Abstract
Ceftobiprole (BAL9141) is an investigational cephalosporin with broad in vitro activity against gram-positive cocci, including enterococci. Ceftobiprole MICs were determined for 93 isolates of Enterococcus faecalis (including 16 β-lactamase [Bla] producers and 17 vancomycin-resistant isolates) by an agar dilution method following the Clinical and Laboratory Standards Institute recommendations. Ceftobiprole MICs were also determined with a high inoculum concentration (107 CFU/ml) for a subset of five Bla producers belonging to different previously characterized clones by a broth dilution method. Time-kill and synergism studies (with either streptomycin or gentamicin) were performed with two β-lactamase-producing isolates (TX0630 and TX5070) and two vancomycin-resistant isolates (TX2484 [VanB] and TX2784 [VanA]). The MICs of ceftobiprole for 50 and 90% of the isolates tested were 0.25 and 1 μg/ml, respectively. All Bla producers and vancomycin-resistant isolates were inhibited by concentrations of ≤1 and ≤4 μg/ml, respectively, at the standard inoculum concentration. Ceftobiprole MICs at a high inoculum concentration for a subset of five Bla+ E. faecalis isolates were ≤1 μg/ml. Bactericidal activity was observed against four isolates tested at concentrations as low as 1 μg/ml regardless of the production of β-lactamase or vancomycin resistance. A combination of ceftobiprole (0.5 μg/ml) and streptomycin (25 μg/ml) was synergistic against Bla+ TX0630 and TX5070. Ceftobiprole (0.5 μg/ml) plus gentamicin (10 μg/ml) was synergistic against VanB isolate TX2484 and showed enhanced killing, but not synergism, against TX2784 (VanA), despite the absence of high-level resistance to gentamicin. In conclusion, ceftobiprole exhibited good in vitro activity against E. faecalis, including Bla+ and vancomycin-resistant strains, and exhibited synergism with aminoglycosides against selected isolates.
Enterococcal infections continue to be a challenge in clinical practice due to the fact that these organisms have the ability to quickly acquire and disseminate resistance genes. The introduction of new agents into clinical practice (e.g., linezolid and daptomycin, among others) has been shortly followed by development of resistance (10). Furthermore, the treatment of certain enterococcal infections (e.g., endocarditis) requires the use of bactericidal (14) agents which decrease the choice of antimicrobials even further.
Ceftobiprole (BPR) is a novel, broad-spectrum parenteral cephalosporin with high affinities for gram-negative and gram-positive penicillin-binding proteins (PBPs), including PBP 2a from methicillin-resistant Staphylococcus aureus (MRSA) (2, 6) and PBP 2x from resistant pneumococci (5, 11). The in vitro spectrum of activity includes both gram-positive and gram-negative organisms, including S. aureus (both MRSA and MSSA isolates) (2, 7), pneumococci (including penicillin- and ceftriaxone-resistant isolates) (5), Streptococcus pyogenes and other streptococci (11), Haemophilus influenzae and Moraxella catarrhalis (including β-lactamase producers) (1), Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Serratia marcescens, Citrobacter freundii, Pseudomonas aeruginosa, and Proteus mirabilis lacking extended spectrum β-lactamases (11, 19). Against anaerobes, BPR had good in vitro activity against Propionibacterium acnes, Peptostreptococcus anaerobius, Clostridium innocuum, Finegoldia magna, Porphyromonas asaccharolytica (including a β-lactamase-producing isolate), and Porphyromonas somerae (9), although Bacteroides fragilis group isolates have been found to be resistant (9, 23).
Against enterococci, BPR was reported to have in vitro bactericidal activity against most strains of ampicillin- and vancomycin-susceptible Enterococcus faecalis, with MICs for 50 and 90% of a small collection of E. faecalis isolates tested (MIC50 and MIC90, respectively) of 0.5 and 4 μg/ml (6, 11). BPR exhibited bactericidal activity at concentrations of 4, 8, and 16 μg/ml against an ampicillin- and vancomycin-susceptible E. faecalis isolate (E. faecalis ATCC 29212) (6). Time-kill studies failed to show synergism when BPR (8 μg/ml) was combined with gentamicin (at one-fourth of the MIC) against two clinical isolates of E. faecalis (ampicillin and vancomycin susceptible) (6). BPR had a MIC90 of 8 μg/ml against ampicillin-susceptible E. faecium (11), but it lacked activity against ampicillin-resistant E. faecium (6, 11).
