptide (30 residues in humans) (2). Galanin-like peptide (GALP), the second member of the galanin family, is 60 aa and was originally discovered as an endogenous ligand for galanin receptors in the porcine hypothalamus (3). Residues 9–21 of GALP are identical to the first 13 aa of galanin, and they function to activate galanin receptors. Both peptides are encoded by single-copy genes organized into six small exons spanning ≈6 kb of genomic DNA (3). The peptides have potent species-specific and time-dependent effects in the CNS and can affect such diverse processes as feeding and reproduction. For example, CNS infusions of GALP stimulate the secretion of both gonadotropin-releasing hormone and luteinizing hormone in female rats and induce sexual behavior in males (4). Thus, GALP establishes a link between metabolism and reproduction (5).
The effects of galanin and GALP are mediated by galanin receptors. These receptors belong to the G protein-coupled receptor superfamily. Galanin has high affinity for all three galanin receptor subtypes, whereas GALP (1–60) displays high affinity only for galanin receptors (GalR) 2 and 3 (3, 6). GalR1 is mainly expressed in the CNS (7). GalR2 is abundant and widely expressed in both the CNS and peripheral tissues, and GalR3 is expressed in significant quantities in the peripheral tissues and is discrete and highly restricted in the CNS (8–11). Differences of in vivo responses upon galanin and GALP treatment indicate that effects of GALP may also be mediated via a novel, as yet unknown receptor (12, 13).
The analysis of alternative splicing has attracted interest, because the number of expressed proteins is substantially larger than the number of genes encoded in the human genome (14, 15). Alternative posttranscriptional splicing is an important mechanism for increasing the potential number of gene products. The resulting proteomic diversity is particularly important in the nervous system, where the peptide/protein/receptor isoforms increase biological diversity (16). The splice variants may be differentially regulated in a tissue-specific and developmental stage-specific manner (17). However, in the regulatory peptide system, tissue-specific splicing is a rather uncommon mechanism. The difficulty to detect alternative gene transcripts from the GALP gene is supported by the fact that GALP ESTs have been reported in only murine but not in human libraries (www.ncbi.nlm.nih.gov/BLAST). Recently, the expression of a splice variant of the GALP gene, excluding exon 3, was observed in gangliocytes of human neuroblastic tumors (18). However, no biological function for that novel peptide has been demonstrated. To our knowledge, no data are available about differential splicing of the murine GALP gene.
The presence of galanin and galanin binding sites in skin, especially around dermal blood vessels, indicates that the galanin peptide family regulates the plasticity of the cutaneous microvasculature (19). Precisely how vasodilation in human skin is regulated remains enigmatic. In the case of response to thermal challenge, a connection between cholinergic sudomotor activity and nervous regulation of vasodilation via nonadrenergic innervation has been suggested (20). In neurogenic inflammation the neuropeptide substance P (SP) has been shown to induce vasodilation and plasma extravasation, whereas the calcitonin gene-related peptide (CGRP) leads to vasodilation only (21). Accordingly, SP- and CGRP-induced edema formation can be inhibited by galanin (22) and GALP (23), thus providing evidence that members of the galanin family may be potential antiinflammatory peptides in a range of skin diseases such as psoriasis, atopic dermatitis, urticaria, and pruritus (24–27).
Here we describe alternative transcripts of GALP mRNA in murine tissues. One of these transcripts encodes a novel regulatory peptide, alarin, with vasoactive biological activity in murine skin.
Results
Differential Splicing of the GALP Gene.
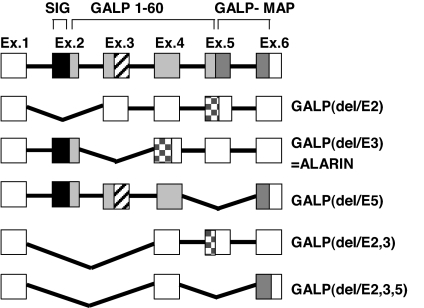
RT-PCR analysis using sets of primers spanning exons 1–6 revealed several GALP cDNAs of different lengths. Sequencing of these putative murine splice variants showed exclusion of exons 2, 3, or 5 singly or a combination of exons 2 and 3 or exons 2, 3, and 5 (Fig. 1). Only the translated GALP(del/E5) peptide would still harbor the galanin receptor binding domain, although it would lack 12 aa of the C-terminal part of GALP and the first 14 aa of the GALP message-associated peptide.
Fig. 1.
Murine prepro-GALP gene splice variants. Filled boxes indicate translated peptides. Open boxes indicate 3′ and 5′ untranslated mRNA regions. Black boxes represent the signal peptide (SIG). Light gray squares indicate the mature GALP peptide with homology to galanin (cross-striped boxes). Dark gray boxes represent the GALP message-associated peptide (GALP-MAP), and checkered boxes represent the putative novel peptide sequences. Exon sizes are not drawn to scale. GALP(delE3) was termed alarin.
The splice variants that exclude exon 2 [GALP(del/E2), GALP(del/E2,3), and GALP(del/E2,3,5)] would lead to putative peptides of <20 aa. These peptides would not contain a signal peptide; therefore, it is unlikely that they would be processed and secreted.
