Abstract
Various inhibitors of metallo-β-lactamases have been reported; however, none are effective for all subgroups. Those that have been found to inhibit the enzymes of subclass B2 (catalytically active with one zinc) either contain a thiol (and show less inhibition towards this subgroup than towards the dizinc members of B1 and B3) or are inactivators behaving as substrates for the dizinc family members. The present work reveals that certain pyridine carboxylates are competitive inhibitors of CphA, a subclass B2 enzyme. X-ray crystallographic analyses demonstrate that pyridine-2,4-dicarboxylic acid chelates the zinc ion in a bidentate manner within the active site. Salts of these compounds are already available and undergoing biomedical testing for various nonrelated purposes. Pyridine carboxylates appear to be useful templates for the development of more-complex, selective, nontoxic inhibitors of subclass B2 metallo-β-lactamases.
β-Lactam antibiotics have long been used to fight bacterial infections in medicine and agriculture. Bacteria have evolved to hydrolyze β-lactams, thus rendering them ineffective by the production of β-lactamases (16, 19, 31). Metallo-β-lactamases (MBLs) constitute one of four classes of β-lactamases, namely, class B. However, unlike the other classes (A, C, and D), which all contain a nucleophilic serine residue in their active site, the MBLs utilize zinc to enable hydrolysis (1, 4, 6) of all the β-lactam antibiotics (with the exception of monobactams) (31). There are three subclasses of MBLs, B1, B2, and B3, which differ in their zinc dependency (17). Subclass B1 enzymes (such as BcII of Bacillus cereus, IMP-1, VIM-2, and VIM-4) can employ one or two zinc ions at their active site for full activity, and subclass B3 enzymes (L1 enzyme produced by Stenotrophomonas maltophilia and FEZ-1 of Legionella gormanii) contain two zinc ions (35, 36), but while subclass B2 enzymes (such as CphA from Aeromonas hydrophila) are active with one zinc ion, they are inhibited upon binding of a second zinc ion (22).
The heterogeneity of the β-lactamases has proved problematic in the search for a generic inhibitor. All the clinically used inhibitors are effective only against the active-site serine β-lactamases as they are hydrolyzed by MBLs. Various inhibitors of MBLs have been reported; in general, their side chains bind in a predominantly hydrophobic pocket while their functional groups interact with both zinc ions, e.g., in the case of thiol inhibitors, the sulfur displaces the hydroxide ion that bridges the two zinc ions (21, 34, 40). The search for inhibitors has understandably focused on the plasmid-encoded IMP and VIM variant MBLs (26, 29, 39). However, with respect to subclass B2 enzymes, the reported inhibitors either have exhibited a lower efficiency than that shown towards the other subgroups (21, 34) or were products of substrate hydrolysis acting as irreversible inactivators (44).
Subclass B2, which contains CphA from Aeromonas hydrophila, is characterized by a narrow specificity profile (limited to the efficient hydrolysis of carbapenems) and a noncompetitive inhibition by a second zinc ion binding to a low-affinity site (22, 24). We are interested in identifying MBL inhibitor templates that can be developed into broad-spectrum inhibitors of the MBLs. Pyridine dicarboxylates are known to be inhibitors of monometallic enzymes (25). Here, we reveal that CphA is competitively inhibited by 2-picolinic acid and one of its derivatives, pyridine-2,4-dicarboxylic acid (2,4-PDCA). The crystal structure of the latter in complex with CphA-Zn(II) is described. The activities of these inhibitors were also tested against those of other enzymes, including the CphA N116H-N220G mutant, an enzyme with a broader specificity than wild-type CphA and without a significant loss of activity upon binding of a second zinc, making it similar to subclass B1 enzymes (2).
MATERIALS AND METHODS
Chemicals.
Buffers, bovine serum albumin (BSA), 2-picolinic acid, and its derivatives were obtained from Sigma-Aldrich (Steinheim, Germany) or BDH Chemicals (Poole, United Kingdom). Imipenem was obtained from Merck Sharpe and Dohme Research Laboratories (Rahway, NJ), nitrocefin was obtained from Unipath Oxoid (Basingstoke, United Kingdom), and cefotaxime was obtained from Sigma (St. Louis, MO). Dimethyl sulfoxide (DMSO) and ZnCl2 were obtained from Merck (Darmstadt, Germany).
Enzyme preparation.
IMP-1 (30), CphA (23), L1 (32, 42), FEZ-1 (33), and the CphA N116H-N220G double mutant (2) were produced and purified as described previously. BcII (35) was a kind gift from O. Jacquin (Université de Liège) and the VIM enzymes (11) from P. Lassaux (Université de Liège).
Screening of inhibitors.
Hydrolysis of nitrocefin (FEZ-1) or imipenem (all other enzymes) was monitored by following the variation in absorbance at 300 nm or 482 nm, respectively, using a Uvikon 860 spectrophotometer connected to a microcomputer via an RS232 serial interface. IMP-1, BcII, CphA, L1, and FEZ-1 enzymes were used at fixed concentrations between 0.03 and 0.7 nM. The enzyme and inhibitor (100 μM) were preincubated for 30 min at room temperature before the substrate (100 μM) was added. The activity was tested using the initial rate of three samples and given as a mean percentage of the rate without inhibitor present. The inhibitors were prepared as 10 mM stock solutions in DMSO before dilution with 20 mM HEPES buffer, pH 7.0, containing 20 μg/ml BSA (and 100 μM ZnCl2 where indicated). It was verified that the concentrations of DMSO present had no inhibitory effects and that the rate remained the same upon the addition of 1% DMSO.
Determination of the inhibition constant.
