Abstract
Treatment of hepatitis C virus (HCV) replicon cells with any single specific anti-HCV inhibitor in vitro leads to a rapid selection of resistant mutants. However, the source and the kinetic evolution of these resistant mutants during treatment are poorly understood. In this study we developed allele-specific real-time PCR assays for quantitative detection of the M414T mutant that was selected by a number of benzothiadiazine HCV polymerase inhibitors. Low levels of preexisting M414T mutants were detected in both 1b-con1 (0.22%) and 1b-N (0.18%) subgenomic replicon cell lines, as well as in 6 of 15 HCV RNA isolated from the sera of treatment-naive HCV-infected patients ranging from 0.11 to 0.60%. The proportion of M414T mutants in replicons rapidly increased in a dose-dependent manner upon treatment with benzothiadiazine inhibitor A-782759. After 4 days of treatment, 2.5, 26, or 60% of the replicon population contained M414T mutants with the use of A-782759 at 1×, 10×, or 100× its 50% effective concentration, respectively. In addition, the short 4-day treatment resulted in significant changes in inhibitor susceptibility in the replicon cells. Our results indicated that the resistant mutant preexisted as a minor population in replicon cells and that the mutant was selected within days of treatment with the inhibitor. The findings from this study suggested that early application of combination therapy of an HCV-specific inhibitor with interferon-based regimens or other classes of available inhibitors will be necessary to avoid quick viral rebound or treatment failure.
Hepatitis C virus (HCV) affects 170 million people worldwide (prevalence rate of 3%), and it is the most common type of chronic viral hepatitis in the industrialized world. The progression of HCV disease varies between individuals; some progress quickly from infection to liver disease, while the vast majority take 20 to 30 years before the appearance of severe liver problems (1, 6, 31, 32). During this chronic infection, HCV replicates at an estimated rate of production and clearance of 1012 virions per day, even higher than the current estimates for viral production in human immunodeficiency virus (HIV)-infected individuals (4, 5, 17). The larger production rate, decades of chronic infection, and highly error-prone HCV RNA-dependent RNA polymerase result in high diversity of virus in HCV-infected individuals. Within an infected subject, HCV is found as a collection of genetically distinct but closely related variants known as quasispecies. This pool of genetic variants is thought to serve as a reservoir from which resistant viruses can emerge when a patient is treated with a specifically targeted antiviral drug (3, 7, 13, 20, 21, 26, 29).
Indeed, selection and characterization of resistant mutants to HCV inhibitors against HCV principal targets, including NS3/NS4A serine protease and NS5B RNA-dependent RNA polymerase, have been reported (8, 9, 11, 12, 15, 16, 18, 27, 28, 30). In all cases, a single mutation in the corresponding gene resulted in remarkable loss of susceptibility to the inhibitor. Significantly, a mutation at NS3 amino acid position 156 was reported to confer resistance to protease inhibitors BILN 2061 (macrocyclic peptide), VX950, or SCH503034 (peptidic ketoamides) (9, 11, 28). In addition, the NS5B mutation M414T conferred resistance to several benzothiadiazine polymerase inhibitors (16, 18). However, little information is available on the prevalence of preexisting resistant mutants and how this prevalence will change in response to treatment with a specific anti-HCV inhibitor.
A major obstacle in detecting or measuring preexisting resistant mutants is that they are generally present as minor populations. Traditional direct PCR population sequencing methods are unable to detect the minor resistant variants present in the mixture. The standard clonal sequencing methods can detect a minor population of resistant variants; however, these methods are time-consuming and labor-intensive. Recently, several groups in the HIV fields have used allele-specific real-time PCR assays to detect minor population variants (10, 14, 19). In the present study, we developed novel allele-specific real-time PCR assays for the quantitative detection of HCV variants containing the M414T mutation in the NS5B gene. We detected preexisting minor population M414T mutants in treatment-naive HCV replicon cells and in virus isolated from the sera of HCV genotype 1b-infected individuals. In addition, we monitored the proportional changes of M414T mutants upon treatment with A-782759 in replicon cells.
MATERIALS AND METHODS
Compounds and HCV replicon cells.