Exclusion of exon 3 [GALP(del/E3)] is predicted to result in a precursor protein still harboring the signal sequence of prepro-GALP and the first 5 aa of the mature GALP peptide, followed by 20 aa that do not show homology to any other protein found in murine sequence databases. Because the N-terminal part of the precursor protein is encoded by exon 2, the proteolytic cleavage site of GALP is maintained. Therefore, proteolytic processing should result in a 25-aa-long neuropeptide (GenBank accession no. DQ155644) (Fig. 2). We termed the peptide alarin because of its N-terminal alanine and the C-terminal serine. In contrast to GALP, alarin has no homology to galanin. Searching EST databases (www.ncbi.nlm.nih.gov/BLAST) using the alarin sequence did not reveal any corresponding EST clones or significant homology to other peptides. Interspecies comparison of the alarin splice variant would also lead to a similar frame shift in rats, macaques, and humans (Fig. 2) (18). Sequence identity between murine and rat alarin is 92%, between human and macaque is 96%, and between primate and rodent is ≈60%. The C-terminal serine of the peptide will be most likely amidated in vivo.
Fig. 2.
Amino acid sequence comparison of murine, rat, macaque, and human alarin. The arrow indicates a putative N-terminal proteolytic cleavage site of dipeptidyl dipeptidase IV. The solid underline indicates the GALP/alarin shared residues.
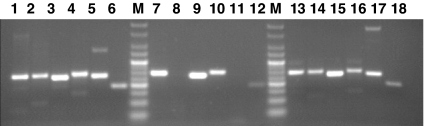
The occurrence of these splice forms in skin, thymus, and brain was further analyzed by RT-PCR by using optimized splice variant-specific primer combinations, which are listed in supporting information (SI) Table 1. In brain and thymus all splice variants were observed. In the skin, predominantly GALP, alarin, and GALP(del/E5) mRNAs were present (Fig. 3). The relative amounts of the different splice variants and the wild-type GALP cannot be directly compared in these assays. The first 2 aa of the mature GALP and alarin peptides could be potentially removed by dipeptidyl dipeptidase IV (28). We therefore examined the effect of alarin (1–25) as well as alarin (3–25) in in vivo assays.
Fig. 3.
Expression of GALP mRNAs in different murine tissues. RT-PCR was carried out by using total RNA extracted from different tissues of normal mice. Specific primer sets, as shown in SI Table 1, were used to amplify specific splice variants. PCR products were visualized by ethidium bromide staining after agarose gel electrophoresis. Lanes 1–6, murine brain; lanes 7–12, murine skin; lanes 13–18, murine thymus. Specific PCR amplifications for full-length GALP (lanes 1, 7, and 13), GALP(del/E2) (lanes 2, 8, and 14), GALP(del/E3) = alarin (lanes 3, 9, and 15), GALP(del/E5) (lanes 4, 10, and 16), GALP(del/E2,3) (lanes 5, 11, and 17), and GALP(del/E2,3,5) (lanes 6, 12, and 18) are shown. Lane M, Alpha Quant 5 DNA Ladder (Alpha Innotech).
Vascular Expression of Alarin.
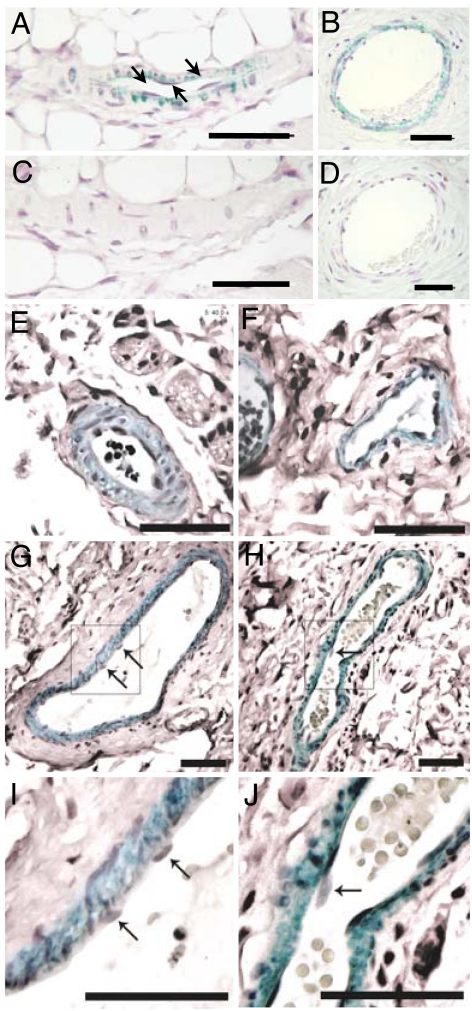
We further analyzed the expression of the alarin peptide in the dermal vascular system by immunohistochemistry. For the generation of the antisera, alarin peptides from amino acids 6–24 were used to avoid cross-reactivity to the first 5 aa of the peptide that are identical with the first 5 aa of GALP. Murine and human skin specimens were stained by using affinity-purified anti-alarin antisera. Because the homology between the human and the murine peptide is only 60%, two antisera, one directed against the murine alarin peptide (6–24) and one directed against the human homologue, were used. Alarin-like immunoreactivity (alarin-LI) was detected in pericytes covering microvascular arterioles and venules on their abluminal surfaces in the murine as well as in the human dermis, whereas in larger vessels alarin-LI was detected in layers of smooth muscle cells (Fig. 4). No alarin-LI was present in endothelial cells of blood vessels in any of these tissues (Fig. 4). This finding further supports the hypothesis that endogenous expressed alarin peptide has a potent vascular activity in vivo.