The objective of this work was to evaluate the in vitro activity of BPR against a larger collection of E. faecalis isolates and use time-kill curves and synergism studies (with aminoglycosides) to specifically assess BPR bactericidal activity against β-lactamase-producing (Bla+) and vancomycin-resistant isolates.
MATERIALS AND METHODS
Bacterial strains.
A total of 93 isolates of E. faecalis were included in this study. They were isolated from five different countries (the United States, Chile, Argentina, Lebanon, and Thailand), and the majority of the isolates were obtained from patients' clinical samples (mainly blood [from endocarditis and nonendocarditis patients] and urine). The collection included 17 vancomycin-resistant isolates and 15 β-lactamase-producing E. faecalis. The Bla+ isolates had previously been characterized by pulsed-field gel electrophoresis and multilocus sequence typing (17), and confirmation of β-lactamase activity was performed with nitrocefin disks following the Clinical and Laboratory Standards Institute recommendations (3). Isolates obtained from fecal samples of healthy volunteers (two isolates) and animals and from animal feed (seven isolates) and laboratory strains E. faecalis JH2-2 (12) and OG1RF (16) were also included in this study. All isolates were kept at −80°C and recovered from frozen stocks.
MIC determinations.
MICs of vancomycin, ampicillin, BPR (BAL9141), gentamicin, and streptomycin were determined by an agar dilution method on Mueller-Hinton agar II (Becton Dickinson & Company, Cockeysville, MD) as recommended by the Clinical and Laboratory Standards Institute (3). BPR was diluted in 9.9% glacial acetic acid and 1% high quality dimethyl sulfoxide as recommended by the manufacturer (Johnson & Johnson, Raritan, NJ). Susceptibilities of isolate TX5070 (a Bla+ transconjugant of E. faecalis JH2-7 [Table 1], which is a thymine auxotroph and cannot grow on Mueller-Hinton broth) (12), were performed on brain heart infusion (BHI) agar with a starting inoculum concentration of 104 CFU/spot. For selected Bla+ E. faecalis isolates, BPR and ampicillin MIC determinations were performed at a high inoculum concentration (107 CFU/ml) in Mueller-Hinton broth (Becton Dickinson & Company, Cockeysville, MD). Control strains included E. faecalis ATCC 29212, S. aureus ATCC 29213, and E. coli 25922.
TABLE 1.
E. faecalis strains used in time-kill and synergism studies
| Strain | Relevant characteristic(s) | Reference | Bla+ | MIC (μg/ml)
|
HLR to aminoglycosides
|
Synergisma | |||
|---|---|---|---|---|---|---|---|---|---|
| BPR | AMPd | VANe | GENf | STRg | |||||
| TX0630 | Blood isolate from Argentina recovered in 1990 | 4 | Yes | 0.25 | 1 | 0.5 | Yes | No | Yes |
| TX5070 | JH2-7 transconjugant resulting from mating with a bla+ strain (HH-22), thymine auxotroph | 12 | Yes | 0.125b | 8b | 8b | Yes | No | Yes |
| TX2484 | Houston blood isolate, 1994, VanB | 4 | No | 0.5 | 0.5 | >256 | No | Yes | Yes |
| TX2784 | Human fecal isolate from Spain, 1998, VanA | 18 | No | 0.25 | 0.5 | >256 | No | Yes | Noc |
With either 10 μg/ml gentamicin or 25 μg/ml streptomycin when appropriate.
Determined with BHI agar since TX5070 does not grow on Mueller-Hinton agar. E. faecalis ATCC 29212 was used as a control.
Decrease in the number of CFU per milliliter of >1 log10 but <2 log10 versus BPR at 24 h.
AMP, ampicillin.