HCV NS5B polymerase inhibitor quinolone benzothiadiazine A-782759 and protease inhibitor BILN 2061 were synthesized as described previously (11, 16). The concentrations for 50% inhibition (EC50) of 1b-N strain replicons were 70 nM for A-782759 and 4 nM for BILN 2061. HCV genotype 1b-N strain and 1b-con1 strain subgenomic replicon cell lines were obtained from UTMB and Apath, respectively. Replicon cells were cultured in Dulbecco modified Eagle medium (Invitrogen) supplemented with 10% fetal bovine serum, 2 μg of blasticidin/ml, and 400 μg of G418/ml.
RNA extraction, cDNA synthesis, and RNA transcription.
Total RNA was extracted from HCV replicon cells by using the RNeasy minikit (QIAGEN) or from HCV-infected patient sera with QIAamp viral RNA minikit (QIAGEN) according to the manufacturer's instructions. cDNA was synthesized from HCV RNA by using the HCV-specific primer p8084 (5′-GGAAATGGCCTATTGGCCTGGAGTGTTTAGCTC-3′ encompassing nucleotides 9427 to 9395; accession no. AF139594) and the SuperScript III first-strand synthesis system for reverse transcription-PCR (RT-PCR) kit (Invitrogen) according to the manufacturer's instructions. Briefly, 0.5 to 1 μg of RNA was reverse transcribed at 50°C for 50 min, followed by 85°C for 5 min. The transcripts of HCV mutant replicons were generated by using XbaI-linearized replicon plasmids and the Megascript T7 kit (Ambion) according to the manufacturer's instructions.
Sequence analysis of the NS5B genes of the individual colonies.
Amplification of NS5B was performed on cDNA synthesized as described above by using the primers 5′-ACGCTGAGTCATGCTCCTCT-3′ (nucleotides 7498 to 7517 of AF139594) and 5′-GCCTATTGGCCTGGAGTGTT-3′ (nucleotides 9420 to 9401 of AF139594) and Platinum Pfx DNA polymerase (Invitrogen) under the following PCR conditions: 94°C for 5 min, followed by 40 cycles of 94°C for 15 s, 58°C for 30 s, and 68°C for 2 min, followed in turn by a hold at 68°C for 7 min. At least three independent PCRs were performed for each sample, and the resulting PCR products were pooled. Amplified DNA fragments were purified by using a QIAquick PCR purification kit (QIAGEN) and then cloned into the pCR-Blunt II-TOPO vector (Invitrogen) according to the manufacturer's instructions. Nucleotide sequences of the cloned products were determined by automated sequencing using BigDye terminator v3.1 (Applied Biosystems).
Allele-specific real-time PCR.
A 255-bp fragment was amplified by first-round PCR on cDNA synthesized from HCV RNA as described above by using Platinum Pfx DNA polymerase (Invitrogen) and the primers M414-S (sense primer, 5′-CTTCACGGAGGCTATGACTAGGTACTCCGC-3′, nucleotides 8627 to 8656 of AF139594) and M414-AS (antisense primer, 5′-ATCCTTGCCCATAAGGTGGGCGCATAC-3′, nucleotides 8881 to 8855 of AF139594). PCRs were performed in the reaction buffer supplied with the polymerase, plus 1.25 mM MgCl2, 0.3 mM concentrations of each deoxynucleoside triphosphate, 0.3 μM concentrations of each primer, 0.1 μl of PCRx enhancer (Invitrogen)/μl, 0.05 U of Platinum Pfx polymerase (Invitrogen)/μl, and 2 μl of cDNA in a volume of 20 μl. The PCR conditions consisted of 94°C for 5 min, followed by 25 cycles of 94°C for 15 s, 58°C for 30 s, and 68°C for 40 s. The reactions were then held at 68°C for 7 min. The PCR products were treated with ExoSAP-IT (USB, Ohio) to remove excess primers according to the manufacturer's instructions. SYBR green-based real-time PCR was performed on the first-round PCR products as follows. A total of 10 μl of 2× SYBR green PCR master mix (Applied Biosystems), 0.4 μl of 5 μM primer M414-S (sense primer), 0.4 μl of 5 μM primer 414M (antisense primer, 5′-AAGGTGGGCGCATACA-3′, nucleotides 8869 to 8854 of AF139594) or 414T (antisense primer, 5′-AAGGTGGGCGCATACG-3′), and 2 μl of DNA template plus water were added to a final volume of 20 μl per reaction and cycled as follows: 95°C for 10 min, followed by 45 cycles of 95°C for 15 s, 61°C for 20 s, and 72°C for 30 s in ABI Prism 7700 or 7900 instruments (Applied Biosystems). Cycle threshold (CT) values were determined by using the ABI Prism software installed in the instruments. PCRs without DNA standards or first-round PCR products were also performed as negative controls in the same PCR plates.
