Abstract
Dietary seaweed fucoidan delays the onset of disease of enterally infected mice with scrapie when given orally for 6 days after infection, but not when given before the infection. This effect was not modified at a tested fucoidan dose range and appeared to reach the maximum level at a concentration of 2.5% or less in feed. Daily uptake of fucoidan might be prophylactic against prion diseases caused by ingestion of prion-contaminated materials, although further evaluation of its pharmacology remains to be done.
Transmissible spongiform encephalopathies, or prion diseases, are fatal neurodegenerative disorders that include Creutzfeldt-Jakob disease (CJD) and Gerstmann-Sträussler-Scheinker syndrome in humans and scrapie, bovine spongiform encephalopathy (BSE), and chronic wasting disease in animals. Recent outbreaks of BSE and variant CJD (vCJD), both of which are considered to occur through ingestion of BSE-contaminated materials (reviewed in reference 21), have necessitated the development of preventive measures against these diseases.
Sulfated polysaccharides, such as heparin, dextran sulfate, and pentosan polysulfate (PPS), are known either to prolong incubation periods in animals with prion diseases or to inhibit formation of pathogen-related abnormal prion protein (PrP) in prion-infected cells (reviewed in reference 3). Their therapeutic effects are attributed to inhibition of the conversion of normal PrP to abnormal PrP by either competitively binding to the normal PrP (4) or reducing normal PrP on the cell surface through stimulation of endocytosis (20). These large-molecule compounds are not taken up well from the gut to blood or from blood to the brain (a target organ of prion diseases). Therefore, these compounds are effective in cases of peripheral infection when given intraperitoneally, intravenously, or subcutaneously (8) and even in cases of intracranial infection when given intracerebroventricularly (5). Recently, PPS intracerebroventricular injection has been utilized for clinical trials of patients; the clinical outcome remains to be determined (17).
Fucoidans, complex sulfated fucosylated polysaccharides, are known to have various biological activities: anticoagulant, antiviral, antiparasital, anti-inflammatory, contraceptive, and so on, because of their ability to imitate patterns of sulfate substitution on glycosaminoglycans and other sulfated glycans (2). Some fucoidans are present in large quantities in dietary brown seaweed food products, which are eaten frequently in Asian countries (9). Here, we report that fucoidan from popularly eaten brown algae has antiprion activity and delays disease onset when it is ingested after the enteral prion infection.
Fucoidan was prepared from the brown seaweed Cladosiphon okamuranus Tokida (Fig. 1A) and subsequently tested as described previously (15). Briefly, the brown seaweed was suspended in distilled water adjusted to pH 3.0 with 30% HCl and heated at 100°C for 30 or 60 min. The suspension was centrifuged (10,000 × g) at room temperature, and the supernatant was filtered using Microza UF membrane (Asahi Kasei Chemicals, Japan). Then the retentate was washed with distilled water and lyophilized. The levels of fucose, uronic acid, and sulfate in the lyophilized preparation were determined by examining the results of the phenol-H2SO4 reaction and carbazole reaction and by ion chromatography, respectively. The purity and molecular mass of the lyophilized preparation were determined by gel filtration high-performance liquid chromatography. Two fucoidan preparations were used in the experiment: sample 1, with an average mass of 42.6 kDa and 87.8% fucoidan content; and sample 2, with an average mass of 140.4 kDa and 87.1% fucoidan content.
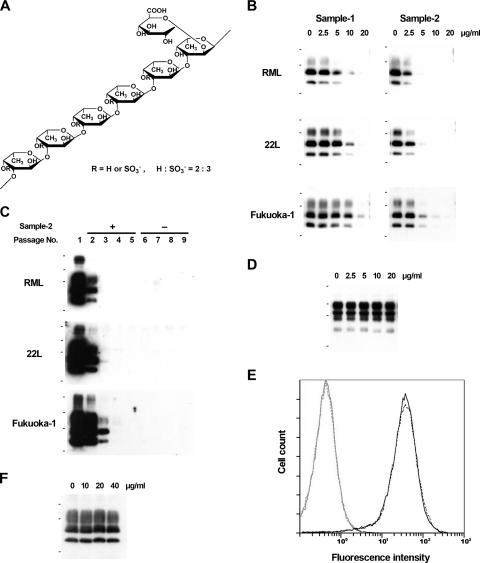
FIG. 1.
