Abstract
The meiotic cycle reduces ploidy through two consecutive M phases, meiosis I and meiosis II, without an intervening S phase. To maintain ploidy through successive generations, meiosis must be followed by mitosis after the recovery of diploidy by fertilization. However, the coordination from meiotic to mitotic cycle is still unclear. Mos, the c-mos protooncogene product, is a key regulator of meiosis in vertebrates. In contrast to the previous observation that Mos functions only in vertebrate oocytes that arrest at meiotic metaphase II, here we isolate the first invertebrate mos from starfish and show that Mos functions also in starfish oocytes that arrest after the completion of meiosis II but not at metaphase II. In the absence of Mos, meiosis I is followed directly by repeated embryonic mitotic cycles, and its reinstatement restores meiosis II and subsequent cell cycle arrest. These observations imply that after meiosis I, oocytes have a competence to progress through the embryonic mitotic cycle, but that Mos diverts the cell cycle to execute meiosis II and remains to restrain the return to the mitotic cycle. We propose that a role of Mos that is conserved in invertebrate and vertebrate oocytes is not to support metaphase II arrest but to prevent the meiotic/mitotic conversion after meiosis I until fertilization, directing meiosis II to ensure the reduction of ploidy.
In sexually reproducing organisms, genomic integrity through successive generations is maintained by the coordination of meiosis and mitosis (1). Meiotic cell cycles are characterized by two consecutive M phases, meiosis I and meiosis II, without an intervening S phase, and result in the production of haploid gametes. Fertilization recovers full ploidy, and after the completion of meiosis II, the embryonic mitotic cell cycle that consists of alternating S phase and M phase proceeds in a zygote cell. It is unclear, however, how the conversion from the meiotic to the embryonic mitotic cell cycle is directed.
Mos, the c-mos protooncogene product, is one of the central regulators of meiosis in vertebrate oocytes (1). It was originally described as a cellular homolog of viral Mos that, when expressed ectopically, could induce the oncogenic transformation of vertebrate somatic cells (2). Biochemically, Mos functions as an mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) kinase [MEKK or mitogen-activated protein kinase kinase (MAPKK) kinase, MAPKKK] that directly phosphorylates and activates MEK, which in turn phosphorylates and activates MAP kinase (3–6). To date, normal Mos expression and function have been restricted to vertebrate oocytes that arrest at metaphase of meiosis II (metaII) during maturation (see refs. 1 and 6). It has been well established that a universal role of Mos in vertebrate oocytes is to cause metaII arrest by acting as an essential component of a cytostatic factor (1, 6–9). In Xenopus oocytes, Mos is further required for meiosis reinitiation (10, 11) and for normal meiosis I to II transition (12, 13). Mos accomplishes the meiosis I to II transition through premature reactivation of cyclin B-Cdc2 kinase, a universal inducer of M phase (14), thereby preventing DNA replication (i.e., entry into S phase) after the completion of meiosis I (15). On the basis of these observations, it has been proposed that the ultimate biological function of Mos during meiosis is to prevent undesirable DNA replication or parthenogenetic activation before fertilization, thus enabling the reduction of chromosome number (1, 6, 15). However, it is still unclear how the meiotic and the embryonic mitotic cycles are coordinated by Mos. Furthermore, in invertebrate oocytes that differ from vertebrate oocytes in that they lack metaII arrest, there has been no evidence for the existence of Mos or any other factor that could regulate the conversion from the meiotic cycle to the embryonic mitotic cycle.
Fully grown immature oocytes of the starfish are arrested at prophase of meiosis I (16, 17). Once meiosis is reinitiated, in the absence of fertilization, starfish oocytes proceed completely through meiosis I and II to produce interphase- (egg pronucleus stage) arrested haploid eggs. In these mature eggs, MAP kinase activity is required for preventing onset of the embryonic mitotic cycle (16, 18–21). This notion is supported by observations that fertilization causes its immediate inactivation; in the absence of fertilization, a MAP kinase cascade inhibitor enables starting of the embryonic mitotic cycle; and constitutive activation of MAP kinase prevents release from arrest at the egg pronucleus stage even though fertilization occurs. However, MAP kinase is activated much earlier than the egg pronucleus stage, that is, soon after the activation of cyclin B-Cdc2 at meiosis reinitiation, and remains at elevated levels of activity throughout meiosis I and II (18–20), suggesting another role(s) for MAP kinase in starfish meiotic cycles.
In the present study, we have searched for an upstream regulator of MAP kinase in starfish oocytes and have isolated, to our knowledge, the first invertebrate homolog of mos. The ablation and restoration of Mos revealed that starfish oocytes already have a competence to progress through embryonic mitotic cycles at the end of meiosis I, yet are detoured to meiosis II by Mos, which prevents conversion to the embryonic mitotic cycle. On the basis of these findings, we propose that a role of Mos that is conserved in vertebrate and invertebrate oocytes is to coordinate the conversion from the meiotic to the embryonic mitotic cycle.