More-detailed kinetics were performed with 2-picolinic acid and 2,4-PDCA, which showed significant inhibition for the CphA MBL. The buffer and enzyme concentrations were the same as those used in the screening. Substrate concentrations varied between 20 and 200 μM at three inhibitor concentrations and in the absence of inhibitor. Cells of 2-mm or 10-mm path length were used depending on the substrate concentration. Preincubation was found to be unnecessary, and the experiments were performed at 30°C in thermostatically controlled cells. The initial rate conditions were used to study the inhibition with imipenem by using the Hanes linearization of the Henri-Michaelis equation and the KaleidaGraph 3.5 program. The competitive inhibition constant, Ki, was then calculated using the following equation (9):
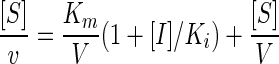 |
where [S] is substrate concentration, v is reaction rate, V is maximum velocity, and [I] is inhibitor concentration. The Ki values were determined for the CphA N116H-N220G double mutant with 2-picolinic acid and 2,4-PDCA under the same conditions as those for the wild type. The Ki value was also determined for the dizinc enzyme with cefotaxime as the substrate in 20 mM sodium cacodylate buffer, pH 6.5, containing 20 μg/ml BSA and 100 μM ZnCl2, conditions under which the enzyme is in the dizinc form (2). The enzyme dilution was performed in the described buffer but without ZnCl2, since the enzyme is not stable for a long period of time when Zn(II) is present.
pH study of the inhibition constant.
The Ki values for picolinic acid and 2,4-PDCA with CphA were determined at different pH values from pH 7 to pH 10. Experiments were performed in a mixed buffer (containing 40 mM sodium acetate, 20 mM sodium cacodylate, 20 mM MOPS [morpholinepropanesulfonic acid], 20 mM TAPS {[2-hydroxy-1,1-bis(hydroxymethyl)ethyl)amino]-1-propanesulfonic acid}, 20 mM CHES, [2-(cyclohexylamino)ethanesulfonic acid], 20 mM CAPS [3-(cyclohexylamino)-1-propanesulfonic acid], and 20 μg/ml BSA) adjusted with HCl or NaOH to the desired pH. Km and kcat for imipenem with CphA were also determined under the same conditions.
Crystallization and data collection.
Crystallization of the wild-type CphA was performed as described previously (18). In brief, crystals were grown at 8°C using the hanging drop method. The reservoir solution contained 30% (wt/vol) polyethylene glycol 8000, 0.6 M ammonium sulfate, and 100 mM Na-citrate, pH 6.5. The drops were obtained by mixing 2 μl of protein solution (∼10 mg/ml) with an equal volume of reservoir solution. The CphA-Zn(II)-2,4-PDCA complex was obtained by adding an excess of 2,4-PDCA powder directly to a drop containing CphA crystals.
Before data collection, crystals were transferred to a cryoprotectant solution (reservoir solution containing 20% [vol/vol] glycerol) and then mounted rapidly in loops and flash-cooled. X-ray data were collected in-house using a Nonius FR591 rotating anode X-ray generator coupled to a Mar Research Imagine Plate detector. Data were processed using CCP4 programs (Table 1) (7).
TABLE 1.
Data collection and refinement statistics
| Parameter | Value for CphA-2,4-PDCA complexa |
|---|---|
| Data collection | |
| Temperature (K) | 100 |
| Wavelength (Å) | 1.54179 |
| Maximum resolution (Å) | 1.86 (1.91-1.86) |
| No. of observed reflections | 260,971 |
| No. of unique reflections | 21,647 |
| Space group | C2221 |
| Unit cell (Å) | |
| a | 42.6 |
| b | 101.2 |
| c | 117.1 |
| Completeness (%) | 99.4 (99.4) |
| Multiplicity | 6.4 (6.0) |
| Rsym (%)b | 8 (39.5) |
| I/σ(I) | 7.4 (4.1) |
| Refinement statistics | |
| Rfactor/Rfree (%)c | 15.7/19.0 |
| Root mean square deviations | |
| Bond length (Å) | 0.013 |
| Bond angle (°) | 1.4 |
| No. of atoms (non-H) | |
| Protein | 1,770 |
| Zn | 1 |
| Pyridine-2,4-dicarboxylate | 12 |
| Glycerol | 6 × 3d |
| Water | 194 |
| Average B factor (Å2) | 14.2 |
Numbers in parentheses are for the highest-resolution shell.
Rsym = ∑khl | I (hkl) − <I (hkl)>/∑khl <I (hkl)>.
Rfactor = ∑khl | Fo (hkl) − Fc (hkl)/∑khl | Fo (hkl). Rfree was calculated based on 5% of the total data omitted during structure refinement.
Six molecules of glycerol per three CphA molecules.
Structure determination and refinement.
Initial phases for the CphA-Zn(II)-2,4-PDCA complex structure were generated by molecular replacement, using the structure of the wild-type CphA as a starting model (Protein Data Bank accession code 1X8G). Refinement and model building were carried out using O (27) and REFMAC (CCP4) (7). The calculation of the first (Fo − Fc) electron density map clearly showed the presence of the inhibitor molecule in the active site. 2,4-PDCA was modeled in the map after most of the protein main chain and side chain atoms and most of the water molecules were built and refined. Conformational torsion-angle restraints and charge assignments for the 2,4-PDCA molecule were obtained using CCP4i Libcheck. Refinement statistics are shown in Table 1.
Protein structure accession number.
Coordinates and structure factors have been deposited in the Protein Data Bank (3) with accession code 2GKL.
RESULTS AND DISCUSSION
Inhibitory activities of picolinic acid and its derivatives.