VAN, vancomycin.
GEN, gentamicin.
STR, streptomycin.
Time-kill and synergism studies.
The bactericidal activity of BPR was evaluated by time-kill curves. Synergism studies were performed with a subinhibitory concentration of either gentamicin (10 μg/ml) or streptomycin (25 μg/ml) (14). The following E. faecalis isolates were chosen for time-kill and synergism studies (Table 1): Bla+ strains E. faecalis TX0630, a clinical blood isolate originally recovered in Argentina (4), and TX5070, a laboratory strain obtained in a mating experiment with the first ever discovered Bla+ enterococcal isolate (E. faecalis HH22, used as a donor) (15); E. faecalis JH2-7, a thymine auxotroph (12); and vancomycin-resistant, ampicillin-susceptible isolates TX2484 and TX2784. Bacteria were grown in flasks in a final volume of 20 ml of BHI broth with a starting inoculum concentration of 107 CFU/ml from an overnight culture. BPR was added at concentrations of 1 and 2 μg/ml for time-kill curve studies and 0.5 μg/ml for synergism studies, which are below the therapeutic levels achieved in humans (22). The concentrations of aminoglycosides used in the synergy studies were below the MICs for the organisms and produced no significant growth inhibition in the absence of BPR. Concentrations of 10 μg/ml gentamicin and 25 μg/ml streptomycin were found to yield the best killing activity. Viable counts were determined at 0, 4, and 24 h by plating appropriate dilutions of the cultures on BHI agar plates. Antibiotic carryover was eliminated by centrifuging 1-ml samples of the culture and resuspending the pelleted bacteria in 0.9% saline before plating. Time-kill and synergism studies were performed two to four times per strain. The level of detection was 10 CFU/ml, assuming maximum plating efficiency. Bactericidal activity was defined as a ≥3-log10 decrease in the number of CFU per milliliter between 0 and 24 h. Synergism was defined as a ≥2-log10 decrease in the number of CFU per milliliter between the combination of BPR plus an aminoglycoside (gentamicin or streptomycin) and BPR alone at 24 h, with a concentration of the aminoglycoside that did not affect the growth curve of the test organism when used alone.
RESULTS
BPR MICs.
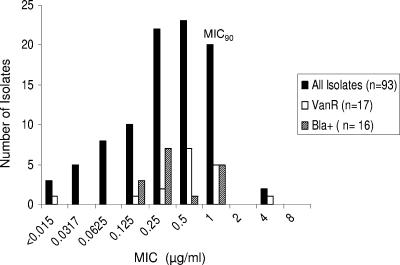
The BPR MIC distribution of all isolates, including the subgroups of vancomycin-resistant and Bla+ isolates, is shown in Fig. 1. The BPR MIC90 and MIC50 for all isolates were 1 and 0.25 μg/ml, respectively, ranging from <0.015 to 4 μg/ml. The presence of vancomycin resistance did not influence BPR susceptibility; for 94% of the vancomycin-resistant isolates, BPR MICs were ≤1 μg/ml. Among the 16 β-lactamase producers, the BPR MIC100 was 1 μg/ml. Table 2 shows the BPR MICs for a subset of five β-lactamase producers from different clonal origins at two inocula. The use of a high inoculum concentration (107 CFU/ml) of these isolates for MIC determination in broth resulted in an increase of two- to eightfold in the MIC, but all MICs were ≤2 μg/ml (Table 2). A similar increase occurred in isolates TX2484 and TX2784, which lack the β-lactamase enzyme (two- and fourfold increases in the MIC at a high inoculum concentration).
FIG. 1.
Distribution of MICs of BPR (BAL9141) for clinical isolates of E. faecalis, including vancomycin-resistant isolates. VanR, vancomycin resistant.
TABLE 2.