Materials and Methods
Oocytes and Eggs.
Immature oocytes were isolated from the starfish Asterina pectinifera and were treated with 1 μM 1-methyladenine (1-MeAde) to undergo maturation as described (19). Some of 1-MeAde-treated oocytes were further treated with 50 μM U0126, an MEK inhibitor, during the limited period from metaI to metaII. In some cases, mature eggs with female pronuclei were inseminated. Oocyte extracts for immunoblots and histone H1 kinase assays were prepared as described (22).
cDNA Cloning.
Partial cDNA clones of starfish Mos were first isolated by reverse transcription–PCR (RT-PCR). Total RNA from starfish oocytes was reverse transcribed with Superscript II (GIBCO/BRL), and PCR was performed by using degenerate primers derived from two highly conserved amino acid sequences among vertebrate Mos proteins: GAFIIMEY, 5′-GGNGCNTTYATHATHATGGARTA-3′ for forward and TKADIYSYG, 5′-CCRTANSWRTADATRTCNGCYTTNGT-3′ for reverse. The RT-PCR products labeled with [α-32P]dCTP were used as probes for screening starfish egg cDNA library in λZapII vector (Stratagene). The starfish Mos sequence has been deposited in the DNA Data Base in Japan/European Molecular Biology Laboratory/GenBank under accession no. AB040102.
Preparation of Recombinant Protein.
To prepare the glutathione S-transferase (GST) fusion protein of Mos (GST-Mos), BamHI sites were introduced into starfish Mos cDNA at the first ATG and immediately downstream of the stop codon by using PCR with primers 5′-GCGGGATCCATGCCTTGCGACACGGCAG-3′ and 5′-GCGGGATCCTCAAGTCGTCCCATGGCG-3′. The PCR product digested with BamHI was ligated into the BamHI site of pGEX-4T-2 (Amersham Pharmacia). The GST-Mos was expressed in Escherichia coli (BL21) for 16 h with 0.5 mM isopropyl-d-thiogalactoside at 18°C, purified with glutathione–Sepharose 4B (Amersham Pharmacia), and concentrated to 0.5 mg/ml in 20 mM Hepes (pH 6.8)/88 mM NaCl/7.5 mM MgCl2 with Microcon 50 (Amicon). Recombinant ΔN-STE11 protein of budding yeast (23) (a gift from E. Nishida of Kyoto University) was dissolved at 1.28 mg/ml in 20 mM NaOH-Hepes and 2 mM DTT, pH 7.4.
Histone H1 Kinase Assay.
The kinase assays contained 2 μl of an oocyte suspension, 6 μl of extraction buffer (see ref. 22), and 4 μl of a histone H1-ATP mixture at final concentrations of 0.3 mg/ml histone H1 (Boehringer Mannheim), 10 μM cold ATP, and 0.16 mCi/ml [γ-32P]ATP. The kinase assay mixture was incubated for 30 min at 25°C, and the reaction was terminated by the addition of 4 μl of 4× concentrated SDS/PAGE sample buffer followed by boiling for 5 min. Samples were run on a 12.5% SDS/PAGE, and the gel was autoradiographed with x-ray film (RX-U, Fuji Film). The radioactivity of the excised histone H1 bands was quantified by using a liquid scintillation counter. In starfish oocyte extracts, histone H1 kinase activity represents the Cdc2 activity, and that derived from Cdk2 constitutes less than 1/100 of the total activity (see ref. 22).
DNA Synthesis Assay.
Aliquots of 15–20 oocytes were pulse labeled with 1 mM BrdUrd in seawater for 30 min. The oocytes were then extracted and fixed for immunofluorescence as described (19). After the denaturation of DNA with 2 N HCl, the incorporated BrdUrd was visualized to monitor DNA synthesis.
Microinjection.
Microinjection was performed as described (24). Oligonucleotide phosphorothioates used for sense and antisense mos were 5′-ATGCCTTGCGACACGGC-3′ and 5′-GCCGTGTCGCAAGGCAT-3′, respectively. Immature oocytes were injected with 35 pg of one or the other oligonucleotide dissolved in 13 pl of sterilized and distilled water, 50 pg (in 100 pl) of the GST-Mos recombinant protein, or 295 pg (in 230 pl) of the ΔN-STE11 protein. At various times after 1-MeAde addition, 6 recipient oocytes were recovered in 5 μl of seawater for immunoblots, mixed with 5 μl of 2× concentrated SDS/PAGE sample buffer, and boiled for 5 min. For histone H1 kinase assay, 5 recipient oocytes were recovered in 2 μl of seawater, added with 6 μl of extraction buffer, and immediately frozen in liquid nitrogen. For immunofluorescence against BrdUrd and β-tubulin, 15–20 recipient oocytes were recovered.