Fig. 4.
Alarin-LI in the dermal microvasculature. Alarin-LI in paraffin sections of murine (A and C) and human (B, D–G, and I) dermal blood vessels. Immunohistochemistry was performed with affinity-purified anti-murine alarin (6–24) antiserum (A and C) and anti-human alarin (6–24) antiserum (B, D–G, and I). Immunohistochemistry was performed with antisera preabsorbed with 3 μM synthetic murine (C) and human (D) alarin 1–25 peptide. Alarin-LI in precapillary arterioles (E) and postcapillary venules of different size (F, G, and I) are shown. Comparison of alarin-LI (G and I) with actin immunoreactivity (H and J) indicates staining of alarin-LI in perivascular cell types (pericytes and smooth muscle cells) but not endothelial cells of human microvessels. I and J represent higher magnification of the boxed area in G and H, respectively. Arrows point toward vascular endothelial cells. Green staining indicates alarin-LI and actin immunoreactivity. (Scale bars: 50 μm.)
Vasoactive Properties of Alarin.
Because the alarin mRNA was detected in the skin, a possible vascular function for alarin (1–25) was investigated (Figs. 5 and 6). The effect of alarin (1–25) on inflammatory edema formation in murine dorsal skin was examined after coinjection of alarin with the edema-inducing combination of SP, a potent mediator of increased microvascular permeability, and the vasodilator CGRP (29). The effects of the full-length mature GALP (1–60), galanin, as well as the peptide fragment GALP (19–37), which lacks the galanin receptor-binding domain, were tested for their ability to modulate edema formation (Fig. 5A). Whereas alarin (1–25), GALP (1–60), and galanin demonstrated a similar profound inhibitory effect on edema formation, GALP (19–37) was inactive (Fig. 5A). Alarin (1–25) inhibited inflammatory edema formation in a dose-dependent manner, at picomolar doses, when coinjected locally into the cutaneous microvasculature (Fig. 5B). Furthermore, alarin (3–25) was also able to inhibit inflammatory edema formation, albeit in a less potent manner (Fig. 5C).
Fig. 5.
Effects on inflammatory edema of alarin. (A) Edema formation was induced by intradermal SP (300 pmol) and CGRP (10 pmol), and the effect of coinjected alarin and different galanin peptides is shown (n = 6). The response of increasing doses of alarin (1–25) (B) and alarin (3–25) (C) coinjected with SP plus CGRP is shown (n = 8). Results are expressed as plasma extravasation (μl/g), mean ± SEM, measured by the 125I-BSA method. Responses that are significantly different from the corresponding SP plus CGRP-treated sites are indicated (∗, P < 0.05; ∗∗, P < 0.01).
Fig. 6.
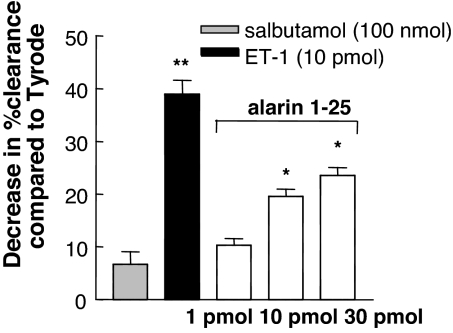
The dose-related effect of alarin on blood flow in cutaneous dorsal microvasculature. The responses of salbutamol and endothelin-1 (ET-1) as controls are shown alongside responses for alarin 1–25. The dose–response effect is shown as a decrease in percentage clearance (mean ± SEM, n = 8) compared with vehicle (Tyrode-injected) skin. Results that are significantly different from Tyrode-injected sites are indicated (∗, P < 0.05; ∗∗, P < 0.01).
There are two major mechanisms whereby regulatory peptides may be acting to modulate inflammatory edema formation. First, by a reduction of skin blood flow (e.g., the vasoconstrictor endothelin), and, second, by inhibition of microvascular permeability (e.g., the antipermeability β2-adrenoreceptor agonist salbutamol). Alarin was able to decrease cutaneous blood flow as determined by a 99m technetium (99mTc) clearance technique (Fig. 6). In this experimental setting, the expected lack of effect of the permeability-decreasing substance salbutamol (30) and the potent vasoconstrictor response to endothelin-1 (31) are also shown.
Receptors.
Both galanin and GALP exert their cellular effects via activation of galanin receptors (32). The lack of the galanin receptor-binding domain in the alarin peptide suggests that alarin is not able to exert its biological function via galanin receptor activation. In keeping with this hypothesis, the synthetic human alarin (1–25) was not able to bind to membrane preparations of either human GalR1- or GalR2-expressing neuroblastoma cells. The displacement of [125I]galanin binding is listed in SI Table 2. This finding was further supported by the observation that alarin was not able to alter the extracellular acidification of GalR2 produced by GalR2-expressing neuroblastoma cells (data not shown). Therefore, the biological function of alarin might be mediated by specific receptors for this peptide.
Discussion
Here we report for the first time extensive splicing of the GALP gene. One of the splice variants leads to a novel peptide, alarin, with biological activity in the vascular system.
