Abstract
Daptomycin monotherapy was superior to ceftriaxone monotherapy and was highly efficacious in experimental pneumococcal meningitis, sterilizing the cerebrospinal fluid (CSF) of three of three rabbits after 4 to 6 h. With daptomycin therapy only a negligible release of [3H]choline as marker of cell wall lysis was detectable in the CSF, peaking around 250 cpm/min after 4 h, compared to a peak of around 2,400 cpm/min after 4 to 6 h for the ceftriaxone-treated rabbits.
Streptococcus pneumoniae has been the major cause of bacterial meningitis in the United States (about 1.1 cases per 100,000 population per year) since the introduction of the conjugated Haemophilus influenzae type b vaccine (10). The mortality from pneumococcal meningitis remains high, at around 20%, and up to 50% of the survivors present with an adverse neurological outcome. These sequelae are mainly due to the overwhelming reaction of the immune response in the cerebrospinal fluid (CSF), which is triggered by pneumococcal virulence factors (8). It has been well known for years that bacterial cell wall components are potent inducers of the immune response (7). β-Lactam antibiotics (e.g., ceftriaxone), which belong to the standard regimen of therapy for pneumococcal meningitis, activate the crucial pneumococcal autolysin, the N-acetyl-muramyl-l-alanine amidase, resulting in an abrupt release of cell wall components into the CSF and inducing the inflammatory burst during bacterial meningitis (11, 12). A highly bactericidal but nonbacteriolytic antibiotic would represent a major progress in the treatment of meningitis. We have recently demonstrated that daptomycin, a new lipopeptide antibiotic, was highly efficacious against penicillin-resistant pneumococci in experimental meningitis, sterilizing the CSF of rabbits within 4 to 6 h (2). Its bactericidal activity is based on various effects on the plasma membrane that do not jeopardize the integrity of the cell wall (1). The aims of this study were to determine the effect of daptomycin on the cell wall of pneumococci during meningitis by measuring [3H]choline release in the CSF and to detect daptomycin-induced morphological changes by electron microscopy.
Strains and MIC determination.
The pneumococcal strain WB4 was originally isolated from a patient with pneumonia at the University Hospital of Bern, Switzerland. MICs for WB4 were as follows: penicillin, 4 mg/liter; ceftriaxone, 0.5 mg/liter; cefotaxime, 1 mg/liter; vancomycin, 0.12 to 0.25 mg/liter; levofloxacin, 1 mg/liter; moxifloxacin, 0.12 mg/liter; and daptomycin, 0.06 mg/liter. MICs were determined by the broth macrodilution method. The MIC was defined as the lowest concentration that inhibited visible growth after 12 and 24 h of incubation at 37°C.
Labeling of growing pneumococci with [3H]choline.
Pneumococci were labeled with [3H]choline as previously described by Fischer and Tomasz (4). A 150-μl portion of the WB4 stock were added to 2 ml C+Y medium (conditioned medium plus yeast) and incubated at 37° until the optical density at 590 nm was 0.2 to 0.3. An aliquot of 40 μl of the culture was then added to 2 ml of fresh C+Y medium containing 8 μl of [3H]choline ([methyl-3H]choline chloride; 3.11 TBq/mmol, 84.0 Ci/mmol) (Amersham Biosciences). This culture was incubated at 37°C until the optical density was 0.2 to 0.3. The culture was centrifuged, the supernatant removed, and the pellet resuspended in fresh C+Y medium. An aliquot was removed and added to fresh medium to obtain about 105 CFU/200 μl. This inoculum was then injected into the CSF of rabbits.
Rabbit meningitis model.