Antibodies, Immunoblotting, and Immunofluorescence.
Polyclonal anti-starfish Mos antibodies were raised in rabbits against the GST-Mos fusion protein and purified as described (22). Other antibodies used were anti-starfish cyclin A, cyclin B, and Cdc25 (22), anti-MAP kinase (19), antiphospho MAP kinase, and antiphospho-Tyr-15 of Cdc2 (New England BioLabs). After incubation of Immobilon (polyvinylidene difluoride) membrane (Millipore) with alkaline phosphatase- or peroxidase-conjugated secondary antibodies, signals were visualized by a 5-bromo-4-chloro-3-indolyl phosphate/NBT phosphatase substrate system or enhanced chemiluminescence (Amersham Pharmacia). For tubulin immunofluorescence, 15–20 oocytes were extracted for 15 min at room temperature with a buffer containing 21.25 mM imidazole, 8.5 mM KCl, 8.5 mM EGTA, 0.85% Triton X-100, 0.17 mM PMSF, 17% glycerol, and 15% methanol, pH 6.9, and then attached to BioBond- (2% in acetone; British Bio Cell, Cardiff, U.K.) coated coverslip, followed by the procedure as described (25). The specimens were labeled by monoclonal anti-β-tubulin antibody (N357, Amersham) and then FITC-conjugated goat anti-mouse IgG (Cappel). The DNA in the extracted oocytes was then stained with 0.1 mg/ml 4′,6-diamidino-2-phenylindole.
Results and Discussion
Identification of Starfish Mos.
In starfish oocytes, the MAP kinase activity depends on continuous protein synthesis throughout meiotic cycles, although MAP kinase and its direct activator, MEK, are already present in immature oocytes (ref. 19; K.T., unpublished observations). As a possible MEKK in starfish oocytes, we initially isolated cDNA clones of starfish homolog of raf, but the activity of Raf was independent of continuous protein synthesis (K.T., unpublished observations). Alternatively, a major protein synthesis-dependent MEKK in vertebrate oocytes is Mos, and its possible presence in invertebrates has been discussed (1, 7, 15). We have then screened a starfish oocyte cDNA library and isolated cDNA clones encoding starfish homolog of mos. The longest clone encoded a protein of 351 amino acids with a predicted molecular mass of 39 kDa (Fig. 1). Starfish Mos shared about 40% identity with vertebrate Mos. It is noted that the third amino acid residue is Cys in starfish Mos instead of Ser, which is conserved among vertebrate Mos and whose phosphorylation is required for stability of Mos in vertebrate oocytes (26). However, the injection of the GST-starfish Mos fusion protein induced meiosis reinitiation in immature Xenopus oocytes and also cleavage arrest in two-cell stage Xenopus blastomeres (data not shown). These observations indicate that starfish Mos is a functional homolog of Xenopus Mos.
Figure 1.
Deduced amino acid sequence of starfish Mos and its alignment with mouse and Xenopus Mos proteins. Identical amino acids are shaded, and gaps introduced for optimal alignment are indicated by dashes. The starfish (sf) Mos sequence has been deposited in DNA Data Base of Japan/European Molecular Biology Laboratory/GenBank under accession no. AB040102. Mm, mouse (accession no. J00372); Xl, Xenopus laevis (accession no. X13311).
In addition to starfish mos, a search for the Drosophila genome sequence database indicated the presence of a presumptive mos homolog (personal communication, Christian Lehner of University Bayreuth; DNA Data Base in Japan/European Molecular Biology Laboratory/GenBank, accession no. AAF58463). Thus, the presence of Mos in invertebrates, whose oocytes do not arrest at metaII but stop at metaI (Drosophila) (see ref. 1) or at the egg pronucleus stage (starfish) unless fertilized (see ref. 16) necessitates a reconsideration of the role for Mos in meiotic cell cycle progression.
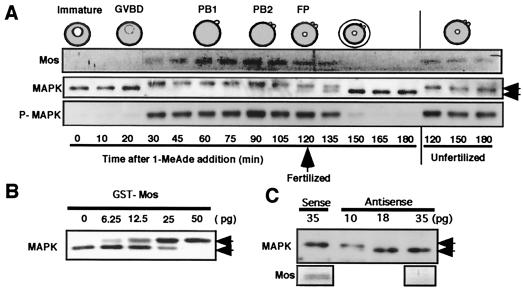
Immunoblots with anti-starfish Mos antibody revealed that starfish Mos was newly synthesized just after germinal vesicle breakdown (GVBD), an indication of meiosis reinitiation, and its appearance coincided with the initial activation of MAP kinase during meiotic maturation (Fig. 2A). Thereafter, the presence of Mos was accompanied by MAP kinase activity. Mos remained through both meiotic cycles, unless fertilization occurred, and disappeared after fertilization when MAP kinase was also inactivated. The injection of the recombinant GST-starfish Mos protein into immature starfish oocytes induced MAP kinase activation (Fig. 2B) but not GVBD (data not shown). In contrast, injection of an antisense mos oligonucleotide into immature oocytes prevented both Mos synthesis and MAP kinase activation after stimulation with 1-MeAde, the starfish maturation-inducing hormone, although GVBD occurred normally in the injected oocytes (Fig. 2C; see also Fig. 3A). Thus, Mos is a MAPKKK in starfish oocytes, indicating that biochemically Mos functions as a universal MAPKKK in both vertebrate and invertebrate oocytes, irrespective of whether they arrest at metaII.