Fucoidan and its effects on prion-infected or noninfected cells. (A) Chemical structure of fucoidan from Cladosiphon okamuranus Tokida. (B) Immunoblot analyses of abnormal PrP in the prion-infected NB cells treated with fucoidan. The small black bars to the left of the blots indicate the positions of molecular size markers at 42, 32, and 17 kDa. (C) Immunoblot analyses of abnormal PrP in the prion-infected NB cells serially passaged in the presence (+) and subsequently in the absence (−) of fucoidan. Overexposed images are shown. The small black bars to the left of the blots indicate the positions of molecular size markers at 81, 42, 32, and 17 kDa. (D) Immunoblot analysis of total normal PrP in noninfected NB cells treated with fucoidan. The molecular size markers to the left of the blot are the same as in panel C. (E) Flow cytometric analysis of normal PrP on the cell surface in noninfected NB cells treated with fucoidan. The solid line and broken line indicate fucoidan-treated cells and nontreated cells, respectively. Gray line peaks on the left show their respective isotype controls. (F) Immunoblot analysis of abnormal PrP from RML-infected cell lysate incubated with fucoidan prior to protease digestion. The molecular size markers to the left of the blot are the same as in panel B. All immunoblot data shown here are of SAF83.
Inhibition of abnormal PrP synthesis in vitro was investigated as described previously (7, 12) in three different prion-infected neuroblastoma (NB) cells, each of which was persistently infected with a distinct prion strain from scrapie (RML or 22L) or human prion disease (Fukuoka-1). The cells were cultured for 3 days in the presence of fucoidan, and proteinase K-resistant abnormal PrP in the cell lysate was recovered by ultracentrifugation and analyzed by immunoblotting with three different anti-PrP antibodies, SAF83 against human PrP142-160 (SPI-BIO, France), PrP-2B against mouse/hamster PrP89-103 (5), and PrP-3B against mouse/hamster PrP218-232 (not published). The three antibodies produced the same immunoblot data in the following studies. Therefore, all immunoblot figures presented are of SAF83. The results from prion-infected NB cells showed that sample 2 of the higher-molecular-mass fucoidan more strongly inhibited abnormal PrP formation at half-maximum effective dosages ranging from 2.5 μg/ml to 5.0 μg/ml in all cells (Fig. 1B), suggesting that fucoidan exerts its antiprion activity in a prion strain-independent manner. The inhibition was irreversible, and even the most effective abnormal PrP preparation technique, sodium phosphotungstic acid precipitation (18), never demonstrated that abnormal PrP signals that had once disappeared after treatment with 10 μg/ml of sample 2 fucoidan could reappear after serial passages in the absence of fucoidan (Fig. 1C). The inhibition mechanism included no alteration of either the total or cell surface level of normal PrP. That fact was demonstrated in noninfected NB cells treated with 20 μg/ml sample 2 fucoidan by either immunoblot analysis of the cell lysate without protease digestion or flow cytometric analysis performed as described previously (6) (Fig. 1D and E). In addition, apparent modification of the abnormal PrP with fucoidan was not observed in the immunoblot data when the RML-infected cell lysate was incubated with 20 μg/ml sample 2 fucoidan at 37°C for 1 h and processed to obtain the abnormal PrP as described (Fig. 1F). The findings are consistent with those of heparan sulfate mimetics (1, 19), but not those of PPS and dextran sulfate, which stimulate endocytosis of normal PrP on the cell surface and engender reduction of the total and cell surface normal PrP (20). Thus, reduction of normal PrP is not necessarily responsible for the antiprion action of sulfated polysaccharides.
In one antiprion in vivo test, prion homogenate was mixed with a test compound prior to intracerebral inoculation and injected into the animal brain to elucidate increased incubation times attributable either to inactivation of the inoculum or its presence in the brain at the time of infection. Sample 2 was more effective in vitro. Therefore, it was tested in this manner using an animal model comprising hamster scrapie prion strain 263K and Tg7 mice expressing hamster PrP (16). That model was chosen because it gives the shortest incubation times of all experimental animal models available and because antiprion activity of fucoidan was observed, irrespective of prion strains. In an initial experiment, immediately after 20 μl of 1% 263K prion homogenate equivalent to an infectivity titer of about 108 50% lethal dose (LD50)/g of tissue (5) was mixed with sample 2 fucoidan at its final concentration of 0 to 200 μg/ml, five to eight-week-old Tg7 mice per group were inoculated intracerebrally with the mixture. Only the mixture containing the largest amount of sample 2 significantly increased the incubation period (P < 0.05) compared to that of the control (experiment 1 in Table 1).
