Abstract
The physiological ligand for the vitamin D receptor (VDR) is 1,25-dihydroxyvitamin D3. Lithocholic acid (LCA), a bile acid implicated in the progression of colon cancer, was recently shown to bind to VDR with low affinity and increase expression of the xenobiotic enzymes of the CYP3A family. Thus, LCA can induce its own catabolism through the VDR. We have now found that LCA can substitute for vitamin D in the elevation of serum calcium in vitamin D-deficient rats. Further, LCA in the diet will also replace vitamin D in the mobilization of calcium from bone. Further, LCA induces CYP24-hydroxylase mRNA gene expression in the kidney of vitamin D-deficient rats. It is clear, therefore, that LCA can be absorbed into the circulation to bind to the VDR at extra-intestinal sites. These findings lend support for the idea that the VDR may have evolved from an original role in detoxification.
Keywords: 24-hydroxylase, calcium, vitamin D receptor
The principal function of the hormonal form of vitamin D is the maintenance of calcium and phosphate homeostasis. These actions occur through the vitamin D receptor (VDR) by influencing target gene expression (1). Other roles for the vitamin D hormone have been discovered in cell proliferation and differentiation, keratinocyte function, osteoclastogenesis, and the immune system (1) and in inducing genes directed to metabolism of xenobiotics (2–4). In the intestine, anaerobic bacteria metabolize cholic and chenodeoxycholic acids into deoxycholic acid and lithocholic acid (LCA), respectively. A high-fat diet is associated with an increase in fecal bile acids, the most toxic of which is LCA. At high concentrations, LCA is poorly absorbed in the intestine and passes into the colon where it accumulates. Evidence from animal and human trials support an important role of fecal bile acids in carcinogenesis (3, 5–7), and LCA is doubled in feces of colorectal cancer patients (8).
An important discovery revealed that the bile acid LCA can serve as a ligand for VDR in intestine to induce not only CYP3A11 genes but also the vitamin D-induced calbindin D9k gene (2), raising the question of whether LCA could substitute for vitamin D in other functions. We can now clearly show that LCA in the diet can, indeed, substitute for vitamin D in one of its essential functions, i.e., the elevation of serum calcium. Further, it can induce the osteoclastic-mediated mobilization of bone calcium and induce CYP24 in kidney.
Results
LCA Increases Serum Calcium in Vitamin D-Deficient Rats.
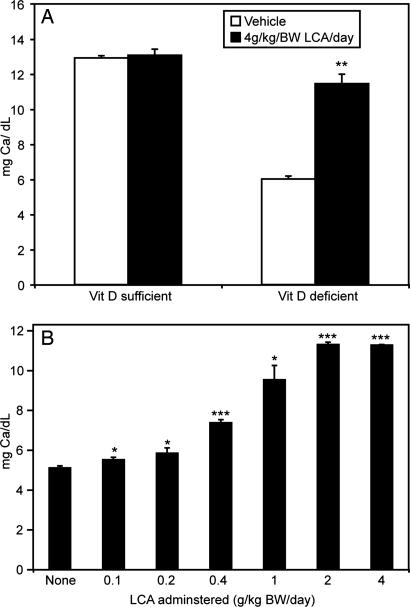
As shown in Fig. 1A, serum calcium levels of vitamin D-deficient rats are increased by administration of 4 g/kg body weight (BW) LCA given in the diet. The same is not observed for vitamin D-sufficient rats where control rats have a basal level of serum calcium that is not further increased by LCA. Interestingly, F344 rats fed a normal diet had higher circulating levels of calcium (≈12 mg/dl) compared with SD/Harlan rats whose levels are generally ≈10 mg/dl (9, 10) when fed the 0.47% calcium diet. Fig. 1B shows that serum calcium is elevated in a dose-dependent manner, and a significant increase in serum calcium is observed at a dietary LCA level as low as 0.1 g/kg BW.
Fig. 1.
Serum calcium response of vitamin D-deficient rats to LCA. (A) Comparison between vitamin D-deficient (n = 10) and vitamin D-sufficient F344 rats (n = 7) fed a 0.47% calcium-purified diet with or without 4 g/kg LCA for 2 weeks. The data are expressed as mean ± SEM. Significantly different from vehicle-treated rats, ∗∗, P < 0.001. (B) Serum calcium response of vitamin-D deficient male rats on the 0.47% vitamin D-deficient diet given no LCA (n = 11), 0.1 g/kg (n = 9), 0.2 g/kg (n = 8), 0.4 g/kg (n = 11), 1 g/kg (n = 3), 2 g/kg (n = 3), or 4 g/kg (n = 3) LCA for 2 weeks. The values represent the mean ± SEM. The differences between vehicle and LCA-treated rats were significant at ∗, P < 0.05 or ∗∗∗, P < 0.01.