Figure 2.
Mos functions as an MAP kinase activator in starfish oocytes. (A) Dynamics of Mos and MAP kinase through starfish meiotic cycles. At the female pronucleus (FP) stage after the completion of meiosis II, mature eggs were fertilized, resulting in the disappearance of Mos. P-MAPK, antiphospho MAP kinase corresponding to its active form; PB 1 and 2, the first and the second polar bodies, respectively. (B) MAP kinase activation induced by injection of the GST-starfish Mos fusion protein into immature starfish oocytes. Oocytes were injected with various amounts of GST-Mos and recovered at 30 min for immunoblots. (C) Antisense mos prevents MAP kinase activation after 1-MeAde addition in starfish oocytes. Immature oocytes were injected with various amounts of antisense and sense mos oligonucleotides and then treated with 1-MeAde to undergo GVBD. Oocytes were recovered for immunoblots 60 min after 1-MeAde addition. Mos synthesis was undetectable at 35 pg injection of antisense mos. Upper and lower bands of MAP kinase (indicated by arrows) correspond to the active and the inactive forms, respectively (see ref. 19).
Figure 3.
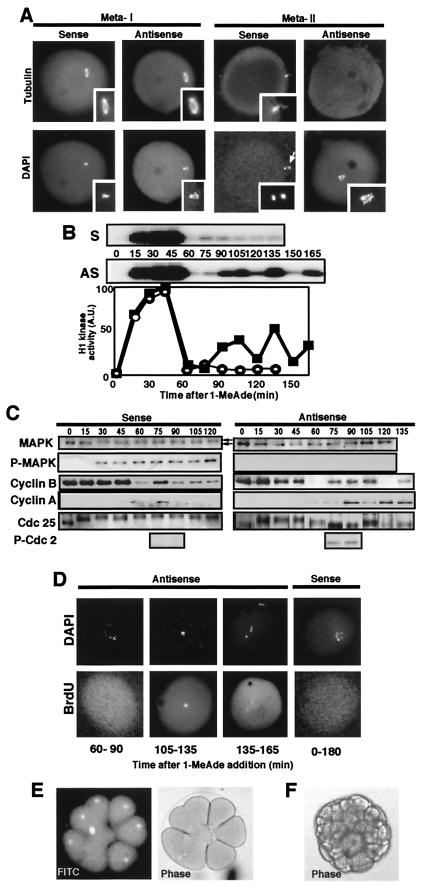
The mitotic type of cell cycles proceed after meiosis I in Mos-deficient 1-MeAde-treated unfertilized starfish oocytes. (A) Abortive spindle formation at a stage corresponding to metaII in Mos-deficient oocytes. After 1-MeAde addition to oocytes that had been injected with either antisense or sense mos, a spindle was frequently detectable at metaI with antitubulin staining but thereafter could not be detected, and condensed chromosomes were located in the middle of each oocyte. (Insets) Meiotic spindles and condensed chromosomes at higher magnification. White arrow indicates the first polar body. (B) Repeated fluctuation of histone H1 kinase activity after 1-MeAde addition in starfish oocytes injected with antisense mos. (Upper) Autoradiograms (AS, antisense mos; S, sense mos). (Lower) Radioactivity of the excised histone H1 bands (closed squares, antisense mos; open circles, sense mos). (C) Dynamics of cyclins B and A, Cdc25, Tyr phosphorylation in Cdc2 and MAP kinase after 1-MeAde addition in starfish oocytes injected with antisense mos. P-MAPK and P-Cdc2, immunoblots with antiphospho MAP kinase and antiphospho-Tyr-15 of Cdc2, respectively. (D) DNA replication occurs when histone H1 kinase activity drops to minimal levels after 1-MeAde addition to starfish oocytes injected with antisense mos. BrdUrd incorporation by pulse labeling for 30 min indicated below each panel was detectable rarely immediately after meiosis I (see also Fig. 4A) and always thereafter in antisense mos-injected oocytes; in contrast, it was undetectable through 180 min continuous labeling in control sense mos-injected oocytes. Each oocyte was double stained with 4′,6-diamidino-2-phenylindole (DAPI). (E) Several rounds of cleavage in antisense mos-injected, 1-MeAde-treated, unfertilized starfish oocytes. Nuclear divisions and furrowing occurred normally in some blastomeres and abnormally in others within an embryo. Thus, although they were abnormal as a whole, these oocytes developed to the two-cell stage almost invariably and to the blastula stage at 10%. Presence of nucleus is shown by accumulation of FITC-conjugated BSA coupled with nucleoplasmin NLS peptide, which was injected into immature oocytes (Left). (F) Development to 64-cell stage embryo in 1-MeAde- and U0126-treated unfertilized starfish oocytes. Almost all of these embryos developed to bipinnaria larvae, even though abnormal cleavages were partially observed. Note the absence of elevation of the fertilization envelope.