The meningitis model originally described by Dacey and Sande (3, 9) was used in this study. The experimental protocol was accepted by the local ethical committee (Veterinäramt des Kantons Bern). Young New Zealand White rabbits weighing 2 to 2.5 kg were anesthetized by intramuscular injections of ketamine (30 mg/kg) and xylazine (15 mg/kg) and were immobilized in stereotactic frames for induction of meningitis and CFU sampling. An inoculum containing approximately 105 CFU of penicillin-resistant strain WB4 (see above) was instilled in the cisterna magna. A long-acting anesthetic drug (ethylcarbamate [urethane] at 3.5 g/rabbit) was injected subcutaneously, and animals were returned to their cages. Fourteen hours later the cisterna magna was punctured again for CSF sampling before and 2, 4, 6, and 8 h after initiation of therapy. Anesthesia was performed by repetitive injections of pentobarbital sodium (Nembutal). Antibiotics were administered by a peripheral ear vein at 125 mg/kg of body weight for ceftriaxone and 15 mg/kg of body weight for daptomycin. Ceftriaxone and daptomycin were injected once at hour zero. All antibiotics and anesthetic drugs were purchased commercially.
An aliquot of the CSF was used for CFU determination, the remaining CSF was centrifuged, and the supernatant was used for measurement of [3H]choline release. The pellet was fixed with 3% glutaraldehyde solution (EMS 16210-S) and further processed for electron microscopy based on a standard procedure.
Bacterial titers were measured by 10-fold serial dilutions of CSF samples, and then samples were plated on blood agar plates containing 5% sheep blood and incubated overnight at 37°C. In parallel, 20 μl of undiluted samples was plated (limit of detection, 50 CFU/ml). Comparison between dilutions of CSF was used to exclude significant carryover effects during therapy. The antimicrobial activities of the different regimens during the 8-h treatment were calculated by linear regression analysis and expressed as change in log10 CFU per milliliter per hour and as change in viable count over 8 h. A value of 1.7 (log10 of the limit of detection) was assigned to the first sterile CSF sample, and a value of 0 was assigned to any following sterile CSF sample. The results are expressed as means ± standard deviations. Statistical significance was determined by the Newman-Keuls test.
Determination of [3H]choline release in the CSF.
Probes (50 μl of rabbit CSF) were added to 1 ml of scintillation solution (Microcint Tm 40; Perkin-Elmer Life and Analytical Sciences, Boston, MA), and radioactivity was measured for 1 min in a beta counter (MR 300 automatic liquid scintillation; Kontron MR 300 System). Experiments were performed in triplicate and expressed as mean cpm ± standard deviation.
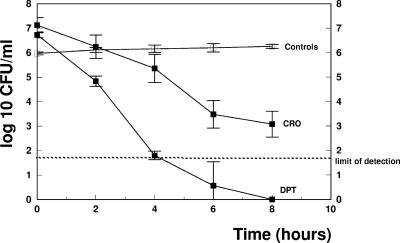
The efficacies of the different regimens are presented in Fig. 1 and summarized in Table 1 Daptomycin monotherapy produced a pronounced antibacterial activity, with a decrease of the viable cell count of around 1 log10 unit per hour (−1.05 ± 0.19 Δlog10 CFU/ml·h) and was able to sterilize the CSF of all rabbits. Interestingly, two out of three CSF samples were already sterile after 4 h. In contrast, ceftriaxone was less efficacious, producing a decrease of the viable cell count of around 0.5 log10 unit per hour (−0.53 ± 0.03 Δlog10 CFU/ml·h). None of the CSF samples from the ceftriaxone group were sterile at the end of the treatment period. The data confirmed the efficacy of daptomycin in experimental meningitis, as demonstrated in previous studies (2, 6). We are aware that the standard regimen for pneumococcal meningitis due to penicillin-resistant strains consists of a combination of ceftriaxone plus vancomycin and not of ceftriaxone monotherapy, but the aim of this study was to compare a bacteriolytic with a nonbacteriolytic regimen.
FIG. 1.
Decrease of bacterial titers in CSF samples from the different treatment groups. CRO, ceftriaxone; DPT, daptomycin. The dashed line represents the limit of detection. Experiments were performed in triplicate, and results are expressed as means ± standard deviations.
TABLE 1.