In evolution the neuropeptide system has used several different avenues to gain diversity of peptides and functions. First, neuropeptides are frequently formed from large precursor preprohormones. Exon duplication within a gene has led to peptides with similar amino acid sequence in precursor proteins. Second, depending on the processing machinery of a cell, these precursor proteins are processed into different peptides. An excellent example is proopiomelaoncortin, which contains three similar peptides, α-, β-, and γ-melanocyte-stimulating hormone, adrenocorticotropic hormone, and β-endorphin. Tissue-specific proteolytic cleavage of the proopiomelaoncortin protein leads to differential production of active peptides (33). Apart from exon duplication, gene duplication of neuropeptide genes has generated additional functional diversity. The NPY gene family is an example of multiple gene duplication events giving rise to a range of structurally related, but functionally distinct, gene products including NPY, pancreatic peptide Y, peptide YY, and seminal plasmin (34).
Alternative splicing seems to play a less prominent role in creation of different neuropeptides. Although it is estimated that in >70% of mammalian multiexon genes differential splicing occurs, this mechanism has been demonstrated for only a minority of neuropeptides, including CGRP, tachykinins, and gonadotropin-releasing hormone. The CGRP primary RNA transcript undergoes tissue-specific alternative processing, resulting in the differential production of calcitonin mRNA in thyroid cells and CGRP mRNA in neurons of the CNS and the peripheral nervous system (35). The gene of the vasoactive intestinal peptide (VIP) is alternatively spliced into a large form encoding both VIP and peptide histidine isoleucine (PHI) 1–27 in the same protein product. The shorter form of chicken and turkey VIP mRNA encodes a protein that does not contain PHI (36, 37). The prepro-tachykinin gene encodes several tachykinin peptides (SP, neurokinin A, neuropeptide P, and neuropeptide K) with distinct pharmacological properties (38). The lack of information on differential splicing of neuropeptides might be due to the predominant interest in the exact topographical and cellular localization of neuropeptides in neurobiology, mostly determined by immunological methods or in situ hybridization, rather than by estimations of the tissue mRNA expression by RT-PCR.
In contrast, a wide variety of alternative splice variants has been reported for neuropeptide receptors, which again results in further diversity and redundancy of the neuropeptide system. The actions of VIP and PACAP are mediated through three G protein-coupled receptors. One of them is the PACAP-preferring PAC1 receptor (39). Further diversity in the number of VIP/PACAP receptors occurs through alternative splicing of the PAC1 receptor gene, giving rise to a large variety of PAC1 receptor isoforms with different expression and signaling properties (40). Other examples for neuropeptide receptor genes with alternative splicing include the cholecystokinin 2 receptor, calcitonin receptor, angiotensin type 1a receptor, and the neuropeptide Y Y5 receptor (41, 42).
The similar genomic organization of the galanin and GALP genes as well as the homology between their encoded peptides indicates that they might have been created by gene duplication. To date, differential splicing of the galanin system has been described only for avian and goldfish galanin (43, 44). The physiological significance of these multiple forms of galanin mRNA in these organisms is unknown. Analysis of a variety of human and murine tissues did not reveal exon-skipping in the galanin gene (unpublished data), indicating that galanin, as the original peptide, retains its function whereas the duplicated gene GALP is free to gain additional functions.
Galanin and GALP originate from precursor peptides that are processed to mature peptides by removal of the signal peptide and endoproteolytic cleavage directed by basic amino acids flanking the mature peptides (3). Because the N-terminal part of the GALP and alarin precursor molecules are identical, harboring the same signal sequence and proteolytic cleavage sites, it is most likely that the same processing and secretion machinery can be used for both peptides.
The presence of alarin mRNA in skin indicates that this peptide might exert physiological functions similar to its ancestral family members galanin and GALP. Accordingly, we were able to show that alarin, in analogy to galanin and GALP, is able to decrease skin blood flow and inflammatory edema with a similar potency. However, alarin (1–25) displayed a more potent vascular activity than the shorter variant alarin (3–25). This is in contrast to the full-length GALP peptide, which showed increased biological activity after removal of the first 2 aa (23). The results demonstrate that alarin is a potent vasoconstrictor and most likely acts to reduce plasma permeability and, in turn, edema formation. This activity is similar to that observed in skin for neuropeptide Y (45). The perivascular location of these vasoactive peptides is indicative of their potential to influence regulatory mechanisms, as well as their participation in pathological conditions attached to vascular pathophysiology such as psoriasis, atopic dermatitis, urticaria, and pruritus. Thus, this activity of alarin provides evidence of its potential to influence blood vessel reactivity at the microvascular level in skin. The immunohistochemical localization of alarin-LI around blood vessels indicates that the alarin mRNA is endogenously translated into a peptide at the appropriate anatomical site. Unlike other neuropeptides the anatomical site of function of alarin is not species-specific. Alarin-LI has been detected in murine as well as human dermal perivascular cells (pericytes and smooth muscle cells) around blood vessels, indicating that alarin might have a similar vascular activity in both species.
Neuropeptides, including galanin, show a wide distribution in the CNS as well as the peripheral nervous system. Consistent with the wide distribution galanin has been shown to have broad range of neuroendocrine and physiological actions. Furthermore, neuropeptides, again including galanin, are known to be expressed in tumors of the CNS and especially neuroendocrine tumors in the periphery (46). Given that alarin might be another member of the increasing family of neuropeptides it is not surprising that its expression has been reported recently in ganglionic cells of human neuroblastic tumors (18), and now we show a vascular function. Future studies might reveal alternative biological effects of this novel peptide dependent on its expression in different tissues.