Role for Mos in Starfish Meiotic Cycle Control.
The above observations also indicate that Mos is not required for meiosis reinitiation in starfish oocytes. This makes a contrast to the previous observations in Xenopus oocytes in which Mos is required for meiosis reinitiation (10, 11), although MAP kinase activation has been recently reported not to be essential for meiosis reinitiation in Xenopus oocytes as well (27, 28). To identify a physiological role for Mos in invertebrates, we monitored the fate of Mos-deficient starfish oocytes after meiosis reinitiation. In oocytes that had been injected with the antisense mos and then treated with 1-MeAde, the first polar body was occasionally emitted after GVBD, and almost normal formation of the first meiotic spindle (Fig. 3A, MetaI; in contrast, see Fig. 4A Left for an unsuccessful emission). Thereafter, however, the second polar body was not detected, and a normal second meiotic spindle failed to form (Fig. 3A, MetaII). Because the chromosomes were condensed but rather dispersed in the cytoplasm of oocytes lacking second meiotic spindle, we have monitored the dynamics of histone H1 kinase activity to follow cell cycle progression. Surprisingly, in 1-MeAde-treated Mos-deficient oocytes, histone H1 kinase activity oscillated through more than three cycles after the initial activation and inactivation corresponding to meiosis I (Fig. 3B). This pattern, which resembled that seen in fertilized eggs undergoing embryonic mitotic cycles, was in marked contrast to control sense mos-injected oocytes, in which oscillation of histone H1 kinase activity after meiosis I was limited to a single small peak.
Figure 4.
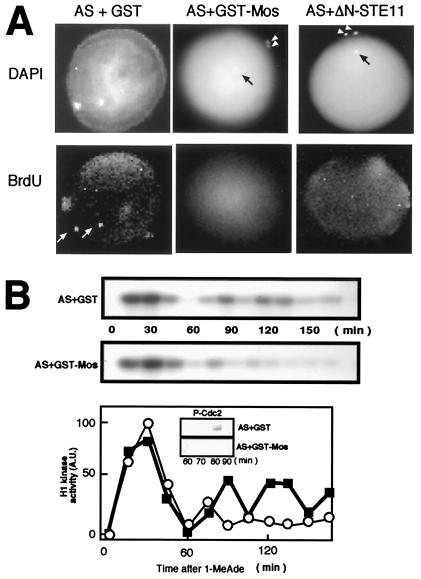
Restoration of meiosis II by Mos in starfish oocytes in which translation of mos is prevented. (A) Formation of two polar bodies along with a female pronucleus after 1-MeAde addition to oocytes that had received the injection of antisense mos (AS) and either GST-Mos or ΔN-STE11. Polar bodies (arrowheads) were detected by DAPI staining. Black arrow indicates the female pronucleus. BrdUrd incorporation was undetectable in female pronuclei of GST-Mos or ΔN-STE11-restored oocytes but could be detected in control oocytes (white arrows). (B) Two limited rounds of H1 kinase activity after 1-MeAde addition to oocytes that had received the injection of antisense mos and the GST-Mos fusion protein. After 1-MeAde addition, oocytes were recovered at indicated times and processed for measurement of H1 kinase activity (Top and Middle for autoradiogram; Bottom for radioactivity of the excised histone H1 bands; open circles, injection with antisense mos plus GST-Mos; closed squares, injection with antisense mos plus GST) and for Tyr phosphorylation in Cdc2 (Bottom, Insets). In both A and B, control oocytes had been injected with antisense mos (AS) and GST before 1-MeAde addition.
Almost in parallel with histone H1 kinase activity, levels of cyclin B and A proteins oscillated after meiosis I in Mos-deficient 1-MeAde-treated oocytes (Fig. 3C), supporting also that the injection of the antisense oligonucleotide has no obviously deleterious effect on protein synthesis. Detailed analysis of the regulation of cyclin B-Cdc2 revealed, however, that Tyr phosphorylation of Cdc2 was detected whenever the levels of histone H1 kinase activity dropped, including the end of meiosis I, and that the levels of Cdc25 phosphorylation oscillated in parallel with histone H1 kinase activity (Fig. 3C). Thus, cyclin B-Cdc2 was inactivated through the inhibitory phosphorylation of Tyr residue in each cycle, including the one immediately after meiosis I. These features in Mos-deficient starfish oocytes are in contrast to the normal meiosis I to II transition, during which Cdc25 remained phosphorylated, Tyr phosphorylation of Cdc2 was undetectable, and only a small peak of cyclins B and A was seen (Fig. 3C; see ref. 22).