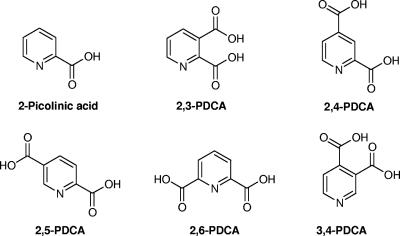
Since pyridine dicarboxylates are known inhibitors of monometallic enzymes (25), they were investigated as MBL inhibitors. Initially, 2-picolinic acid and five pyridine dicarboxylic acid derivatives with 2,3, 2,4, 2,5 2,6, and 3,4 substitution patterns (Fig. 1), each with two carboxylate groups at different positions on the ring, were screened for inhibitory activity against the subclass B1 enzymes IMP-1, BcII, VIM-2, and VIM-4, the subclass B2 enzyme CphA, and the subclass B3 enzymes L1 and FEZ-1. The residual activities are shown in Table 2. When a residual activity is below 50%, the compound is considered an inhibitor. Only inhibitors demonstrating such values will produce Ki values low enough to be considered significant.
FIG. 1.
Structures of 2-picolinic acid and its derivatives, 2,3-PDCA, 2,4-PDCA, 2,5-PDCA, 2,6-PDCA (dipicolinic acid), and 3,4-PDCA, which were tested as MBL inhibitors.
TABLE 2.
Residual activities estimated as initial rates of substrate hydrolysis of MBLs after 30 min of preincubation at room temperature with 2-picolinic acid and its derivativesa
| Enzyme | Residual activity (%) in the presence of:
|
||||||
|---|---|---|---|---|---|---|---|
| No inhibitor | 2,3-PDCA | 2,4-PDCA | 2,5-PDCA | 2,6-PDCA | 3,4-PDCA | 2-Picolinic acid | |
| IMP-1 with no added Zn | 100 | 89 ± 2 | 94 ± 4 | 99 ± 4 | 2 ± 4 | 91 ± 5 | 31 ± 8 |
| IMP-1 | 100 | 67 ± 3 | 78 ± 3 | 81 ± 2 | 72 ± 2 | 88 ± 7 | 54 ± 6 |
| BcII | 100 | 98 ± 4 | 97 ± 3 | 99 ± 2 | 95 ± 3 | 97 ± 3 | 95 ± 5 |
| VIM-2 | 100 | 106 ± 6 | 98 ± 5 | 97 ± 3 | 96 ± 8 | 92 ± 3 | 80 ± 2 |
| VIM-4 | 100 | 73 ± 3 | 90 ± 1 | 97 ± 2 | 88 ± 2 | 100 ± 1 | 59 ± 2 |
| CphA with no added Zn | 100 | 130 ± 10 | 38 ± 4 | 114 ± 14 | 12 + 17 | 116 ± 3 | 8 ± 18 |
| L1 | 100 | 93 ± 2 | 78 ± 2 | 94 ± 5 | 92 ± 14 | 97 ± 2 | 29 ± 13 |
| FEZ-1 | 100 | 62 ± 4 | 65 ± 6 | 80 ± 11 | 77 ± 6 | 93 ± 8 | 62 ± 2 |
The reporter substrates used were nitrocefin (FEZ-1) and imipenem (all other enzymes) at a concentration of 100 μM. Residual activity was tested in the presence of 100 μM inhibitor. Values are means ± standard deviations.
2,6-PDCA (dipicolinic acid) is a known zinc chelator which has been used in the identification (28) and characterization (15, 30) of metalloenzymes, including the class B β-lactamases. The strong zinc chelation ability of 2,6-PDCA is consistent with the large increase in activity seen upon the addition of excess (100 μM) ZnCl2 to the IMP-1 assay in the presence of 2,6-PDCA. It seems likely that in the absence of excess zinc, 2,6-PDCA chelates zinc so strongly that it depletes zinc from the enzyme, thereby inactivating it. The large disparity between residual activities with and without added zinc as tested on IMP-1 was not seen for any of the other pyridine dicarboxylates. In these cases, the position of the second carboxylic group on the pyridine ring limits the ligand to less potent bidentate metal chelation. However, the compounds are able to bind the active-site zinc, causing enzyme inhibition if space and geometry allow.
With the exception of the inhibition of CphA by 2,4-PDCA, the dicarboxylic compounds showed little inhibitory activity against any of the MBLs. CphA was slightly activated by the presence of some compounds, possibly due to their ability to chelate free zinc which inhibits the enzyme (22). 2-Picolinic acid showed inhibitory activity against all the MBLs tested, to various degrees. The activity was greatest for CphA; this may reflect the relatively tight active site of this enzyme, as highlighted by crystallographic studies (18).
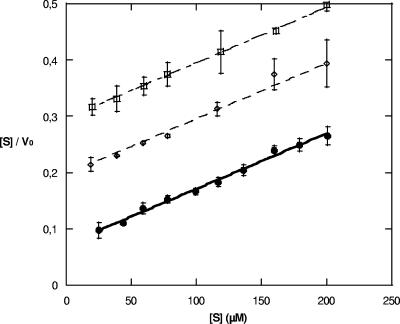
Further investigation of CphA inhibition by 2-picolinic acid and the pyridine dicarboxylates by using the Hanes linearization revealed that they act as competitive inhibitors (Fig. 2 and Table 3). The Ki values indicate that 2,4-PDCA is the best inhibitor and also the most specific for CphA. 2-Picolinic acid did not show the same selectivity for subclass B2 but showed a greater potency across the other MBLs tested (Table 2). The least effective inhibitor with respect to CphA was 3,4-PDCA (Table 3), which is the only compound tested that was unable to use the nitrogen present to chelate the zinc bidentately.
FIG. 2.
Hanes linearization for the inhibition of CphA by 2,4-PDCA at 0, 10, and 20 μM 2,4-PDCA, depicted by graphs of filled circles, open diamonds, and open squares, respectively.