TABLE 1.
Antiprion effects of fucoidan mixed with infectious inoculum
| Expt no. | Fucoidan concn in inoculum (μg/ml) | Incubation period (days) (mean ± SD) (nc) | Statistical significance |
|---|---|---|---|
| Expt 1a | 200 | 57.9 ± 2.5 (8) | P < 0.05d |
| 20 | 55.0 ± 2.5 (6) | ||
| 2 | 54.2 ± 2.7 (5) | ||
| 0.2 | 54.2 ± 3.9 (6) | ||
| 0.02 | 53.7 ± 2.6 (6) | ||
| 0 (control) | 53.0 ± 2.7 (6) | ||
| Expt 2b | 200 | 73.3 ± 3.7 (7) | P < 0.01e |
| 20 | 67.4 ± 7.2 (5) | ||
| 2 | 69.0 ± 2.1 (5) | ||
| 0.2 | 66.8 ± 4.6 (6) | ||
| 0.02 | 64.4 ± 4.5 (7) | ||
| 0 (control) | 62.2 ± 2.2 (6) |
Twenty microliters of 1% 263K prion homogenate mixed with the designated dose of sample 2 fucoidan was inoculated intracerebrally into each Tg7 mouse immediately after the mixture was made.
Twenty microliters of 0.1% 263K prion homogenate with the designated dose of sample 2 fucoidan was inoculated intracerebrally into each Tg7 mouse after the mixture was incubated for 14 h at room temperature.
Number of mice tested in each group.
Statistical significance against the value for the control, analyzed using one-way analysis of variance followed by the Tukey-Kramer method for multiple comparisons.
Statistical significance against the value for either the 0.02 μg/ml group or the control, analyzed as described in footnote d.
Next, to determine whether preincubation of the mixture enhances inactivation of the infectious inoculum, the prion homogenate-fucoidan mixtures, similarly prepared but containing 10 times more diluted homogenate, were incubated for 14 h at room temperature and then injected similarly into five to seven mice per group. Experiment 2 in Table 1 shows that only the mixture containing the largest amount of sample 2 significantly increased the incubation period (P < 0.01), as demonstrated similarly in experiment 1. The findings suggest that fucoidan itself does not modify infectivity of the inoculum, but its presence in the brain might inhibit prion replication or PrP conversion, probably in a manner similar to that observed in vitro. This inference is supported by results described in previous reports that PPS is effective in delaying the onset of disease of prion-infected animals when administered continuously into the brain (5) or even by bolus shots (13). However, there remains another possibility, the possibility that fucoidan can modify the infectivity in the inoculum very rapidly without a 14-h incubation.
Finally, the potential practical utility of fucoidan was investigated, especially its prophylactic effects against peroral and enteral prion infections such as those that occur in BSE and vCJD. Two different timings of fucoidan feeding, where fucoidan powder was given in a mixture with feed powder at three different levels (2.5, 5, or 10%) were designed to reveal its distinct effects in mice. In one, fucoidan feeding started 7 days prior to enteral inoculation into five to seven Tg7 mice per group by gavage feeding over a few hours with a total of 200 μl of 10% 263K prion homogenate (about 109 LD50/g infectivity titer) and ended the day preceding inoculation to elucidate its preinfection prophylactic effects. In the other, fucoidan feeding started the day after the inoculation and continued for 6 days to elucidate its postinfection prophylactic effects. The results demonstrated that fucoidan feeding that commenced after the enteral inoculation delayed the disease onset for about half the time of the control incubation (Table 2). However, fucoidan feeding before the enteral inoculation did not affect the incubation time.
TABLE 2.
Prophylactic effects of fucoidan feeding preinfection or postinfection
| Time of feeding | Fucoidan concn in feed (%) | Incubation period (days) (mean ± SD) (nc) | Statistical significance |
|---|---|---|---|
| Preinfectiona | 2.5 | 215.4 ± 40.5 (5) | |
| 5 | 243.4 ± 31.8 (5) | ||
| 10 | 212.2 ± 37.7 (5) | ||
| Postinfectionb | 2.5 | 346.8 ± 28.0 (5) | P < 0.01d |
| 5 | 368.1 ± 73.9 (7) | P < 0.01d | |
| 10 | 367.4 ± 44.9 (5) | P < 0.01d | |
| Control | 231.1 ± 28.3 (6) |
Fucoidan feeding started 7 days prior to enteral inoculation by gavage feeding with 200 μl of 10% 263K prion homogenate and ended the day before inoculation.