What can be deduced from the cell cycle regulatory features in Mos-deficient starfish oocytes? Typically in early embryonic mitotic cell cycles, which consist of alternating M phase and S phase, histone H1 kinase activity fluctuates repeatedly with a peak at each M phase, and after exit from each M-phase, cyclin B-Cdc2 is reactivated after a lag period that is caused by its inactivation by Tyr phosphorylation of Cdc2 (22, 29, 30; reviewed in refs. 31 and 32). In contrast, in meiotic cell cycles, which consist of two consecutive M phases without an intervening S phase, histone H1 kinase activity exhibits only two limited peaks, and the entry into meiosis II after meiosis I is characterized by a unique regulation in which cyclin B-Cdc2 is immediately reactivated by bypassing the inhibitory Tyr-phosphorylation-dependent lag seen in the mitotic cycles of early embryos (15, 22, 30, 33). Considering these differences between mitotic and meiotic cell cycles, the behavior of Mos-deficient 1-MeAde-treated starfish oocytes indicates that in the absence of Mos, the regulation of cyclin B-Cdc2 might be converted from the meiotic type of control to the mitotic type immediately after the completion of meiosis I. The requirement of Mos for spindle formation needs further study.
In agreement with this notion, DNA replication was occasionally observed immediately after meiosis I in Mos-deficient 1-MeAde-treated unfertilized oocytes and was always seen at later times whenever the minimal levels of histone H1 kinase activity were reached (Fig. 3D). The reason why DNA replication was not always detectable immediately after meiosis I could be explained by the fact that the ability to replicate DNA is acquired around meiosis I to II transition during starfish oocyte maturation (19). Once the ability is acquired, it is presumed that only the absence of the Cdc2 activity results in DNA replication during embryonic mitotic cycles because of continuously active DNA replication machinery (see refs. 15, 30, 34). Our data indicate that in the absence of Mos, embryonic mitotic cell cycles might start immediately after completion of meiosis I in unfertilized oocytes and continue thereafter. Consistently, furrow formation and cleavages that resemble those seen in early embryonic mitotic cycles were observed occasionally in Mos-deficient 1-MeAde-treated unfertilized oocytes (Fig. 3E). Typically, starfish oocytes that were treated with 1-MeAde and then with MEK inhibitor, U0126, during the period from metaI to metaII underwent parthenogenetic development to bipinnaria larvae in the absence of fertilization (see Fig. 3F).
To demonstrate a requirement for Mos in the execution of meiosis II, the GST-Mos fusion protein was introduced into antisense mos-injected immature oocytes. These oocytes exhibited elevated levels of MAP kinase activity (data not shown; see Fig. 2B), and after 1-MeAde addition, they emitted two polar bodies and then arrested at the egg pronucleus stage without DNA synthesis (Fig. 4A; compare Middle with Left), indicating the successful formation of the first and the second meiotic spindles. In these oocytes, histone H1 kinase activity exhibited only two rounds of fluctuation with the first distinct peak followed by the second relatively small peak, and Tyr phosphorylation of Cdc2 was not detected between these two peaks (Fig. 4B). Thus, both the execution of meiosis II and the cell cycle termination after meiosis II were restored by Mos addition into oocytes, in which translation of mos had been prevented by injection of antisense oligonucleotides. Because both the recovery of meiosis II and the arrest at pronucleus stage without DNA synthesis were also seen after injection of budding yeast ΔN-STE11 protein, a constitutively active form of MAPKKK (23), into these oocytes, the effect of Mos is likely to be performed solely through the activation of MAP kinase (Fig. 4A Right). In addition, although we demonstrated previously that sole inhibition of protein synthesis by emetine causes entry into S phase in pronucleus stage eggs (19), the emetine response was blocked by GST-Mos (K.T., unpublished observations). The Mos-MAPK pathway could execute meiosis II through the suppression of Myt1 (35, 36), whereas its downstream leading to arrest at the pronucleus stage needs further study.
Role of Mos Conserved in Vertebrate and Invertebrate Oocytes.
It was previously reported that in c-mos-knockout mice, the progression into meiosis II is apparently normal, and that mature eggs undergo parthenogenetic activation without a distinct metaII arrest, leading to significant development of ovarian teratomas (8, 9). Detailed analysis of these mouse oocytes has revealed, however, that the organization of microtubules and chromosomes becomes interphase like before entry into meiosis II (37, 38), or that parthenogenetic activation (i.e., formation of female pronucleus) occurs most frequently immediately after meiosis I rather than after meiosis II (39). These recent observations are consistent with an earlier report indicating that loss of c-mos function in mouse oocytes leads to the decondensation of metaphase chromosomes, reformation of a nucleus after meiosis I, and cleavage to two cells (40). These changes are all consistent with the premature initiation of mitotic cell cycles immediately after meiosis I.