BPR (BAL9141) MICs against different inocula of Bla-producing E. faecalis from different clonal origins
| Strain | Clonal origin (multilocus sequence type) | BPR MIC inoculumc
|
Aminoglycoside HLR
|
||
|---|---|---|---|---|---|
| High | Standard | GENd | STRe | ||
| TX0921 | BVEa (ST-7) | 1 | 0.25 | Yes | Yes |
| TX0614 | BVE (ST-6) | 2 | 1 | Yes | Yes |
| TX0638 | BVE (ST-7) | 1 | 0.25 | Yes | Yes |
| TX0630 | ACBb (ST-9) | 1 | 0.125 | Yes | No |
| TX0645 | Unrelated | 1 | 0.25 | Yes | No |
BVE, Bla-producing and vancomycin-resistant endocarditis clone.
ACB, Argentina-Connecticut Bla-producing clone.
The standard inoculum concentration was 104 CFU/spot in agar, and the high inoculum concentration was 107 CFU/ml in broth.
GEN, gentamicin.
STR, streptomycin.
Bactericidal activity of BPR.
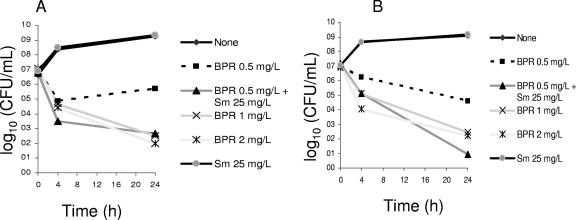
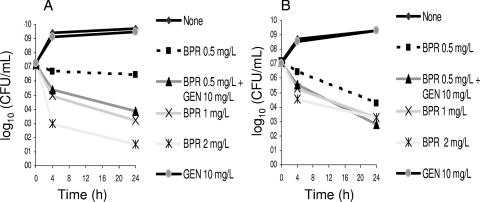
The bactericidal activity of BPR was assessed by time-kill assays against four different isolates of E. faecalis (Table 1). Figure 2 shows the in vitro activity of BPR against two Bla+ strains (TX0630 in panel A and TX5070 in panel B). Both isolates were highly resistant to gentamicin but lacked high-level resistance (HLR) to streptomycin (Table 1). Concentrations of BPR as low as 1 and 2 μg/ml were bactericidal against both Bla+ strains, decreasing the viable bacterial count (CFU per milliliter) ca. 4 log10 from the starting inoculum concentration (time zero) (Fig. 2). Figure 3 shows that BPR was also bactericidal against vancomycin-resistant isolates. For both TX2484 (a VanB isolate) and TX2784 (a VanA isolate), BPR decreased the viable counts >3 log10 CFU/ml at 24 h from the starting inoculum concentration.
FIG. 2.
Time-kill and synergism studies of BPR and streptomycin against two Bla+ isolates of E. faecalis. Panel A, TX0630; panel B, TX5070. Detection limit, 10 CFU/ml. Sm, streptomycin.
FIG. 3.
Time-kill and synergism studies of BPR and gentamicin against two vancomycin-resistant isolates of E. faecalis. Panel A, TX2484 (VanB); panel B, TX2784 (VanA). Detection limit, 10 CFU/ml. GEN, gentamicin.
Synergism between BPR and aminoglycosides.
Synergistic aminoglycoside (either gentamicin or streptomycin) activity was evaluated for two Bla+ and two vancomycin-resistant E. faecalis isolates. For the Bla+ strains (which exhibit HLR to gentamicin but not to streptomycin), addition of streptomycin (25 μg/ml) to BPR was synergistic (Fig. 2). The decrease in viable counts (CFU) at 24 h was >2 log10 (ca. 3 log10 for TX0630 and ca. 4 log10 for TX5070 compared with BPR alone) (Fig. 2A and B). Similarly, the combination of BPR and gentamicin (10 μg/ml) was synergistic against vancomycin-resistant isolate TX2484 (Fig. 3A) (reduction of ca. 2.5 log10 CFU/ml when gentamicin was added compared to BPR alone). Addition of gentamicin (10 μg/ml) decreased counts of isolate TX2784 by less than 2 log10 CFU/ml at 24 h (Fig. 3B). The lack of synergism in strain TX2784 was observed at BPR concentrations of 0.25 and 1 μg/ml and gentamicin concentrations of 5 and 8 μg/ml (data not shown).