Fucoidan feeding started the day after enteral inoculation and continued for 6 days.
Number of mice tested in each group.
Statistical significance against either the value for the preinfection group or the control, analyzed as described in Table 1, footnote d.
Low proportions of fucoidan are absorbed from the gut into blood (11) and excreted in urine (10), although little more is known of the detailed pharmacology of ingested fucoidan. Sulfated polysaccharides injected intraperitoneally or intravenously inhibit prion replication in the lymphoreticular system, which is involved in the delivery of prion from the gut to the brain (8, 14). Therefore, it can be speculated that fucoidan absorbed into blood exerts its effects by inhibiting prion replication in the lymphoreticular system. A gap of fucoidan effects between the preinfection and postinfection fucoidan feeding might be attributable to the rapid clearance of blood fucoidan into urine when this is the case. Another possible mechanism of the postinfectious fucoidan effects might be that it facilitates the excretion of infectious materials from the gut. This inference is supported by the fact that seaweed polysaccharides and other natural polysaccharides alter the bacterial spectrum of the gut and assist detoxification (9). In contrast, fucoidan does not seem to act via a certain factor induced in the host because fucoidan administered until the day before inoculation was never effective.
There was no difference in prolonged incubation times among the three different fucoidan concentrations, although the feed consumption per mouse was not statistically different in each experimental group irrespective of the fucoidan level. This might occur because even the lowest concentration of fucoidan in feed surpasses its absorption threshold from the gut or because blood fucoidan concentrations are not parallel to ingested fucoidan doses. The latter was previously reported in humans, where only a threefold difference in blood plasma fucoidan concentrations was detected despite a 7.5-fold difference in ingested fucoidan doses (11). In addition, the stoichiometric relationship between blood fucoidan concentration and inhibitory activity against prion replication in vivo might also be attributable to the results observed here. However, these inferences remain to be elucidated.
The inoculum used in the study of enteral infection contained an extremely high titer of about 109 LD50/g, although most of the inoculum might be excreted in feces, and presumably, a much lower titer may cause the infection. More satisfactory prophylactic effects by orally ingested fucoidan might be expected when prion infection in BSE or vCJD is presumed to occur through a lower level of infectivity than that used in this study. On the other hand, the data presented cannot exclude the possibility that the in vivo effects of fucoidan on the 263K prion strain are different from those on other strains. However, this did not occur during our previous experiments with a sulfated polysaccharide (5).
Finally, all fucoidan samples used here contained fucoidan at less than 90% of total weight. Therefore, it is possible that ingredients other than fucoidan exert the antiprion activity observed in this study. However, gel-filtrated samples, which contained 99.9% fucoidan with a mass of 100 to 190 kDa produced the same in vitro results (data not shown). Therefore, fucoidan itself of the dietary brown seaweed imparts the antiprion activity. Its daily ingestion has the potential to provide some prophylactic benefit against such oral or enteral prion infections as occurred in BSE and vCJD, but further studies must be done to elucidate the pharmacology of ingested fucoidan.
Acknowledgments
This work was supported by the Ministry of Health, Labor and Welfare (H16-kokoro-024) and the BSE Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan.