Ceftriaxone versus daptomycin against penicillin-resistant S. pneumoniae WB4 in experimental meningitis
| Antibiotic | n | Mean initial titer (log10 CFU/ml) ± SD | Mean killing rate (Δlog10 CFU/ml·h) ± SD | Mean decrease of viable cell count over 8 h (log10 CFU/ml) ± SD |
|---|---|---|---|---|
| None (control) | 3 | 5.96 ± 0.05 | +0.03 ± 0.01a | +0.29 ± 0.11a |
| Ceftriaxone | 3 | 7.12 ± 0.30 | −0.53 ± 0.03b | −4.04 ± 0.46b |
| Daptomycin | 3 | 6.72 ± 0.12 | −1.05 ± 0.19b | −6.64 ± 0.08b |
P < 0.05 versus all groups.
P < 0.05 for daptomycin versus ceftriaxone.
The enhanced activity of daptomycin has also been confirmed in time-killing assays over 8 h in vitro (data not shown). Daptomycin sterilized the cultures already after 4 h and produced a bactericidal activity of 6 log10 units, whereas ceftriaxone led to a decrease of the bacterial titer of around 2 log10 units, using 10 times the MIC.
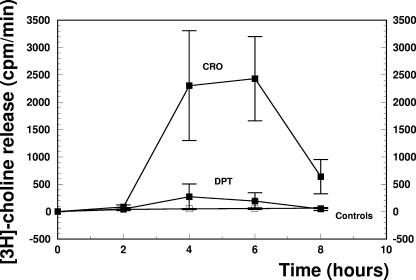
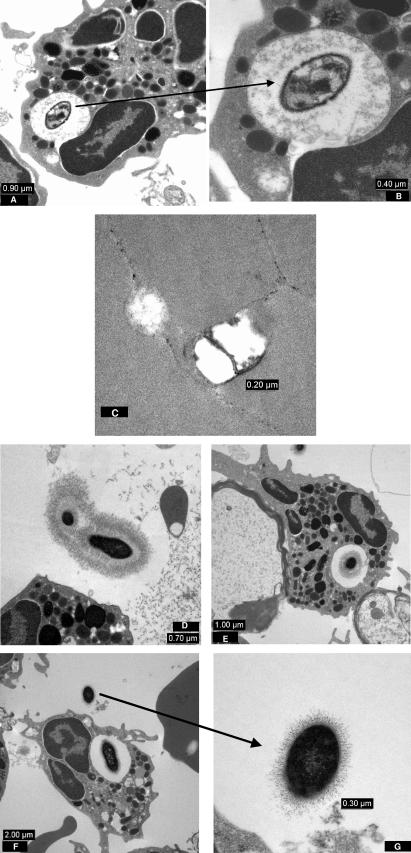
The most interesting feature of the study was the effect of the different regimens on the release of [3H]choline in the CSF. Choline is a main component of teichoic acid, which is bound to the cell wall, and also of lipoteichoic acid, which is anchored on the outer layer of the cytoplasmic membrane. During bacterial cell growth, [3H]choline is mainly incorporated into teichoic acid (5). In this study, we used [3H]choline release in the CSF as a marker of cell wall lysis during antibiotic treatment (Fig. 2). Whereas the [3H]choline release by untreated controls was negligible, ceftriaxone treatment led to a drastic increase of [3H]choline of around 2,500 cpm/min at 4 and 6 h after initiation of therapy. Accordingly, the first morphological alterations of the cell wall of a phagocytosed pneumococcus were detected already at 2 h after one injection of ceftriaxone by electron microscopy (Fig. 3A and B). At hour 6, when the [3H]choline release peaked, pieces of bacterial cell membranes were seen (Fig. 3C). In contrast, [3H]choline peaked at around 250 cpm/min at 4 h after the administration of daptomycin. No essential morphological alterations of the pneumococci could be detected by electron microscopy at hour 2, 4, or 6 (Fig. 3D, E, F, and G). It is interesting to note that these pictures are of pneumococci from rabbit CSF samples which were already sterile at hour 4. The source of the [3H]choline in the daptomycin group is not clear. One may speculate that the [3H]choline is partially due to lipoteichoic acid release from the plasma membrane.