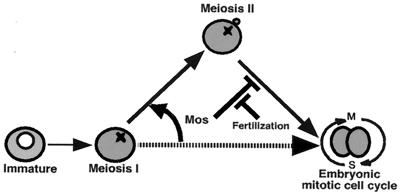
In Mos-ablated oocytes of Xenopus, an interphase nucleus is transiently formed after the completion of meiosis I, and DNA replication occurs in the presence of weak Tyr phosphorylation on Cdc2 (15). Thereafter, a monaster-like structure is formed (15) and, by inference, cyclin B-Cdc2 should be reactivated (K. Ohsumi and T.K., unpublished work). But progression through embryonic mitotic cycles or parthenogenetic development that was seen in Mos-ablated oocytes of starfish and mouse has not been reported in Mos-ablated Xenopus oocytes. Accordingly, it has been discussed that a role for Mos conserved in vertebrate oocytes is solely to prevent undesirable DNA replication or parthenogenetic activation in the meiosis I to II transition and after entry into meiosis II until fertilization (see refs. 1 and 15). However, taking all the above into consideration, the observations in mouse, Xenopus, and starfish could be rather reconciled by the argument that in each of these cases, the ability to undergo mitotic cell cycles, with their specific mechanism of control, is already acquired at the completion of meiosis I, but Mos suppresses the mitotic cell cycles and ensures that the oocyte executes meiosis II (Fig. 5). Mos further remains until fertilization to prevent the start of the embryonic mitotic cell cycle, resulting in well-known cell cycle arrest at either metaII in mouse and Xenopus (reviewed in refs. 1 and 6) or pronucleus stage in starfish (reviewed in ref. 16). The lack or disappearance of Mos cancels this inhibition, leading to recovery of the embryonic mitotic cell cycles.
Figure 5.
A role of Mos is conserved in vertebrate and invertebrate oocytes. At the end of meiosis I, the competence to undergo the embryonic mitotic cell cycle is already acquired, but Mos forces the embryonic mitotic cycle to undergo meiosis II, thus enabling the reduction of ploidy. Thereafter, Mos remains to restrain the return to the embryonic mitotic cycle, thus preventing parthenogenetic development. Fertilization resets the Mos-dependent detour of the cell cycle, leading to the recovery of the embryonic mitotic cycle. Thus, Mos is a key coordinator of meiotic/mitotic conversion.
In conclusion, Mos plays a conserved role in vertebrates and invertebrates as a key coordinator that negatively controls the meiotic/mitotic conversion before and after entry into meiosis II. The ultimate biological consequence of Mos function that enables the production of haploid gametes, which is a major object of meiosis, is accomplished by diverting the cell cycle pattern from embryonic type of mitosis to meiosis II immediately after completion of meiosis I.
Acknowledgments
We thank Drs. E. Nishida for ΔN-STE11, C. Lehner for suggestion of the Drosophila mos sequence, K. Ohsumi for discussion, and M. J. Lohka for critical reading of the manuscript. This work was supported by scientific grants from the Ministry of Education, Science, Sports and Culture (to K.T. and T.K.), and from Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Corporation (to T.K.). T.K. is an investigator of the CREST research project.
Abbreviations
- MEK
mitogen-activated protein kinase/extracellular signal-regulated kinase kinase
- MAPKK
mitogen-activated protein kinase kinase
- metaII
metaphase of meiosis II
- 1-MeAde
1-methyladenine
- GST
glutathione S-transferase
- GVBD
germinal vesicle breakdown
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB040102).