TABLE 3.
Competitive inhibition constant Ki of 2-picolinic acid and its derivatives for CphA and the N116H-N220G double mutanta
| Inhibitor |
Ki (μM)
|
|
|---|---|---|
| Wild type | N116H-N220G mutant | |
| 2,3-PDCA | 200 ± 20 | ND |
| 2,4-PDCA | 4.5 ± 0.5 | 28 ± 4 (35 ± 5) |
| 2,5-PDCA | 420 ± 10 | ND |
| 3,4-PDCA | 1,100 ± 40 | ND |
| 2-Picolinic acid | 5.7 ± 0.9 | 13 ± 2 (NA) |
Ki values were found using 100 μM imipenem. Values are means ± standard deviations. The data shown in parentheses are those obtained with the dizinc form. ND, not determined; NA, not applicable (see text).
pH study.
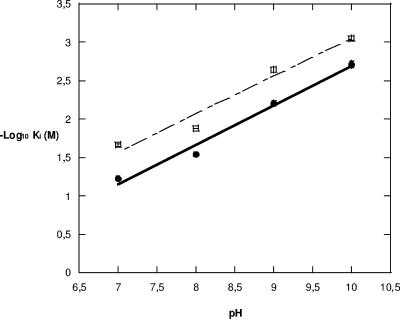
The Ki values increased for both 2-picolinic acid and 2,4-PDCA in the mixed buffer at pH 7 with respect to those determined for sodium cacodylate at pH 7. This effect was more pronounced for 2-picolinic acid, bearing one carboxylate group, as its Ki value is increased approximately eightfold when the buffer concentration is increased seven times whereas 2,4-PDCA shows a fourfold increase only. The differences in Ki values may be due to a shielding effect on the electrostatic interactions between Zn(II) and the inhibitors (5). The Ki values for 2-picolinate and 2,4-PDCA were also determined at pH 8, pH 9, and pH 10. The results (Table 4) indicate that Ki increases with increasing pH values, suggesting that as pH increases the positive charge of the zinc ion is shielded by the increasing number of hydroxide ions present (5), thus decreasing the attraction of the inhibitor to the enzyme's active site. Unfortunately, it was not possible to obtain Ki values at pH 6 and below. When the enzyme was diluted in pH 6 buffer at 4°C, the triplicate points showed that a significant decrease in activity occurred for each subsequent point. This was tested over a longer period of time and showed that the enzyme was unstable at pH 6 (data not shown). To counter the problem of instability, the enzyme was kept at pH 7 and added to the reaction mix, which was at pH 6. However, the initial rate produced was not linear at any time, so the activity was not found. The relationship between pH and pKi (Fig. 3) seems to be linear and interestingly affects 2,4-PDCA and 2-picolinic acid to the same extent, as highlighted by the parallel nature of the graphs.
TABLE 4.
pH dependence of the Ki values for 2,4-PDCA and 2-picolinic acid with CphA by using imipenem as the reporter substrate at a final concentration of 100 μM
| Inhibitor |
Ki (μM) at pHa:
|
|||
|---|---|---|---|---|
| 7.0 | 8.0 | 9.0 | 10.0 | |
| 2,4-PDCA | 17 ± 1 | 35 ± 1 | 160 ± 10 | 520 ± 50 |
| 2-Picolinic acid | 46 ± 2 | 76 ± 1 | 440 ± 40 | 1,100 ± 80 |
Values are means ± standard deviations.
FIG. 3.
pH dependence of the log10 of the competitive inhibition constant Ki with 2,4-PDCA and 2-picolinic acid, depicted by graphs of filled circles and open squares, respectively.
Table 5 shows the Km and kcat values of CphA with imipenem in the pH range 7 to 10. The catalytic efficiency of the enzyme (kcat/Km) decreased with increasing pH values across the entire range. At pH 10, the stability of imipenem was such that the rate of spontaneous hydrolysis was so high that the individual kcat and Km values were unreliable and only the value of kcat/Km was considered accurate. The decrease in the catalytic efficiency is explained by the fact that the decrease in kcat is more pronounced than the concomitant decrease in Km. It is not known how the shielding effect discussed previously affects the constants that make up the Km value; hence, it is able to decrease while the pH increases.
TABLE 5.
pH dependence of the kinetic constants Km and kcat for wild-type CphA by using the substrate imipenema
| pH | Km (μM) | kcat (s−1) | kcat/Km (μM−1 s−1) |
|---|---|---|---|
| 7.0 | 120 ± 10 | 210 ± 8 | 1.8 |
| 8.0 | 83 ± 6 | 140 ± 4 | 1.7 |
| 9.0 | 63 ± 3 | 49 ± 1 | 0.78 |
| 10.0 | ND | ND | 0.44 |
Values are means ± standard deviations. ND, not determined (see text).
CphA N116H-N220G double mutant.
Ki values for 2-picolinate and 2,4-PDCA were also measured with the CphA N116H-N220G double mutant (Table 3), which displays some subgroup B1 characteristics, including the binding of two zinc ions without the inhibition of catalysis. The N116H-N220G mutant was inhibited by both 2-picolinic acid and 2,4-PDCA, as was the wild type. However, the mutations, which broaden the substrate profile of CphA, reversed the potency of the two inhibitors so that the mutant behaved as B1 (and B3) enzymes, which are more inhibited by 2-picolinic acid than by 2,4-PDCA.