The first 10 aa of alarin are conserved between rodents and primates. Therefore, the N-terminal part of the peptide might be crucial for receptor binding. Both the absent galanin receptor-binding domain and our experimental data indicate that alarin is unlikely to exert its function via galanin receptors. Given that alarin shares only five identical amino acids with GALP it is also unlikely that alarin can activate GALP-specific receptors. Because a large number of G protein-coupled receptors are expressed in the vascular system and most neuropeptide receptors are members of the G protein-coupled receptor superfamily, many of them lacking data on a ligand (47), we hypothesize that alarin receptors also belong to this type of receptor family. However, we cannot exclude the presence of an unknown galanin/GALP receptor, which is also activated by alarin.
Compensatory parameter variations and network redundancy are found to be important mechanisms for the robustness of biochemical networks (48). The overall redundancy of neuropeptide function guarantees failsafe systems and built-in compensatory mechanisms.
For the galanin family of peptides a multicomponent failsafe system can be envisaged in which some components of the galanin system are redundant, a common phenomenon in the evolution of the physiological adaptations of skin defense mechanisms. For the necessary homeostasis of the external barrier of the human body it is crucial to have in place a redundant system. For example, thermal challenges lead to the activation of multiple cascades of proinflammatory cytokines. The diversity of antiinflammatory galanin family peptides produced by alternative splicing provides a mechanism for fine-tuning this antiinflammatory activity. In the vascular system alarin might induce a new level of safety because it most likely does not function via the known galanin receptors and second messenger pathways. This establishes alarin as a peptide acting via a new regulatory circuit.
Materials and Methods
Animals.
Experiments involving mice were carried out under the Animals (Scientific Procedures) Act, 1986. Normal female CD-1 mice (22–27 g, 8–12 weeks old) were obtained from Charles River (Kent, U.K.). All mice were maintained on a normal diet, with free access to food and water, in a climatically controlled environment. Animals were anesthetized with urethane (25% w/vol−1; 2.5 g·kg−1 i.p.), and the dorsal skin was shaved. Injection sites were chosen according to a randomized site pattern on the dorsal skin of the anesthetized mouse. Agents (SP, CGRP, endothelin-1, and salbutamol) were from Sigma (Poole, U.K.), as were all other agents unless specified. Galanin (rat) and GALP (1–60) (human) were purchased from Bachem (Bubendorf, Switzerland), and GALP (19–37), alarin (1–25)-amide, and alarin (3–25)-amide were custom-synthesized by NeoMPS (Strasbourg, France). All peptides were dissolved in distilled water. The stock solutions (10 nM) were stored at −20°C and further diluted in Tyrode's solution (137 mM NaCl/2.7 mM KCl/0.5 mM MgCl2/0.4 mM NaH2PO4/11.9 mM NaHCO3/5.6 mM glucose) just before use.
RT-PCR.
Tissues of male C57/BL6 mice (maximum 100 mg) were homogenized directly in Tri Reagent (Molecular Research Center, Cincinnati, OH) using an Ultra-Turrax T25 (IKA Werke, Staufen, Germany). Total RNA was isolated according to the instructions of the manufacturer. Two micrograms of RNA was reverse-transcribed with 140 units of SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD). Fifty nanograms of cDNA was used for PCR amplification with 0.5 units of Thermo Start polymerase (ABgene, Surrey, U.K.), 200 μM dNTPs, 3 mM MgCl2, and 200 nM primers (see SI Table 1) in a 20-μl reaction. GALP splice variant RT-PCRs were performed with a denaturation step at 95°C, followed by 60 cycles each consisting of 15 s at 95°C, a primer annealing step at 64°C for 15 s,, and 72°C for 15 s. The PCR products were analyzed by electrophoresis on a 3% agarose gel. In addition, PCR products were sequenced to confirm their identity.
Generation of Polyclonal Alarin Antibodies.
Rabbit polyclonal antisera were custom-made by using the synthetic human alarin peptide 6–24-Cys (SSTFPKWVTKTERGRQPLRC) and the synthetic murine alarin peptide 6–24-Cys (SSPFPPRPTRAGRETQLLRC) (NeoMPS). Synthetic human and murine alarin 6–24 peptides were coupled via a C-terminal cysteine residue to the carrier protein keyhole limpet hemocyanin. Immunization was carried out on days 0, 14, 28, and 56. The affinity purification of the antisera was carried out as described previously (47). Because of the low homology of 60% between human and murine alarin, no cross-reactivity of the purified antisera was observed to the peptide of the other species. In addition, no cross-reactivity with human galanin or human GALP was detectable.
Immunohistochemistry.