Standard DNA for allele-specific real-time PCR.
Both wild-type and mutant DNA template standards were obtained by PCR from molecular clones of either wild-type 1b-N strain or 1b-N strain containing the M414T mutation introduced by site-directed mutagenesis (16). Briefly, the HCV NS5B region from nucleotides 863 to 1389 was amplified by using the primers P1 (5′-GCTGCGGTAATACCCTCAC-3′, nucleotides 8476 to 8494 of AF139594) and P1A (5′-GGTAGGTCAAGTGGCTCAATG-3′, nucleotides 8992 to 8972 of AF139594) with Platinum Pfx polymerase (Invitrogen). The PCR products were then purified by using the QIAquick PCR purification kit (QIAGEN). The DNA quantity was determined spectrophotometrically, and molecular copy numbers were then calculated according to the following conversions: 50 μg of DNA/ml per A260 unit and 1 μg of 1,000-bp DNA = 1.52 pmole.
Treatment of replicon cells with inhibitors.
For experiments lasting a total of 4 days, 2 × 104 replicon cells were placed in each well of a 24-well plate with various concentrations of inhibitors. At each of the collecting time points, cells in the appropriate wells were washed once with phosphate-buffered saline (PBS) and then collected for total RNA extraction according to the instructions of the RNeasy minikit (QIAGEN). For longer experiments, 2 × 105 replicon cells were placed in a 10-cm cell culture dish with various concentrations of inhibitors. Cells were split 1:10 when they reached 90% confluence. At RNA collecting time points, cells were suspended by using trypsin-EDTA (Invitrogen) and then washed with PBS and used for RNA extraction as described above.
Measurement of HCV RNA copy number and determination of inhibitor susceptibility.
HCV RNA copy numbers were measured by using quantitative TaqMan real-time RT-PCR as described previously (16). The total cellular RNA in each sample was determined by comparing β-actin amount in the sample to a normal human RNA standard using TaqMan β-actin control reagents kit (Applied Biosystems) and ABI 7900 real-time PCR machine (Applied Biosystems) according to the protocols provided by the manufacturer. HCV RNA copy numbers were normalized by the total amount RNA in each sample when appropriate. Inhibitor susceptibility was evaluated by determining inhibitory effects on HCV replicon copy numbers in cells as described previously (16). Briefly, 4,000 cells per well were placed into 96-well plates, and a total of eight serial dilutions of each compound were added to various wells. Total cellular RNA was extracted after 4 days by using an RNeasy-96 kit (QIAGEN) according to the manufacturer's instructions and used for TaqMan quantitative real-time RT-PCR as described above.
RESULTS
Sensitivity and discriminatory ability of allele-specific real-time PCR assay.
The nucleotide codon change for HCV NS5B mutation M414T is from ATG for methionine to ACG for threonine. Allele-specific real-time PCRs were developed to selectively amplify genotype 1b wild-type or M414T mutant DNAs based on their sequence differences. Both wild-type and mutant allele-specific real-time PCRs used the same sense primer, M414-S; however, the two antisense primers differed only at the last nucleotide at the 3′ end, matching either the mutant or the wild-type sequences. An A at the 3′ end of the primer (414M) amplifies the wild-type DNA, whereas a G at the 3′ end of the primer (414T) amplifies the mutant DNA.
In order to overcome the problem of heterogeneity in the templates, the antisense primers (414M or 414T) for real-time PCR are contained within the antisense primer (M414-AS) used for the first round of PCR (Fig. 1), except for the 3′ end nucleotide of each primer that is specific for the 414M or 414T codon. This ensures that if the DNA fragment is amplified in the first round of PCR that product will also be amplified in the second round, the allele-specific real-time PCR. This also ensures that amplification differences between wild-type and mutant by real-time PCR are not due to nucleotide differences elsewhere in the primers.