The Ki values for the dizinc species of the N116H-N220G mutant with 2,4-PDCA cannot be compared with those for the monozinc species, as the experiments were performed under different conditions. However, the result is interesting since it suggests that 2,4-PDCA is specific for CphA rather than for a monozinc enzyme. A different inhibitory profile was observed for 2-picolinic acid. It was unable to inhibit the dizinc form of the N116H-N220G mutant, showing no inhibition until the inhibitor concentration was above the zinc concentration. Above a concentration of 100 μM, inhibition occurred with 2-picolinic acid but it was not competitive, as the Hanes linearization graphs were not parallel (data not shown), suggesting that a mix of mono- and dizinc forms were produced and that the decrease in activity observed with the substrate cefotaxime was due to the decrease in activity which occurs upon the removal of a zinc ion from the dizinc form. At similar zinc concentrations, when imipenem was used as the substrate, an increase in the rate of hydrolysis was seen. Again, this suggests that the monozinc form is being produced since the hydrolysis of imipenem is inhibited upon the binding of a second zinc form (2).
2,4-PDCA-CphA complex structure.
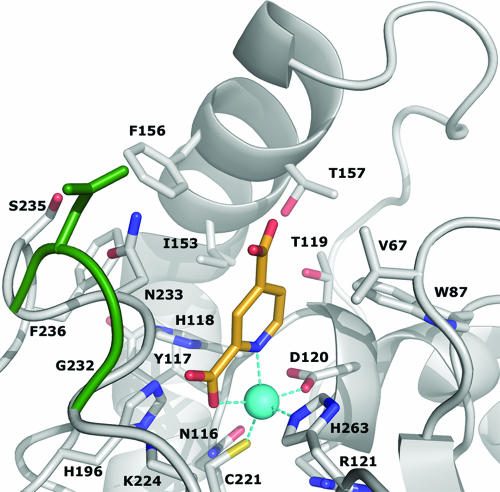
To investigate the structural basis of the inhibition of CphA by 2,4-PDCA, crystals of the inhibitor complexed to the CphA enzyme were produced. The refined CphA-Zn(II)-2,4-PDCA complex structure comprises 227 protein residues, 1 zinc ion, 1 2,4-PDCA, 3 glycerols, and 194 water molecules (Table 1). The protein structure of CphA in the complex is very close to that of the wild-type protein (18), with a root mean square deviation between the 227 Cα atoms of 0.15 Å. Superposition of the two structures shows that the binding of the inhibitor leads to the movement of the Gly232-Asn233 loop located at the active site entrance so that it encloses the active site (Fig. 4).
FIG. 4.
Active site of CphA-Zn(II) in complex with the pyridine-2,4-dicarboxylate inhibitor (carbon atoms colored in orange). The conformational change upon inhibitor binding is represented by superimposition of the wild-type Gly232 and Asn233 residues (green). The zinc ion is represented as a blue sphere. This figure was prepared using the program PYMOL.
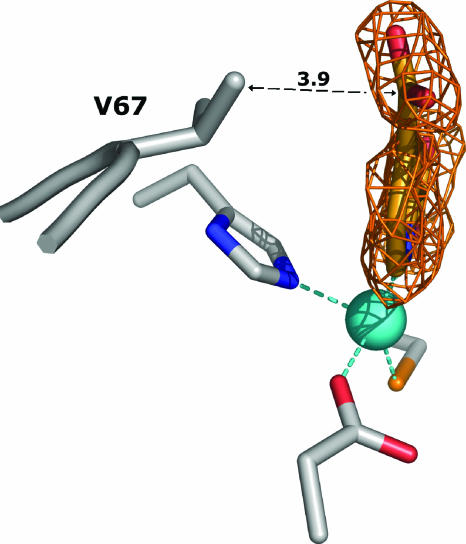
The structure of the CphA-Zn(II)-2,4-PDCA complex shows that the 2,4-PDCA inhibitor binds to the catalytic zinc ion, acts as an NO-chelating ligand, and interacts with the enzyme active site with both electrostatic and hydrophobic contacts (Fig. 4). The zinc ion is coordinated to five ligands: a triad of residues, Asp120-Cys221-His263, the 2,4-PDCA pyridyl nitrogen (N-1), and one oxygen of the carboxylate group in position 2 (O-22). The refined Zn-to-N-1 and Zn-to-O-22 distances are 2.2 and 2.3 Å, respectively. The zinc-bound 2-carboxylate group is in a position to form hydrogen bonds with the side chain of Lys224 and the Asn233 backbone nitrogen and is coplanar with the pyridine ring. In contrast, the 4-carboxylate group is slightly out of plane [χ (C-3-C-4-C-41-O-42) = 23.6°] (Fig. 5). The distance of 3.7 Å between Val67 CG2 and the carboxylate oxygen (O-42) may explain the slight rotation.
FIG. 5.
Difference electron density map (Fcomplex − Fwt) at contoured 2σ (orange) corresponding to the area of the complex structure where the pyridine-2,4-dicarboxylate inhibitor was modeled. (Fcomplex and Fwt are the structure factor amplitudes of the CphA crystal soaked in solution containing 2,4-PDCA and of the uncomplexed crystals, respectively.) The final refined coordinates of the inhibitor molecule, zinc ion, and D120, C221, H263, and V67 side chains have been superimposed. The distance between V67 (CG2) and 2,4-PDCA (C-41) is indicated in angstroms.
In addition to the four strong electrostatic interactions described above, three hydrophobic contacts contribute to the stabilization of the inhibitor in the active site: (i) Asn233 CB and C-3 (3.7 Å), (ii) Trp87 CH2 and C-5 and C-6 (3.7 Å for both), and (iii) Val67 CG2 and C-41 (3.9 Å). (The carboxylic acids' carbon atoms are numbered 21 and 41.)