FIG. 2.
[3H]choline release into the CSF after one injection of ceftriaxone (CRO) or daptomycin (DPT). Experiments were performed in triplicate, and results are expressed as means ± standard deviations.
FIG. 3.
(A) A phagocytosed pneumococcus in the CSF at 2 h after injection of ceftriaxone. (B) At a higher magnification, morphological alterations of the cell wall are already detectable. (C) At 6 h after injection, isolated bacterial cell membranes are present. (D) Two extracellular pneumococci at 2 h after injection of daptomycin. (E and F) Phagocytosed pneumococci are presented at 4 h (E) and 6 h (F) after daptomycin injection. The CSF of rabbits was already sterile after 4 h. (G) A pneumococcus at high resolution at 6 h after injection.
Taken together, these data suggest that daptomycin led to a negligible secretion of cell wall components, a major pneumococcal virulence factor, in the CSF compared to that with ceftriaxone. Recently, Grandgirard et al. (6a) demonstrated in the infant rat model that one injection of daptomycin led to a reduced secretion of MMP9 in the CSF, as a marker of the inflammatory response during pneumococcal meningitis. In addition, cortical damage was detected only in ceftriaxone-treated animals.
In summary, this study confirmed the favorable characteristics of daptomycin as therapeutic agent in pneumococcal meningitis, i.e., a highly bactericidal activity inducing a low inflammatory response of the host.
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Canepari, P., M. Boaretti, M. M. Lleo, and G. Satta. 1990. Lipoteichoic acid as a new target for activity of antibiotics: mode of action of daptomycin (LY146032). Antimicrob. Agents Chemother. 34:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottagnoud, P., M. Pfister, F. Acosta, M. Cottagnoud, L. Flatz, F. Kuhn, H. P. Muller, and A. Stucki. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dacey, R. G., and M. A. Sande. 1974. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob. Agents Chemother. 6:437-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer, H., and A. Tomasz. 1984. Production and release of peptidoglycan and wall teichoic acid polymers in pneumococci treated with beta-lactam antibiotics. J. Bacteriol. 157:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer, W. 2000. Pneumococcal lipoteichoic and teichoic acid, p. 155-177. In A. Tomasz (ed.), Streptococcus pneumoniae, molecular biology and mechanisms of disease. Mary Ann Liebert, Inc., Larmont, NY.
- 6.Gerber, P., A. Stucki, F. Acosta, M. Cottagnoud, and P. Cottagnoud. 2006. Daptomycin is more efficacious than vancomycin against a methicillin-susceptible Staphylococcus aureus in experimental meningitis. J. Antimicrob. Chemother. 57:720-723. [DOI] [PubMed] [Google Scholar]
- 6a.Grandgirard, D., C. Schuerch, P. Cottagnoud, and S. L. Leib. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 742. [DOI] [PMC free article] [PubMed]
- 7.Grimwood, K., V. A. Anderson, L. Bond, C. Catroppa, R. L. Hore, E. H. Keir, T. Nolan, and D. M. Roberton. 1995. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics 95:646-656. [PubMed] [Google Scholar]
- 8.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 9.Nau, R., K. Kaye, M. Sachdeva, E. R. Sande, and M. G. Tauber. 1994. Rifampin for therapy of experimental pneumococcal meningitis in rabbits. Antimicrob. Agents Chemother. 38:1186-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, B. A. Perkins, et al. 1997. Bacterial meningitis in the United States in 1995. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 11.Tuomanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect. Dis. 151:859-868. [DOI] [PubMed] [Google Scholar]
- 12.Tuomanen, E., A. Tomasz, B. Hengstler, and O. Zak. 1985. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J. Infect. Dis. 151:535-540. [DOI] [PubMed] [Google Scholar]