We thank Satoshi Kawatake for valuable suggestions and Kyomi Sasaki for manuscript preparation.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Adjou, K. T., S. Simoneau, N. Salès, F. Lamoury, D. Dormont, D. Papy-Garcia, D. Barritault, J. P. Deslys, and C. I. Lasmézas. 2003. A novel generation of heparan sulfate mimetics for the treatment of prion diseases. J. Gen. Virol. 84:2595-2603. [DOI] [PubMed] [Google Scholar]
- 2.Berteau, O., and B. Mulloy. 2003. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 13:29R-40R. [DOI] [PubMed] [Google Scholar]
- 3.Cashman, N. R., and B. Caughey. 2004. Prion diseases-close to effective therapy? Nat. Rev. Drug Discov. 3:874-884. [DOI] [PubMed] [Google Scholar]
- 4.Caughey, B., K. Brown, G. J. Raymond, G. E. Katzenstein, and W. Thresher. 1994. Binding of the protease-sensitive form of PrP (prion protein) to sulfated glycosaminoglycan and Congo red. J. Virol. 22:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doh-ura, K., K. Ishikawa, I. Murakami-Kubo, K. Sasaki, S. Mohri, R. Race, and T. Iwaki. 2004. Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models. J. Virol. 78:4999-5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doh-ura, K., K. Tamura, Y. Karube, M. Naito, T. Tsuruo, and Y. Kataoka. 19 January 2007. Chelating compound, chrysoidine, is more effective in both antiprion activity and brain endothelial permeability than quinacrine. Cell. Mol. Neurobiol. doi: 10.1007/s10571-006-9122-0. [DOI] [PMC free article] [PubMed]
- 7.Doh-Ura, K., T. Iwaki, and B. Caughey. 2000. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74:4894-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farquhar, C., A. Dickinson, and M. Bruce. 1999. Prophylactic potential of pentosan polysulphate in transmissible spongiform encephalopathies. Lancet 353:117. [DOI] [PubMed] [Google Scholar]
- 9.Fitton, J. H. 2003. Brown marine algae: a survey of therapeutic potentials. Altern. Complem. Ther. 9:29-33. [Google Scholar]
- 10.Guimarães, M. A. M., and P. A. S. Mourão. 1997. Urinary excretion of sulfated polysaccharides administered to Wistar rats suggests a renal permselectivity to these polymers based on molecular size. Biochim. Biophys. Acta 1335:161-172. [DOI] [PubMed] [Google Scholar]
- 11.Irhimeh, M. R., J. H. Fitton, R. M. Lowenthal, and P. Kongtawelert. 2005. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 27:705-710. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa, K., Y. Kudo, N. Nishida, T. Suemoto, T. Sawada, T. Iwaki, and K. Doh-ura. 2006. Styrylbenzoazole derivatives for imaging of prion plaques and treatment of transmissible spongiform encephalopathies. J. Neurochem. 99:198-205. [DOI] [PubMed] [Google Scholar]
- 13.Kocisko, D. A., W. S. Caughey, R. E. Race, G. Roper, B. Caughey, and J. D. Morrey. 2006. A porphyrin increases survival time of mice after intracerebral prion infection. Antimicrob. Agents Chemother. 50:759-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mabbott, N. A., and G. G. MacPherson. 2006. Prions and their lethal journey to the brain. Nat. Rev. Microbiol. 4:201-211. [DOI] [PubMed] [Google Scholar]
- 15.Nagaoka, M., H. Shibata, I. Kimura-Takagi, S. Hashimoto, K. Kimura, T. Makino, R. Aiyama, S. Ueyama, and T. Yokokura. 1999. Structural study of fucoidan from Cladosiphon okamuranus Tokida. Glycoconjugate J. 16:19-26. [DOI] [PubMed] [Google Scholar]
- 16.Race, R. E., S. A. Priola, R. A. Bessen, D. Ernst, J. Dockter, G. F. Rall, L. Mucke, B. Chesebro, and M. B. Oldstone. 1995. Neuron-specific expression of a hamster prion protein minigene in transgenic mice induces susceptibility to hamster scrapie agent. Neuron 15:1183-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainov, N. G., I. R. Whittle, and K. Doh-ura. 2005. Treatment options in patients with prion disease—the role of long term cerebroventricular infusion of pentosan polysulphate, p. 41-66. In T. Kitamoto (ed.), Prions—food and drug safety. Springer-Verlag, Tokyo, Japan.
- 18.Safar, J., H. Wille, V. Itri, D. Groth, H. Serban, M. Torchia, F. E. Cohen, and S. B. Prusiner. 1998. Eight prion strains have PrPSc molecules with different conformations. Nat. Med. 4:1157-1165. [DOI] [PubMed] [Google Scholar]
- 19.Schonberger, O., L. Horonchik, R. Gabizon, D. Papy-Garcia, D. Barritault, and A. Taraboulos. 2003. Novel heparan mimetics potently inhibit the scrapie prion protein and its endocytosis. Biochem. Biophys. Res. Commun. 312:473-479. [DOI] [PubMed] [Google Scholar]
- 20.Shyng, S. L., S. Lehmann, K.L. Moulder, and D. A. Harris. 1995. Sulfated glycans stimulate endocytosis of the cellular isoform of the prion protein, PrPC, in cultured cells. J. Biol. Chem. 270:30221-30229. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, D. M. 2002. Current perspectives on bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease. Clin. Microbiol. Infect. 8:332-339. [DOI] [PubMed] [Google Scholar]