Murine skin was derived from the bellies of normal female CD-1 mice (n = 4). Human foreskin samples were taken from healthy volunteers after informed consent was given (n = 4). The tissues were embedded in paraffin. Paraffin sections (4 μm) were deparaffinized, rehydrated, heated to 90°C for 15 min in 0.01 M citric acid (pH 6.0), and washed with 1× PBS. Immunostaining was performed according to the protocol of Level 2 USA Ultra Streptavidin Detection System (Signet Laboratories, Dedham, MA) with modifications. The endogenous peroxidase was quenched in 3% H2O2 for 5–10 min. Sections were blocked with 3% normal goat serum in PBS for 30 min at room temperature followed by an overnight incubation at 4°C with the affinity-purified anti-alarin antibodies diluted 1:100 in PBS or anti-human actin monoclonal antibody (MCA1905; Serotec, Oxford, U.K.) diluted 1:1,000 in PBS. After three washes with PBS and incubation with linking reagent for 1 h, sections were treated with labeling reagent for 20 min and washed with PBS. Alarin-LI and actin immunoreactivity were visualized with HistoGreen according to the manufacturer's protocol (Linaris, Wertheim-Bettingen, Germany). Sections were counterstained with Mayer's Hemalum solution (Merck, Darmstadt, Germany) and mounted with Assistent-Histokitt (Assistent, Sondheim, Germany). The specificity of the immunostaining was tested by preabsorption of the affinity-purified anti-alarin antisera with 3 μM of the respective peptide for 2–3 h at 37°C. After centrifugation for 10 min at 10,000 × g serial sections were incubated with the preabsorbed sera.
Measurement of Inflammatory Edema Formation.
Plasma extravasation was used as an index of inflammatory edema formation and measured as previously described (49). Briefly, test agents [SP, CGRP, galanin, GALP (1–60), GALP (19–37), alarin (1–25), and alarin (3–25)] were diluted in Tyrode's solution and stored on ice. 125I-BSA (45 kBq in 100 μl of saline) was administered i.v. into the tail vein, and 5 min later test agents (50 μl per site) were injected intradermally. Plasma extravasation was allowed for 30 min, and then a blood sample (0.5 ml), in a heparin-coated syringe, was obtained via cardiac puncture and centrifuged at 10,000 × g for 4 min to obtain plasma. The mice were then killed, the dorsal skin was removed, and the injected sites were punched out (8 mm). The amount of plasma extravasated (μl·g−1 tissue) was calculated by comparing the amount of radioactivity in each skin site with that in 100 μl of plasma from the same animal.
Measurement of Skin Blood Flow.
Blood flow changes were measured by using a 99mTc clearance technique (45). Briefly, test agents [endothelin-1, salbutamol, and alarin (1–25)] were made up in Tyrode's solution, and an equal amount of 99mTc (≈200 kBq) was added to all samples and kept on ice until use. Test agents (50 μl per site) were injected intradermally with an identical amount placed into a vial for measurement of the total radioactivity. The mouse was killed after a clearance period (15 min), the skin was removed, and the injected sites were punched out for measurement of the remaining radioactivity. Initially, the amount of 99mTc cleared from each injection site was calculated by comparing counts in skin with counts in the respective paired aliquot of total radioactivity. From this, the clearance at test agent-injected sites was then calculated by comparing with the Tyrode value (which was normalized to 100 for each experiment) and expressed as percentage change in clearance compared with Tyrode, with positive numbers indicating a decreased blood flow.
Cell Lines.
Stable transfectants of SH-SY5Y neuroblastoma cells with the human galanin receptors (SH-SY5Y/GalR1 and SH-SY5Y/GalR2) were generated and cultivated as recently described (6). Because the galanin receptor expression is under control of a tetracycline-regulated expression system (T-REx System; Invitrogen), receptor expression was induced overnight with 1 μg/ml tetracycline.
Receptor Binding Assay.
Membrane preparations and radioligand binding assays were performed according to Berger et al. (50). Displacement of radiolabeled galanin binding to membrane preparations (15 μg) was carried out in duplicates in a total volume of 120 μl of binding buffer containing 50 pM [125I]galanin [2,000 Ci/mmol; Amersham Pharmacia Biotech (Little Chalfont, U.K.)] and 1 μM synthetic human peptides as indicated. Unspecific binding was defined as binding of [125I]galanin to membranes of mock-transfected human neuroblastoma cells SH-SY5Y. Full-length human galanin was purchased from Phoenix Pharmaceuticals (Belmont, CA), and the human GALP (1–32)-amide, GALP (3–32)-amide, and alarin (1–25)-amide were custom-synthesized by Neosystems (Strasbourg, France).
Statistical Analysis.
Results for functional studies are shown mainly as mean ± SEM. Statistical analyses were performed on original data by one-way ANOVA followed by Dunnett's multiple-comparison test. P < 0.05 was considered significant. n represents the number of animals.
Supplementary Material
Acknowledgments
We thank Anton Hermann (Paris–London University of Salzburg, Salzburg, Austria) for providing the microphysiometer and Michael Emberger (University Hospital Salzburg) for evaluation of immunohistochemical stainings. This study was supported by Austrian Science Foundation Grant P14906, the Salzburg Auslandsstipendium für Kurzfristige Wissenschaftliche Arbeiten im Ausland, the British Heart Foundation, and the Biotechnology and Biological Sciences Research Council.
Abbreviations
- alarin-LI
alarin-like immunoreactivity
- CGRP
calcitonin gene-related peptide
- GALP
galanin-like peptide
- GalRn
galanin receptor n
- SP
substance P
- VIP
vasoactive intestinal peptide
- 99mTc
99m technetium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. DQ155644).
This article contains supporting information online at www.pnas.org/cgi/content/full/0608585104/DC1.