DISCUSSION
BPR is a novel cephalosporin that has been shown to be active against gram-positive organisms (including MRSA) and also maintains the spectrum of extended-spectrum cephalosporins against gram-negative bacteria (2, 11). The basis for BPR's potent activity against many organisms is its high affinity for PBPs (including PBP 2a of MRSA) and stability against hydrolysis by β-lactamases (11). Against enterococci, BPR displays properties unique among the cephalosporins, since it has good activity against isolates of E. faecalis (6). A previous study (11) showed that the MIC90 for a collection of 14 clinical isolates of ampicillin-susceptible E. faecalis was 4 μg/ml. The results of our work support the potent in vitro activity of BPR against E. faecalis from different geographical, clinical, and host origins (MIC90 of 1 μg/ml for our isolates). Furthermore, our findings confirm that susceptibility to BPR in E. faecalis is not affected by the presence of vancomycin resistance or by β-lactamase production in enterococci. Although a modest inoculum concentration effect was seen with BPR for both Bla+ and Bla− isolates, the MIC remained ≤2 μg/ml.
We also evaluated the bactericidal activity of BPR against four strains of E. faecalis exhibiting either vancomycin resistance (VanA and VanB phenotypes, Table 1) or ampicillin resistance due to the production of the β-lactamase enzyme. BPR was bactericidal in time-kill studies against all of the strains at concentrations as low as 1 μg/ml. Pharmacokinetic studies (21, 22) have shown that single infusions of 750 mg of BPR medocaril (a BAL9141 prodrug) led to mean plasma drug concentrations above 4 μg/ml for approximately 7 h (22) (the MIC at which 100% of our E. faecalis isolates were inhibited, including Bla+ isolates at a high inoculum concentration). Our results support the fact that BPR would likely exhibit bactericidal activity against E. faecalis at the dose proposed. Our findings are also in agreement with those of Deshpande et al. (6), who showed that BPR (at concentration of 4, 8, and 16 μg/ml) was bactericidal against E. faecalis ATCC 29212 and two additional E. faecalis clinical isolates (tested at 8 μg/ml). Bactericidal activity against E. faecalis is a characteristic of BPR that is unique among the cephalosporins and is likely to be due to the high affinity for the enterococcal PBPs. BPR has been shown to exhibit increased affinity for PBPs of several gram-positive organisms (particularly PBP 2a of MRSA and S. epidermidis) (11) compared with other β-lactams. Moreover, it has been shown that BPR acylates PBP 2a more rapidly than other β-lactam antibiotics and forms a more stable acyl-enzyme complex through a unique mode of interaction with the protein (11).
Another important feature of BPR is its β-lactamase stability. The production of this enzyme is rare among clinical isolates of E. faecalis, but its presence compromises the use of the most effective antienterococcal β-lactams (e.g., ampicillin). The enterococcal β-lactamase is identical to the staphylococcal class A enzyme encoded by the blaZ gene (15), and BPR is a poor substrate for these enzymes, which explains its excellent activity against β-lactamase-producing E. faecalis.
The combination of β-lactams and aminoglycosides has been widely used in the treatment of enterococcal infections that require bactericidal therapy for optimal cure rates (e.g., endocarditis) (14). Previously, no synergistic activity was observed for two strains of E. faecalis when using concentrations of BPR of 8 μg/ml and gentamicin at one-fourth of the MIC for the strains (6). In contrast with these data, we were able to demonstrate synergism in three out of four isolates of E. faecalis when using a BPR concentration of 0.5 μg/ml (which is equal to or slightly higher than the MIC). An explanation for this discrepancy is that, at concentrations as high as 8 μg/ml (as used by Deshpande et al. [6]), the killing effect of BPR is so marked that it may mask the effect of the aminoglycoside. Consistent with this hypothesis is the fact that we were unable to show any synergism when using BPR concentrations of 1, 2, and 4 μg/ml (data not shown).