The positions of the groups on the 2,4-PDCA pyridine ring may explain its specificity for CphA. The electron-withdrawing effect of a second carboxylate group in position 4 may lower the chelation strength but does not cause a decrease in Ki, probably because of the hydrophobic interaction between the carboxylate group and the Val67 residue. Additionally, the 4-carboxylate group points towards the solvent, interacting with a network of water molecules (not shown). This may (i) reduce the energy of desolvation required for the transfer of 2,4-PDCA from the aqueous buffer to the CphA-Zn(II) active site and therefore contribute to the potency of the inhibitor and (ii) participate in the overall stabilization of the CphA-Zn(II)-2,4-PDCA complex by additional enzyme/ligand interactions through hydrogen bonding via water molecules.
Finally, 2-picolinic acid and 2,4-PDCA have similar Ki values (Table 3), 5.7 and 4.3 μM, respectively. Both molecules bear the same pyridyl nitrogen and C-2 carboxylate functions, which may be involved in the same four strong electrostatic interactions described above. Moreover, superimposition of 2,3-PDCA and 2,5-PDCA with 2,4-PDCA in the CphA-Zn(II)-2,4-PDCA complex structure shows that Asn233 and Trp87 hinder the binding of 2,3-PDCA and 2,5-PDCA, respectively, consistent with their respective Ki values (420 and 200 μM) (Table 3). Based on the CphA-Zn(II)-2,4-PDCA complex structure, derivatization of the 2-picolinic acid molecule at the C-4 position to improve inhibition can be envisaged. MBLs of subclasses B1 and B3 are able to undergo dynamic conformational changes upon substrate binding, i.e., in order to accommodate the hydrophobic side chain of penicillins or potent inhibitors (8); therefore, derivatizations at the picolinic acid C-5 and C-6 positions also cannot be ruled out.
The comparison of the CphA-Zn(II)-2,4-PDCA complex structure with that of the reported CphA-Zn(II)-hydrolyzed biapenem complex (Protein Data Bank code 1X8I) (18) reveals two interesting analogies: (i) a similar Gly232-Asn233 loop closure upon binding of 2,4-PDCA and hydrolyzed biapenem and (ii) analogous positions for the zinc-bound N-1 and 2-carboxylate group of 2,4-PDCA and for the zinc-bound N-4 and 3-carboxylate group of hydrolyzed biapenem.
Conclusion.
Although the compounds analyzed in this study do not give inhibition constants low enough for medicinal studies, they may provide the basis for a new template for MBL inhibition. Previous inhibition studies of MBLs have identified many thiol-containing inhibitors that are generally less potent against subgroup B2 than against B1 and B3 (21, 34, 40). In this study, a different chelating group has been found, forming part of commercially available compounds. 2,4-PDCA is currently being investigated as an inhibitor of Fe(II)-dependent oxygenases involved in collagen biosynthesis and transcriptional regulation (10, 20, 38, 41). 2-Picolinic acid is the body's natural zinc chelator that helps zinc absorption (12, 13). It has already been tested as a dietary supplement and is currently used in salt form with chromium as a muscle enhancer (14, 37, 43). The lower Ki values for these two compounds identified them as the most potent inhibitors, and the reasons for their success rather than for any of the other pyridine carboxylate compounds were found by examining the crystal structure of the CphA-Zn(II)-2,4-PDCA complex. The tightly packed active site also suggested that interactions occurring here might not occur in the more open and flexible B1 and B3 enzymes, explaining the specificity for subclass B2. The inhibition of both the mono- and dizinc forms of the N116H-N220G mutant by 2,4-PDCA showed that, of the compounds tested in this study, this inhibitor is not only the most successful but also the most specific to CphA. Therefore, derivatives of pyridine carboxylate compounds, and in particular 2,4-PDCA, would seem to be useful starting points for the development of more-complex, selective, nontoxic inhibitors of the metallo-β-lactamases of subclass B2.
Acknowledgments
This work was supported by the Belgian Federal Government (PAI P5/33), grants from the FNRS (FRFC grant no. 2.4.524.03 and 2.4.511.06 and Loterie Nationale 9.4538.03), the European Research Training Network (MEBEL contract HPTR-CT-2002-00264), and the targeted program COBRA, financed by the European Commission (grant no. LSHM-CT-2003-503335).
We thank AMURA for a CASE studentship to B.M.R.L.
Footnotes
Published ahead of print on 16 February 2007.
REFERENCES
- 1.Ambler, R. P. 1980. The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B 289:321-331. [DOI] [PubMed] [Google Scholar]
- 2.Bebrone, C., C. Anne, K. De Vriendt, B. Devreese, G. M. Rossolini, J. van Beeumen, J. M. Frere, and M. Galleni. 2005. Dramatic broadening of the substrate profile of the Aeromonas hydrophila CphA metallo-β-lactamase by site-directed mutagenesis. J. Biol. Chem. 280:28195-28202. [DOI] [PubMed] [Google Scholar]
- 3.Berman, H. M., J. Westbrook, Z. Feng, G. Gilliland, T. N. Bhat, H. Weissig, I. N. Shindyalov, and P. E. Bourne. 2000. The Protein Data Bank. Nucleic Acids Res. 28:235-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush, K., G. A. Jacoby, and A. A. Medeiros. 1995. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39:1211-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantor, C. R., and P. R. Schimmel. 1980. Biophysical chemistry. Part III: the behavior of biological macromolecules. W. H. Freeman and Company, New York, NY.