References
- 1.Hoyle CH. Brain Res. 1999;848:1–25. doi: 10.1016/s0006-8993(99)01975-7. [DOI] [PubMed] [Google Scholar]
- 2.Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V. FEBS Lett. 1983;164:124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 3.Ohtaki T, Kumano S, Ishibashi Y, Ogi K, Matsui H, Harada M, Kitada C, Kurokawa T, Onda H, Fujino M. J Biol Chem. 1999;274:37041–37045. doi: 10.1074/jbc.274.52.37041. [DOI] [PubMed] [Google Scholar]
- 4.Krasnow SM, Fraley GS, Schuh SM, Baumgartner JW, Clifton DK, Steiner RA. Endocrinology. 2003;144:813–822. doi: 10.1210/en.2002-220982. [DOI] [PubMed] [Google Scholar]
- 5.Gottsch ML, Clifton DK, Steiner RA. Trends Endocrinol Metab. 2004;15:215–221. doi: 10.1016/j.tem.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Berger A, Lang R, Moritz K, Santic R, Hermann A, Sperl W, Kofler B. Endocrinology. 2004;145:500–507. doi: 10.1210/en.2003-0649. [DOI] [PubMed] [Google Scholar]
- 7.Burgevin MC, Loquet I, Quarteronet D, Habert-Ortoli E. J Mol Neurosci. 1995;6:33–41. doi: 10.1007/BF02736757. [DOI] [PubMed] [Google Scholar]
- 8.Fathi Z, Battaglino PM, Iben LG, Li H, Baker E, Zhang D, McGovern R, Mahle CD, Sutherland GR, Iismaa TP, et al. Brain Res Mol Brain Res. 1998;58:156–169. doi: 10.1016/s0169-328x(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 9.Kolakowski LF, Jr, O'Neill GP, Howard AD, Broussard SR, Sullivan KA, Feighner SD, Sawzdargo M, Nguyen T, Kargman S, Shiao LL, et al. J Neurochem. 1998;71:2239–2251. doi: 10.1046/j.1471-4159.1998.71062239.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Donnell D, Ahmad S, Wahlestedt C, Walker P. J Comp Neurol. 1999;409:469–481. [PubMed] [Google Scholar]
- 11.Mennicken F, Hoffert C, Pelletier M, Ahmad S, O'Donnell D. J Chem Neuroanat. 2002;24:257–268. doi: 10.1016/s0891-0618(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y, Tyszkiewicz JP, Fong TM. J Neurophysiol. 2006;95:3228–3234. doi: 10.1152/jn.01117.2005. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham MJ. J Neuroendocrinol. 2004;16:717–723. doi: 10.1111/j.1365-2826.2004.01221.x. [DOI] [PubMed] [Google Scholar]
- 14.Mironov AA, Fickett JW, Gelfand MS. Genome Res. 1999;9:1288–1293. doi: 10.1101/gr.9.12.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neverov AD, Artamonova II, Nurtdinov RN, Frishman D, Gelfand MS, Mironov AA. BMC Bioinformatics. 2005;6:266. doi: 10.1186/1471-2105-6-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabowski PJ, Black DL. Prog Neurobiol. 2001;65:289–308. doi: 10.1016/s0301-0082(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 17.Enigk RE, Maimone MM. Gene. 1999;238:479–488. doi: 10.1016/s0378-1119(99)00358-3. [DOI] [PubMed] [Google Scholar]
- 18.Santic R, Fenninger K, Graf K, Schneider R, Hauser-Kronberger C, Schilling FH, Kogner P, Ratschek M, Jones N, Sperl W, Kofler J Mol Neurosci. 2006;29:145–152. doi: 10.1385/JMN:29:2:145. [DOI] [PubMed] [Google Scholar]
- 19.Kofler B, Berger A, Santic R, Moritz K, Almer D, Tuechler C, Lang R, Emberger M, Klausegger A, Sperl W, et al. J Invest Dermatol. 2004;122:1050–1053. doi: 10.1111/j.0022-202X.2004.22418.x. [DOI] [PubMed] [Google Scholar]
- 20.Sugenoya J, Ogawa T, Jmai K, Ohnishi N, Natsume K. Eur J Appl Physiol Occup Physiol. 1995;71:33–40. doi: 10.1007/BF00511230. [DOI] [PubMed] [Google Scholar]
- 21.Scholzen T, Armstrong CA, Bunnett NW, Luger TA, Olerud JE, Ansel JC. Exp Dermatol. 1998;7:81–96. doi: 10.1111/j.1600-0625.1998.tb00307.x. [DOI] [PubMed] [Google Scholar]
- 22.Green PG, Basbaum AI, Levine JD. Neuroscience. 1992;50:745–749. doi: 10.1016/0306-4522(92)90461-a. [DOI] [PubMed] [Google Scholar]
- 23.Schmidhuber SM, Santic R, Tam CW, Bauer JW, Kofler B, Brain SD. J Invest Dermatol. 2007;127:716–721. doi: 10.1038/sj.jid.5700569. [DOI] [PubMed] [Google Scholar]
- 24.Holmberg K, Kuteeva E, Brumovsky P, Kahl U, Karlstrom H, Lucas GA, Rodriguez J, Westerblad H, Hilke S, Theodorsson E, et al. Neuroscience. 2005;133:59–77. doi: 10.1016/j.neuroscience.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 25.Jancso G, Santha P, Horvath V, Pierau F. Regul Pept. 2000;95:75–80. doi: 10.1016/s0167-0115(00)00140-3. [DOI] [PubMed] [Google Scholar]