Synergistic activity was evident in the presence of β-lactamase in different host backgrounds. BPR combined with streptomycin at a concentration of 25 μg/ml each exhibited synergism against both TX0630 (a Bla+ clinical strain) and TX5070 (a Bla+ laboratory strain). Both isolates have HLR to gentamicin but not to streptomycin. Similarly, the combination of BPR (0.5 μg/ml) and gentamicin (10 μg/ml) was synergistic against one vancomycin-resistant (VanB) isolate of E. faecalis. However, although a decrease in viable counts caused by the combination of BPR and gentamicin compared to BPR alone was observed at 24 h in isolate TX2484 (a VanA human fecal isolate from Spain), the reduction in the number of CFU per milliliter did not reach the cutoff for synergism (>1 log10 but ≤2 log10). These results indicate that, with selected isolates of Bla+ or vancomycin-resistant E. faecalis, a combination of BPR and an aminoglycoside could be potentially useful in clinical settings where bactericidal therapy is important. In vivo studies are of paramount importance to clarify this issue.
As opposed to E. faecalis isolates, E. faecium isolates have developed different strategies for β-lactam resistance which include hyperproduction of PBP 5, which has a low affinity for β-lactams and is capable of substituting for the functions of β-lactam-susceptible PBPs (8), and by introducing amino acid substitutions into the penicillin-binding domain of PBP 5 (13, 20, 24). Previous studies (6) have shown that BPR had no activity against ampicillin-resistant E. faecium, indicating that it is likely that the affinity of BPR for PBP 5 of E. faecium is low and therefore not clinically useful for the treatment of ampicillin-resistant E. faecium infections.
In conclusion, we demonstrated that BPR has potent in vitro activity against the largest collection of E. faecalis isolates tested to date. The activity was not affected by vancomycin resistance or production of β-lactamase, and synergism with aminoglycosides can be achieved for selected strains. Therefore, BPR emerges as a promising agent with potential for future use to treat Bla+ and vancomycin-resistant E. faecalis infections.
Acknowledgments
This study was supported by a grant from Johnson & Johnson Pharmaceutical Research and Development, L.L.C. D.P. was partially funded by a graduate scholarship from The Instituto Colombiano para el Desarrollo de la Ciencia y Tecnología, Francisco José de Caldas, Colciencias.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Bogdanovich, T., C. Clark, L. Ednie, G. Lin, K. Smith, S. Shapiro, and P. C. Appelbaum. 2006. Activities of ceftobiprole, a novel broad-spectrum cephalosporin, against Haemophilus influenzae and Moraxella catarrhalis. Antimicrob. Agents Chemother. 50:2050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambers, H. F. 2005. Evaluation of ceftobiprole in a rabbit model of aortic valve endocarditis due to methicillin-resistant and vancomycin-intermediate Staphylococcus aureus. Antimicrob. Agents Chemother. 49:884-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2005. Performance standard for antimicrobial susceptibility testing; 15th informational supplement M100-S15. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 4.Coque, T. M., and B. E. Murray. 1995. Identification of Enterococcus faecalis strains by DNA hybridization and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:3368-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies, T. A., W. Shang, and K. Bush. 2006. Activities of ceftobiprole and other beta-lactams against Streptococcus pneumoniae clinical isolates from the United States with defined substitutions in penicillin-binding proteins PBP 1a, PBP 2b, and PBP 2x. Antimicrob. Agents Chemother. 50:2530-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deshpande, L. M., and R. N. Jones. 2003. Bactericidal activity and synergy studies of BAL9141, a novel pyrrolidinone-3-ylidenemethyl cephem, tested against streptococci, enterococci and methicillin-resistant staphylococci. Clin. Microbiol. Infect. 9:1120-1124. [DOI] [PubMed] [Google Scholar]