- 6.Carfi, A., S. Pares, E. Duee, M. Galleni, C. Duez, J. M. Frere, and O. Dideberg. 1995. The 3-D structure of a zinc metallo-β-lactamase from Bacillus cereus reveals a new type of protein fold. EMBO J. 14:4914-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaborative Computational Project. 1994. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. Sect. D 50:760-763. [DOI] [PubMed] [Google Scholar]
- 8.Concha, N. O., C. A. Janson, P. Rowling, S. Pearson, C. A. Cheever, B. P. Clarke, C. Lewis, M. Galleni, J. M. Frere, D. J. Payne, J. H. Bateson, and S. S. Abdel-Meguid. 2000. Crystal structure of the IMP-1 metallo-β-lactamase from Pseudomonas aeruginosa and its complex with a mercaptocarboxylate inhibitor: binding determinants of a potent, broad-spectrum inhibitor. Biochemistry 39:4288-4298. [DOI] [PubMed] [Google Scholar]
- 9.Cornish-Bowden, A. 2001. Fundamentals of enzyme kinetics. Portland Press Ltd., London, United Kingdom.
- 10.Derian, C. K., W. VanDusen, C. T. Przysiecki, P. N. Walsh, K. L. Bekner, R. J. Kaufman, and P. A. Friedman. 1989. Inhibitors of 2-ketoglutarate-dependent dioxygenases block aspartyl β-hydroxylation of recombinant human factor IX in several mammalian expression systems. J. Biol. Chem. 264:6615-6618. [PubMed] [Google Scholar]
- 11.Docquier, J. D., J. Lamotte-Brasseur, M. Galleni, G. Amicosante, J. M. Frere, and G. M. Rossolini. 2003. On functional and structural heterogeneity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 51:257-266. [DOI] [PubMed] [Google Scholar]
- 12.Evans, G. W., C. I. Grace, and H. J. Votava. 1975. A proposed mechanism of zinc absorption in the rat. Am. J. Physiol. 228:501-505. [DOI] [PubMed] [Google Scholar]
- 13.Evans, G. W., and P. E. Johnson. 1980. Characterization and quantitation of a zinc-binding ligand in human milk. Pediatr. Res. 14:876-880. [DOI] [PubMed] [Google Scholar]
- 14.Fox, G. N., and Z. Sabovic. 1998. Chromium picolinate supplementation for diabetes mellitus. J. Fam. Pract. 46:83-86. [PubMed] [Google Scholar]
- 15.Franceschini, N., B. Caravelli, J.-D. Docquier, M. Galleni, J.-M. Frère, G. Amicosante, and G. M. Rossolini. 2000. Purification and biochemical characterization of the VIM-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:3003-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frere, J. M. 1995. β-Lactamases and bacterial resistance to antibiotics. Mol. Microbiol. 16:385-395. [DOI] [PubMed] [Google Scholar]
- 17.Galleni, M., J. Lamotte-Brasseur, G. M. Rossolini, J. Spencer, O. Dideberg, and J.-M. Frère. 2001. Standard numbering scheme for class B β-lactamases. Antimicrob. Agents Chemother. 45:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garau, G., C. Bebrone, C. Anne, M. Galleni, J. M. Frere, and O. Dideberg. 2005. A metallo-β-lactamase enzyme in action: crystal structures of the monozinc carbapenemase CphA and its complex with biapenem. J. Mol. Biol. 345:785-795. [DOI] [PubMed] [Google Scholar]
- 19.Ghuysen, J. M. 1991. Serine β-lactamases and penicillin-binding proteins. Annu. Rev. Microbiol. 45:37-67. [DOI] [PubMed] [Google Scholar]
- 20.Hanauske-Abel, H. M. 1991. Prolyl-4-hydroxylase, a target enzyme for drug development. Design of suppressive agents and the in vitro effects of inhibitors and proinhibitors. J. Hepatol. 13(Suppl. 3):S8-S15. [DOI] [PubMed] [Google Scholar]
- 21.Heinz, U., R. Bauer, S. Wommer, W. Meyer-Klaucke, C. Papamichaels, J. Bateson, and H. W. Adolph. 2003. Coordination geometries of metal ions in d- or l-captopril-inhibited metallo-β-lactamases. J. Biol. Chem. 278:20659-20666. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Valladares, M., A. Felici, G. Weber, H. W. Adolph, M. Zeppezauer, G. M. Rossolini, G. Amicsante, J. M. Frere, and M. Galleni. 1997. Zn(II) dependence of the Aeromonas hydrophila AE036 metallo-β-lactamase activity and stability. Biochemistry 36:11534-11541. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Valladares, M., M. Galleni, J. M. Frere, A. Felici, M. Perilli, N. Franceschini, G. M. Rossolini, A. Oratore, and G. Amicosante. 1996. Overproduction and purification of the Aeromonas hydrophila CphA metallo-β-lactamase expressed in Escherichia coli. Microb. Drug Resist. 2:253-256. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Valladares, M., M. Kiefer, U. Heinz, R. P. Soto, W. Meyer-Klaucke, H. F. Nolting, M. Zeppezauer, M. Galleni, J. M. Frere, G. M. Rossolini, G. Amicosante, and H. Adolph. 2000. Kinetic and spectroscopic characterization of native and metal-substituted β-lactamase from Aeromonas hydrophila AE036. FEBS Lett. 467:221-225. [DOI] [PubMed] [Google Scholar]
- 25.Ivan, M., T. Haberberger, D. C. Gervasi, K. S. Michelson, V. Gunzler, K. Kondo, H. F. Yang, I. Sorokina, R. C. Conaway, J. W. Conaway, and G. Kaelin. 2002. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 99:13459-13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin, W., Y. Arakawa, H. Yasuzawa, T. Taki, R. Hashiguchi, K. Mitsutani, A. Shoga, Y. Yamaguchi, H. Kurosaki, N. Shibata, M. Ohta, and M. Goto. 2004. Comparative study of the inhibition of metallo-β-lactamases (IMP-1 and VIM-2) by thiol compounds that contain a hydrophobic group. Biol. Pharm. Bull. 27:851-856. [DOI] [PubMed] [Google Scholar]
- 27.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. Sect. A 47:110-119. [DOI] [PubMed] [Google Scholar]
- 28.Kimura, S., Y. Ishii, and K. Yamaguchi. 2005. Evaluation of dipicolinic acid for the detection of IMP- or VIM-type metallo-β-lactamase-producing Pseudomonas aeruginosa clinical isolates. Diagn. Microbiol. Infect. Dis. 53:241-244. [DOI] [PubMed] [Google Scholar]
- 29.Kurosaki, H., Y. Yamaguchi, T. Higashi, K. Soga, S. Matsueda, H. Yumoto, S. Misumi, Y. Yamagata, Y. Arakawa, and M. Goto. 2005. Irreversible inhibition of metallo-β-lactamase (IMP-1) by 3-(3-mercaptopropionylsulfanyl)-propionic acid pentafluorophenyl ester. Angew. Chem. Int. Ed. 44:3861-3864. [DOI] [PubMed] [Google Scholar]
- 30.Laraki, N., N. Franceschini, G. M. Rossolini, P. Santucci, C. Meunier, E. de Pauw, G. Amicosante, J. M. Frère, and M. Galleni. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matagne, A., A. Dubus, M. Galleni, and J. M. Frere. 1999. The β-lactamase cycle: a tale of selective pressure and bacterial ingenuity. Nat. Prod. Rep. 16:1-19. [DOI] [PubMed] [Google Scholar]
- 32.Mercuri, P. S. 2002. Ph.D thesis. Université de Liège, Liège, Belgium.