Effect of LCA on Genes Involved in Calcium Absorption.
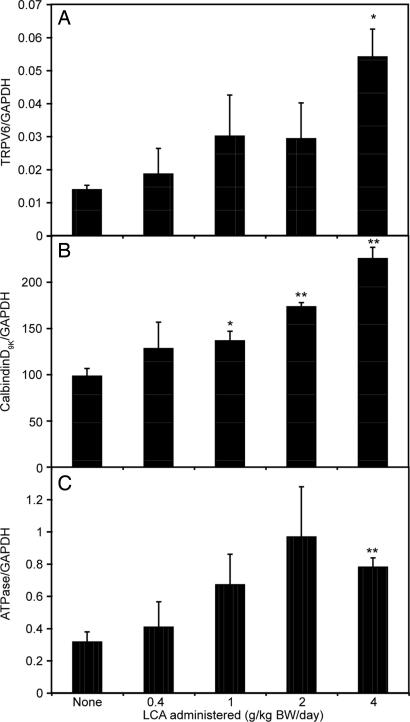
Consistent with the serum calcium data, dietary LCA increases the expression of TRPV6, calbindin D9k, and Ca2+ATPase mRNA in the intestine, although at 0.4 g/kg BW LCA, which causes a significant increase in serum calcium, none of the calcium transport mRNAs was significantly increased (Fig. 2).
Fig. 2.
The effect of LCA on the mRNA levels of intestinal calcium absorption-associated genes. Male vitamin D-deficient rats were fed the 0.47% calcium-purified diet providing 0.4, 1, 2, or 4 g/kg LCA per day (n = 3) for 2 weeks. Quantitative PCR was performed on RNA from duodenal mucosa. (A) TRPV6 mRNA. (B) Calbindin D9k mRNA. (C) ATPase mRNA. The difference between vehicle and the treatment groups was significant at ∗, P < 0.05; ∗∗, P < 0.01.
LCA Stimulates 24-Hydroxylase Expression in Intestine and Kidney.
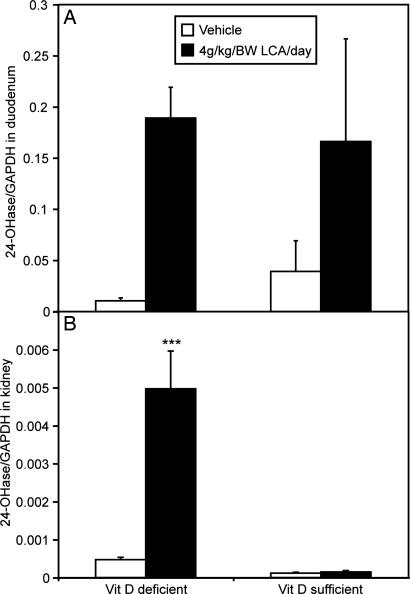
The effect of LCA on the activation of the 24-hydroxylase (CYP24) gene was studied in intestine and kidney. LCA up-regulates intestinal CYP24 mRNA in both vitamin D-deficient and vitamin D-sufficient rats (Fig. 3A). In kidney, we observed a significant up-regulation of CYP24 only in vitamin D-deficient rats (Fig. 3B).
Fig. 3.
The effect of LCA on CYP24 (24-hydroxylase) mRNA in the duodenum and kidney of vitamin D-deficient and vitamin D-sufficient rats. (A) Duodenum (n = 8–11). (B) Kidney (n = 8–11). The difference between vehicle and the treatment groups was significant at ∗∗∗, P < 0.001.
LCA Acts on Bone to Mobilize Calcium.
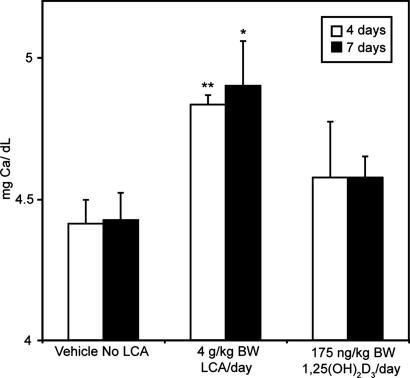
Because LCA in the diet produced a measurable response in kidney, we determined the effect of LCA on calcium mobilization from bone. Vitamin D-deficient rats fed diets containing 0.02% calcium had increased levels of serum calcium in response to LCA. Under these circumstances, the source of calcium in blood is from bone, thus demonstrating that LCA is able to act on bone resulting in the mobilization of calcium (Fig. 4). However, it should be noted that LCA is ≈4,000 times less potent than 1,25-(OH)2D3 in this activity.
Fig. 4.