- 26.El-Nour H, Lundeberg L, Boman A, Theodorsson E, Hokfelt T, Nordlind K. Acta Derm Venereol. 2004;84:428–432. doi: 10.1080/00015550410017290. [DOI] [PubMed] [Google Scholar]
- 27.Pincelli C, Fantini F, Massimi P, Girolomoni G, Seidenari S, Giannetti A. Br J Dermatol. 1990;122:745–750. doi: 10.1111/j.1365-2133.1990.tb06261.x. [DOI] [PubMed] [Google Scholar]
- 28.Kreil G, Haiml L, Suchanek G. Eur J Biochem. 1980;111:49–58. doi: 10.1111/j.1432-1033.1980.tb06073.x. [DOI] [PubMed] [Google Scholar]
- 29.Brain SD, Williams TJ. Br J Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwan ML, Gomez AD, Baluk P, Hashizume H, McDonald DM. Am J Physiol. 2001;280:L286–L297. doi: 10.1152/ajplung.2001.280.2.L286. [DOI] [PubMed] [Google Scholar]
- 31.Brain SD, Crossman DC, Buckley TL, Williams TJ. J Cardiovasc Pharmacol. 1989;13:147–149. discussion S150. [PubMed] [Google Scholar]
- 32.Lang R, Berger A, Santic R, Geisberger R, Hermann A, Herzog H, Kofler B. Neuropeptides. 2005;39:179–184. doi: 10.1016/j.npep.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi A, Nakata O, Kasahara M, Sower SA, Kawauchi H. Gen Comp Endocrinol. 2005;144:174–181. doi: 10.1016/j.ygcen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Couzens M, Liu M, Tuchler C, Kofler B, Nessler-Menardi C, Parker RM, Klocker H, Herzog H. Genomics. 2000;64:318–323. doi: 10.1006/geno.2000.6132. [DOI] [PubMed] [Google Scholar]
- 35.Rosenfeld MG, Emeson RB, Yeakley JM, Merillat N, Hedjran F, Lenz J, Delsert C. Ann NY Acad Sci. 1992;657:1–17. doi: 10.1111/j.1749-6632.1992.tb22754.x. [DOI] [PubMed] [Google Scholar]
- 36.You S, Silsby JL, Farris J, Foster DN, el Halawani ME. Endocrinology. 1995;136:2602–2610. doi: 10.1210/endo.136.6.7750483. [DOI] [PubMed] [Google Scholar]
- 37.Talbot RT, Dunn IC, Wilson PW, Sang HM, Sharp PJ. J Mol Endocrinol. 1995;15:81–91. doi: 10.1677/jme.0.0150081. [DOI] [PubMed] [Google Scholar]
- 38.Lai JP, Douglas SD, Rappaport E, Wu JM, Ho WZ. J Neuroimmunol. 1998;91:121–128. doi: 10.1016/s0165-5728(98)00170-2. [DOI] [PubMed] [Google Scholar]
- 39.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 40.Lutz EM, Ronaldson E, Shaw P, Johnson MS, Holland PJ, Mitchell R. Mol Cell Neurosci. 2006;31:193–209. doi: 10.1016/j.mcn.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Ji H, Fabucci ME, Falconetti C, Zheng W, Sandberg K. Gene. 2004;341:93–100. doi: 10.1016/j.gene.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez M, Audinot V, Dromaint S, Macia C, Lamamy V, Beauverger P, Rique H, Imbert J, Nicolas JP, Boutin JA, et al. Biochem J. 2003;369:667–673. doi: 10.1042/BJ20020739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unniappan S, Lin X, Peter RE. Mol Cell Endocrinol. 2003;200:177–187. doi: 10.1016/s0303-7207(02)00301-5. [DOI] [PubMed] [Google Scholar]
- 44.Kohchi C, Tsutsui K. J Exp Zool. 2000;287:183–190. [PubMed] [Google Scholar]
- 45.Chu DQ, Cox HM, Costa SK, Herzog H, Brain SD. Br J Pharmacol. 2003;140:422–430. doi: 10.1038/sj.bjp.0705452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger A, Santic R, Hauser-Kronberger C, Schilling FH, Kogner P, Ratschek M, Gamper A, Jones N, Sperl W, Kofler B. Neuropeptides. 2005;39:353–359. doi: 10.1016/j.npep.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Maguire JJ, Davenport AP. Trends Pharmacol Sci. 2005;26:448–454. doi: 10.1016/j.tips.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Chen BS, Wang YC, Wu WS, Li WH. Bioinformatics. 2005;21:2698–2705. doi: 10.1093/bioinformatics/bti348. [DOI] [PubMed] [Google Scholar]
- 49.Cao T, Gerard NP, Brain SD. Am J Physiol. 1999;277:R476–R481. doi: 10.1152/ajpregu.1999.277.2.R476. [DOI] [PubMed] [Google Scholar]
- 50.Berger A, Tuechler C, Almer D, Kogner P, Ratschek M, Kerbl R, Iismaa TP, Jones N, Sperl W, Kofler B. Neuroendocrinology. 2002;75:130–138. doi: 10.1159/000048229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.