- 7.Entenza, J. M., P. Hohl, I. Heinze-Krauss, M. P. Glauser, and P. Moreillon. 2002. BAL9141, a novel extended-spectrum cephalosporin active against methicillin-resistant Staphylococcus aureus in treatment of experimental endocarditis. Antimicrob. Agents Chemother. 46:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontana, R., M. Ligozzi, F. Pittaluga, and G. Satta. 1996. Intrinsic penicillin resistance in enterococci. Microb. Drug Resist. 2:209-213. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. A. Warren, K. L. Tyrrell, and H. T. Fernandez. 2006. In vitro activity of ceftobiprole against aerobic and anaerobic strains isolated from diabetic foot infections. Antimicrob. Agents Chemother. 50:3959-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales, R. D., P. C. Schreckenberger, M. B. Graham, S. Kelkar, K. DenBesten, and J. P. Quinn. 2001. Infections due to vancomycin-resistant Enterococcus faecium resistant to linezolid. Lancet 357:1179. [DOI] [PubMed] [Google Scholar]
- 11.Hebeisen, P., I. Heinze-Krauss, P. Angehrn, P. Hohl, M. G. Page, and R. L. Then. 2001. In vitro and in vivo properties of Ro 63-9141, a novel broad-spectrum cephalosporin with activity against methicillin-resistant staphylococci. Antimicrob. Agents Chemother. 45:825-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ligozzi, M., F. Pittaluga, and R. Fontana. 1996. Modification of penicillin-binding protein 5 associated with high-level ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 40:354-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moellering, R. C., Jr., C. Wennersten, and A. N. Weinberg. 1971. Synergy of penicillin and gentamicin against enterococci. J. Infect. Dis. 124(Suppl.):S207-S209. [DOI] [PubMed] [Google Scholar]
- 15.Murray, B. E., and B. Mederski-Samaroj. 1983. Transferable beta-lactamase: a new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J. Clin. Investig. 72:1168-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray, B. E., K. V. Singh, R. P. Ross, J. D. Heath, G. M. Dunny, and G. M. Weinstock. 1993. Generation of restriction map of Enterococcus faecalis OG1 and investigation of growth requirements and regions encoding biosynthetic function. J. Bacteriol. 175:5216-5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nallapareddy, S. R., H. Wenxiang, G. M. Weinstock, and B. E. Murray. 2005. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J. Bacteriol. 187:5709-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robredo, B., K. V. Singh, F. Baquero, B. E. Murray, and C. Torres. 2000. Vancomycin-resistant enterococci isolated from animals and food. Int. J. Food Microbiol. 54:197-204. [DOI] [PubMed] [Google Scholar]
- 19.Rouse, M. S., M. M. Hein, P. Anguita-Alonso, J. M. Steckelberg, and R. Patel. 2006. Ceftobiprole medocaril (BAL5788) treatment of experimental Haemophilus influenzae, Enterobacter cloacae, and Klebsiella pneumoniae murine pneumonia. Diagn. Microbiol. Infect. Dis. 55:333-336. [DOI] [PubMed] [Google Scholar]
- 20.Rybkine, T., J. L. Mainardi, W. Sougakoff, E. Collatz, and L. Gutmann. 1998. Penicillin-binding protein 5 sequence alterations in clinical isolates of Enterococcus faecium with different levels of beta-lactam resistance. J. Infect. Dis. 178:159-163. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt-Hoffmann, A., L. Nyman, B. Roos, M. Schleimer, J. Sauer, N. Nashed, T. Brown, A. Man, and E. Weidekamm. 2004. Multiple-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmitt-Hoffmann, A., B. Roos, M. Schleimer, J. Sauer, A. Man, N. Nashed, T. Brown, A. Perez, E. Weidekamm, and P. Kovacs. 2004. Single-dose pharmacokinetics and safety of a novel broad-spectrum cephalosporin (BAL5788) in healthy volunteers. Antimicrob. Agents Chemother. 48:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wootton, M., K. E. Bowker, H. A. Holt, and A. P. MacGowan. 2002. BAL9141, a new broad-spectrum pyrrolidinone cephalosporin: activity against clinically significant anaerobes in comparison with 10 other antimicrobials. J. Antimicrob. Chemother. 49:535-539. [DOI] [PubMed] [Google Scholar]
- 24.Zorzi, W., X. Y. Zhou, O. Dardenne, J. Lamotte, D. Raze, J. Pierre, L. Gutmann, and J. Coyette. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948-4957. [DOI] [PMC free article] [PubMed] [Google Scholar]