LCA induces bone calcium mobilization in vivo. Serum calcium response of vitamin D-deficient rats on a 0.02% calcium diet given either 4 g/kg BW LCA or 175 ng per day of 1,25-(OH)2D3. Each group contained at least four animals, and the values represent the mean ± SEM. The difference between the vehicle and LCA was significant at ∗, P < 0.05 and ∗∗, P < 0.01.
Discussion
The surprising finding that LCA can serve as a low-affinity ligand for VDR led to the suggestion that VDR could serve as a bile acid sensor and could induce the catabolic enzyme CYP3A11 (2). This group also found that LCA activates the calbindin D9k gene. Our results build on that work (2) and show that LCA can substitute for vitamin D in its classical functions, i.e., elevation of serum calcium originating from intestinal absorption and bone mobilization. As little as 0.1 g/kg or ≈30 μg per day can raise serum calcium in a vitamin D-deficient rat. LCA is poorly absorbed so it is likely that <10 μg per day is absorbed. From these calculations, it would appear that LCA has in vivo activity of ≈1/1,000 that of 1,25-(OH)2D3. In terms of calcium regulation, therefore, it seems LCA physiologically does not play a significant role. This view is further supported by the fact that at very high levels LCA does not affect serum calcium of vitamin D-sufficient rats, presumably because of the presence of the much more potent 1,25-(OH)2D3 ligand competing with the low-affinity LCA. By the same reasoning, it seems unlikely, at the present stage of evolution, that VDR serves as a bile acid sensor, because in the presence of sufficient quantities of 1,25-(OH)2D3 available under normal circumstances LCA would be unable to activate the CYP3A11 through VDR.
The target gene most responsive to 1,25-(OH)2D3 is the CYP24 (11–13). It is of considerable interest that even in the kidney LCA is able to induce this gene in vitamin D-deficient rats, where the gene is largely silent under circumstances of vitamin D deficiency (13). It also shows that significant amounts of LCA must have been absorbed to reach the kidney. Again, in vitamin D-sufficient animals, no stimulation of CYP24 by LCA was seen in kidney. The amount of LCA reaching the kidney was probably unable to compete with the highly potent endogenous 1,25-(OH)2D3. In the intestine, higher levels of LCA were likely achieved to produce marginal stimulation. Similarly, significant amounts of LCA must have reached osteoblasts to activate RANKL that, in turn, activates both osteoclastogenesis and osteoclasts themselves to mobilize bone (14).
Because of the rise in serum calcium as a result of LCA's action, it is not surprising that TRPV6 and ATPase genes are activated by LCA. That these genes are activated confirms that high-dose LCA can substitute for vitamin D in the vitamin D-deficient animal.
Perhaps the most important finding is that LCA can act as a vitamin D substitute. This finding argues strongly that VDR may, indeed, have originally played a significant role in a detoxifying system inducing cytochrome P450 (CYP) enzymes that inactivate xenobiotics. Evolutionarily, it later acquired its now obvious calcium and phosphorus homeostasis roles. Because VDR still retains the ability to up-regulate the CYPs and other biotransformation systems (4), this role may well contribute significantly to human health, as, for example, the reported finding that vitamin D reduces the risk of colon and other cancers.
Materials and Methods
Materials.
1,25(OH)2D3 was purchased from SAFC (Madison, WI), and LCA was purchased from Sigma (St. Louis, MO).
Animals.
Four- to 5-week-old male F344 rats were obtained from Harlan (Indianapolis, IN) and housed in overhanging wire cages in a vivarium under incandescent lighting. The rats were fed a purified vitamin D-deficient diet (9) supplemented with vitamins A, E, and K as described (9). To obtain vitamin D-deficient rats, the animals were fed a 0.47% calcium diet ad libitum for 1 week, followed by 3 weeks of a 0.02% calcium diet. The cycle was then repeated by feeding a 0.47% calcium diet for 1 week, followed by 2 weeks of a 0.02% calcium diet. For the final week, the rats were fed a 0.47% calcium diet. Vitamin D deficiency was confirmed by serum calcium analysis. All vitamin D-sufficient animals were kept on a purified diet containing 0.47% calcium supplemented with vitamins A, E, D, and K as described (9).
After the 8-week regimen, the rats were fed the purified diet with normal calcium levels (0.47%) containing various levels of LCA per kg BW. After 2 weeks, the animals were weighed and killed by CO2 asphyxiation, and blood was immediately collected via heart puncture. Kidneys and duodenum were collected for further analysis. All of the procedures described were reviewed and approved by the University of Wisconsin Research Animal Resources Center Committee Review Board.
Serum Calcium Analysis.
Serum calcium was measured in 0.1% lanthanum chloride with an atomic absorption spectrometer (model 3110; PerkinElmer, Wellesley, MA). All test values were compared with those of reference standards measured on the same day.