References
- 1.Sagata N. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- 2.Oskarsson M, McClements W L, Blair D G, Maizel L V, Vande Woude G F. Science. 1980;207:1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- 3.Posada J, Yew N, Ahn N G, Vande Woude G F, Cooper J A. Mol Cell Biol. 1993;13:2546–2553. doi: 10.1128/mcb.13.4.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibuya E K, Ruderman J V. Mol Biol Cell. 1993;4:781–790. doi: 10.1091/mbc.4.8.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nebreda A R, Hunt T. EMBO J. 1993;12:1979–1986. doi: 10.1002/j.1460-2075.1993.tb05847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagata N. BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- 7.Sagata N, Watanabe N, Vande Woude G F, Ikawa Y. Nature (London) 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 8.Colledge W H, Carlton M B L, Udy G B, Evans M J. Nature (London) 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, Aizawa S. Nature (London) 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- 10.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude G F. Nature (London) 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 11.Sagata N, Daar I, Oskarsson M, Showalter S D, Vande Woude G F. Science. 1989;245:643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- 12.Daar I, Paules R S, Vande Woude G F. J Cell Biol. 1991;114:329–335. doi: 10.1083/jcb.114.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanki J P, Donoghue D. Proc Natl Acad Sci USA. 1991;88:5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nurse P. Nature (London) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 15.Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishimoto T. Semin Cell Dev Biol. 1998;9:549–557. doi: 10.1006/scdb.1998.0249. [DOI] [PubMed] [Google Scholar]
- 17.Kishimoto T. Dev Biol. 1999;214:1–8. doi: 10.1006/dbio.1999.9393. [DOI] [PubMed] [Google Scholar]
- 18.Picard A, Galas S, Peaucellier G, Doree M. EMBO J. 1996;15:3590–3598. [PMC free article] [PubMed] [Google Scholar]
- 19.Tachibana K, Machida T, Nomura Y, Kishimoto T. EMBO J. 1997;16:4333–4339. doi: 10.1093/emboj/16.14.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrieu A, Fisher D, Simon M-N, Doree M, Picard A. EMBO J. 1997;16:6407–6413. doi: 10.1093/emboj/16.21.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sadler K C, Ruderman J V. Dev Biol. 1998;197:25–38. doi: 10.1006/dbio.1998.8869. [DOI] [PubMed] [Google Scholar]
- 22.Okano-Uchida T, Sekiai T, Lee K, Okumura E, Tachibana K, Kishimoto T. Dev Biol. 1998;197:39–53. doi: 10.1006/dbio.1998.8881. [DOI] [PubMed] [Google Scholar]
- 23.Gotoh Y, Matsuda S, Takenaka K, Hattori S, Iwamatsu A, Ishikawa M, Kosako H, Nishida E. Oncogene. 1994;9:1891–1898. [PubMed] [Google Scholar]
- 24.Kishimoto T. Methods Cell Biol. 1986;27:379–394. doi: 10.1016/s0091-679x(08)60359-3. [DOI] [PubMed] [Google Scholar]
- 25.Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T. EMBO J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishizawa M, Okazaki K, Furuno N, Watanabe N, Sagata N. EMBO J. 1992;11:2433–2446. doi: 10.1002/j.1460-2075.1992.tb05308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher D L, T, Brassac T, Galas S, Doree M. Development (Cambridge, UK) 1999;126:4537–4546. doi: 10.1242/dev.126.20.4537. [DOI] [PubMed] [Google Scholar]
- 28.Gross S D, Schwab M S, Taieb F E, Lewellyn A L, Qian Y-W, Maller J L. Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 29.Kim S H, Li C, Maller J L. Dev Biol. 1999;212:381–391. doi: 10.1006/dbio.1999.9361. [DOI] [PubMed] [Google Scholar]
- 30.Iwabuchi M, Ohsumi K, Yamamoto T M, Sawada W, Kishimoto T. EMBO J. 2000;19:4513–4523. doi: 10.1093/emboj/19.17.4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nigg E A. BioEssays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 32.Lew D J, Kornbluth S. Curr Opin Cell Biol. 1996;8:795–804. doi: 10.1016/s0955-0674(96)80080-9. [DOI] [PubMed] [Google Scholar]
- 33.Ohsumi K, Sawada W, Kishimoto T. J Cell Sci. 1994;107:3005–3013. doi: 10.1242/jcs.107.11.3005. [DOI] [PubMed] [Google Scholar]
- 34.Murray A W, Kirschner M W. Science. 1989;246:614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- 35.Palmer A, Gavin A C, Nebreda A R. EMBO J. 1998;17:5037–5047. doi: 10.1093/emboj/17.17.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nebreda A R, Gavin A C. Science. 1999;286:1309–1310. doi: 10.1126/science.286.5443.1309. [DOI] [PubMed] [Google Scholar]
- 37.Verlhac M H, Kubiak J Z, Weber M, Geraud G, Colledge W H, Evans M J, Maro B. Development (Cambridge, UK) 1996;122:815–822. doi: 10.1242/dev.122.3.815. [DOI] [PubMed] [Google Scholar]
- 38.Choi T, Fukasawa K, Zhou R, Tessarollo L, Borror K, Resau J, Vande Woude G F. Proc Natl Acad Sci USA. 1996;93:7032–7035. doi: 10.1073/pnas.93.14.7032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araki K, Naito K, Haraguchi S, Suzuki R, Yokoyama M, Inoue M, Aizawa S, Toyoda Y, Sato E. Biol Reprod. 1996;55:1315–1324. doi: 10.1095/biolreprod55.6.1315. [DOI] [PubMed] [Google Scholar]
- 40.O'Keefe S J, Wolfes H, Kiessling A A, Cooper G M. Proc Natl Acad Sci USA. 1989;86:7038–7042. doi: 10.1073/pnas.86.18.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]