- 33.Mercuri, P. S., F. Bouillenne, L. Boschi, J. Lamotte-Brasseur, G. Amicosante, B. Devreese, J. van Beeumen, J.-M. Frère, G. M. Rossolini, and M. Galleni. 2001. Biochemical characterization of the FEZ-1 metallo-β-lactamase of Legionella gormanii ATCC 33297T produced in Escherichia coli. Antimicrob. Agents Chemother. 45:1254-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollard, C., C. Moali, C. Papamicael, C. Damblon, S. Vessilier, G. Amicosante, C. J. Schofield, M. Galleni, J. M. Frere, and G. C. Roberts. 2001. Thiomandelic acid, a broad spectrum inhibitor of zinc β-lactamases: kinetic and spectroscopic studies. J. Biol. Chem. 276:45015-45023. [DOI] [PubMed] [Google Scholar]
- 35.Paul-Soto, R., R. Bauer, J. M. Frere, M. Galleni, W. Meyer-Klaucke, H. Nolting, G. M. Rossolini, D. de Seny, M. Hernandez-Valladares, M. Zeppezauer, and H. W. Adolph. 1999. Mono- and binuclear Zn2+-β-lactamase. Role of the conserved cysteine in the catalytic mechanism. J. Biol. Chem. 274:13242-13249. [DOI] [PubMed] [Google Scholar]
- 36.Paul-Soto, R., M. Hernandez-Valladares, M. Galleni, R. Bauer, M. Zeppezauer, J. M. Frere, and H. W. Adolph. 1998. Mono- and binuclear Zn-β-lactamase from Bacteroides fragilis: catalytic and structural roles of the zinc ions. FEBS Lett. 438:137-140. [DOI] [PubMed] [Google Scholar]
- 37.Reading, S. A. 1996. Chromium picolinate. J. Fla. Med. Assoc. 83:29-31. [PubMed] [Google Scholar]
- 38.Sharir, M., and T. J. Zimmerman. 1993. In vitro inhibition of collagen formation by 2,4-pyridine dicarboxylate and minoxidil in rabbit corneal fibroblasts. Curr. Eye Res. 12:553-559. [DOI] [PubMed] [Google Scholar]
- 39.Siemann, S., A. J. Clarke, T. Viswanatha, and G. I. Dmitrienko. 2002. Thiols as classical and slow-binding inhibitors of IMP-1 and other binuclear metallo-β-lactamases. Biochemistry 42:1673-1683. [DOI] [PubMed] [Google Scholar]
- 40.Siemann, S., D. P. Evanoff, L. Marrone, A. J. Clarke, T. Viswanatha, and G. I. Dmitrienko. 2002. N-arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 46:2450-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tschank, G., D. G. Brocks, K. Engelbart, J. Mohr, E. Baader, V. Gunzler, and H. M. Hanauske-Abel. 1991. Inhibition of prolyl hydroxylation and procollagen processing in chick-embryo calvaria by a derivative of pyridine-2,4-dicarboxylate. Characterization of the diethyl ester as a proinhibitor. Biochem. J. 275:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ullah, J. H., T. R. Walsh, I. A. Taylor, D. C. Emery, C. S. Verma, S. J. Gamblin, and J. Spencer. 1998. The crystal structure of the L1 metallo-β-lactamase from Stenotrophomonas maltophilia at 1.7 Å resolution. J. Mol. Biol. 284:125-136. [DOI] [PubMed] [Google Scholar]
- 43.Vincent, J. B. 2003. The potential value and toxicity of chromium picolinate as a nutritional supplement, weight loss agent and muscle development agent. Sports Med. 33:213-230. [DOI] [PubMed] [Google Scholar]
- 44.Zervosen, A., M. H. Valladares, B. Devreese, C. Prosperi-Meys, H. W. Adolph, P. S. Mercuri, M. Vanhove, G. Amicosante, J. van Beeumen, J. M. Frere, and M. Galleni. 2001. Inactivation of Aeromonas hydrophila metallo-β-lactamase by cephamycins and moxalactam. Eur. J. Biochem. 268:3840-3850. [DOI] [PubMed] [Google Scholar]