RNA Isolation, Reverse Transcription, and Quantitative PCR.
RNA was isolated from rat duodenum by using TRI Reagent (Molecular Research Center, Cincinnati, OH). Five micrograms of total RNA was transcribed at 42°C by using random hexamers and avian myeloblastosis virus reverse transcriptase according to the manufacturer's protocol (Promega, Madison, WI). After reverse transcription, samples were diluted 8-fold with water to 200 μl final volume and heated to 90°C for 5 min. Real-time PCR was performed with the LightCycler FastStart DNA Master SYBR Green l kit (Roche, Indianapolis, IN) with 5 μl of the reverse-transcribed material per reaction on a LightCycler 2.0 Instrument (Roche). For quantitation, serial dilutions of plasmid DNA containing the genes of interest were used as standards.
The amplification primers and conditions used were as follows: (i) rat CYP24 (909–1177), 5′-GCA TGG ATG AGC TGT GCG A-3′ and 5′-AAT GGT GTC CCA AGC CAG C-3′, denaturing at 95°C for 5 s, annealing at 63°C for 5 s, and elongating at 72°C for 12 s; and (ii) rat GAPDH, 5′-TGA AGG TCG GTG TGA ACG GAT TTG GC-3′ and 5′-CAT GTA GGC CAT GAG GTC CAC CAC-3′, denaturing at 95°C for 15 s, annealing at 58°C for 5 s, and elongating at 72°C for 38 s. All PCRs were preceded by an initial 10-min denaturation step at 95°C.
Bone Calcium Mobilization.
Four- to 5-week-old male F344 rats were made vitamin D-deficient as described above with an exception. In the fifth day of the last 0.02% diet change, animals were fed a purified diet containing either vehicle, 4 g/kg BW LCA, or 1 μg/kg 1,25(OH)2D3 for 7 days. Blood was collected from the tail after 4 days, and serum calcium levels were determined. Rats were killed after 7 days, and blood was collected for a final serum calcium measurement.
Statistics.
Values are shown as mean ± SEM. The unpaired two-group Student's t test was performed to assess significant differences (P < 0.05).
Acknowledgments
This work was supported in part by the Wisconsin Alumni Research Foundation.
Abbreviations
- VDR
vitamin D receptor
- LCA
lithocholic acid
- BW
body weight.
Footnotes
The authors declare no conflict of interest.
References
- 1.Jones G, Strugnell SA, DeLuca HF. Physiol Rev. 1998;78:1193–1231. doi: 10.1152/physrev.1998.78.4.1193. [DOI] [PubMed] [Google Scholar]
- 2.Makishima M, Lu TT, Xie W, Whitfield GK, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 3.Baijal PK, Fitzpatrick DW, Bird RP. Nutr Cancer. 1998;31:81–89. doi: 10.1080/01635589809514685. [DOI] [PubMed] [Google Scholar]
- 4.Kutuzova GD, DeLuca HF. Toxicol Appl Pharmacol. 2007;218:37–44. doi: 10.1016/j.taap.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Kishida T, Taguchi F, Feng L, Tatsuguchi A, Sato J, Fujimori S, Tachikawa H, Tamagawa Y, Yoshida Y, Kobayashi M. J Gastroenterol. 1997;32:306–311. doi: 10.1007/BF02934485. [DOI] [PubMed] [Google Scholar]
- 6.Narisawa T, Magadia NE, Weisburger JH, Wynder EL. J Natl Cancer Inst. 1974;53:1093–1097. doi: 10.1093/jnci/53.4.1093. [DOI] [PubMed] [Google Scholar]
- 7.Reddy BS, Watanabe K. Cancer Res. 1979;39:1521–1524. [PubMed] [Google Scholar]
- 8.Owen RW, Dodo M, Thompson MH, Hill MJ. Nutr Cancer. 1987;9:73–80. doi: 10.1080/01635588709513914. [DOI] [PubMed] [Google Scholar]
- 9.Suda T, DeLuca HF, Tanaka Y. J Nutr. 1970;100:1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- 10.Steenbock H, Herting DC. J Nutr. 1955;57:449–468. doi: 10.1093/jn/57.4.449. [DOI] [PubMed] [Google Scholar]
- 11.Kutuzova GD, DeLuca HF. Arch Biochem Biophys. 2004;432:152–166. doi: 10.1016/j.abb.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zierold C, Darwish HM, DeLuca HF. J Biol Chem. 1995;270:1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 13.Endres B, Kato S, DeLuca HF. Biochemistry. 2000;39:2123–2129. doi: 10.1021/bi9923757. [DOI] [PubMed] [Google Scholar]
- 14.Suda T, Takahashi N, Martin TJ. Endocr Rev Monogr. 1995;4:266–270. [Google Scholar]