FIG. 1.
Alignment of 21 genotype 1b sequences in the primer regions. The M414-S and M414-AS primers used for the first-round PCR are indicated by arrows at the top of the sequences. The arrow at the bottom of the right-hand sequence indicates the location of the antisense primers used in the allele-specific real-time PCRs. The shaded nucleotide indicates the position that differs between the 414M and 414T primers.
To assess the sensitivity of the real-time PCR assay, serial dilutions of wild-type or mutant DNA standard were used. Both wild-type and mutant allele-specific real-time PCRs were able to detect as few as 10 copies per reaction and had a 7-log10 linear dynamic range from 10 to 108 copies (Fig. 2).
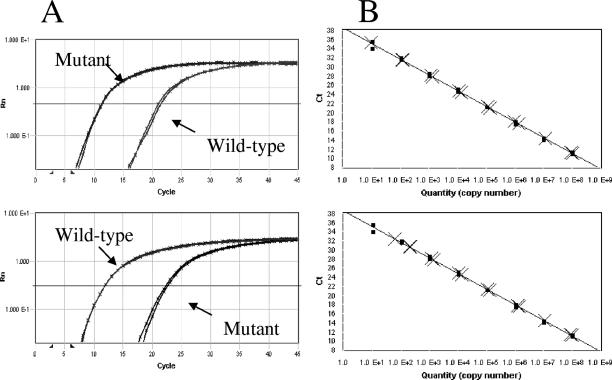
FIG. 2.
(A) Equal amounts of mutant or wild-type standard DNA templates were amplified with mutant-specific primers (upper panel) or wild-type-specific primers (lower panel), respectively. Both assays have a discriminatory ability of about 11 cycles. (B) Discriminatory ability of M414T-specific real-time PCR. A total of 104 (upper panel) or 105 (lower panel) copies of wild-type standard DNA were added to each serial dilution (108 to 101 copies) of mutant standard DNA. In each case, the CT was compared to PCRs performed without the addition of wild-type DNA. •, M414T standard only; ×, M414T mixed with 104 (upper panel) or 105 (lower panel) copies of wild-type DNA.
In order to evaluate the discriminatory ability of each assay, wild-type and mutant DNA standards derived from the genotype 1b-N strain were used for amplification with corresponding and noncorresponding primers. The threshold for detecting wild-type standard DNA by the M414T-specific primer was about 11 PCR cycles later than for the same amount of mutant standard DNA (Fig. 2A, upper panel). A similar discriminatory ability was observed for the amplification of both targets with the wild-type specific primer (Fig. 2A, lower panel). In addition, the discriminatory ability of the M414T-specific assay was tested in reciprocal mixing experiments by adding 104 (Fig. 2B, upper panel) or 105 (Fig. 2B, lower panel) copies of wild-type standard DNA to each serial dilution (108 to 10 copies) of mutant standard DNA. In each case, the CT was compared to PCRs performed without the addition of wild-type DNA. The discriminatory ability was about 3 log10 in each experiment. Furthermore, low amounts of mutant standard DNA were spiked into 105 copies of wild-type DNA. The copy numbers of mutant DNA detected from the mixtures by M414T-specific real-time PCR closely matched the amounts spiked into the reactions. Overall, the estimated discriminatory ability of allele-specific real-time PCRs for detection of mutant M414T as a minority population is 0.1%.
To evaluate whether RT and/or first-round PCR could change the ratio of mutant to wild type in an RNA sample, we mixed synthesized M414T mutant RNA with wild-type RNA such that the mutant RNA was present at 0, 0.1, 0.2, 0.4, 0.6, 0.8, 1, or 100%. After RT and first-round PCR, the M414T mutant/wild-type ratios in the PCR products were determined by using allele-specific real-time PCR. The results were very close to the actual ratios in the RNA samples, indicating that the RT and first-round PCR conditions used in the present study have very limited impact on M414T mutant/wild-type ratio (Table 1).
TABLE 1.
Detection of M414T mutant from RNA samplesa
| Actual mutant ratio (%) in RNA | Mean measured %M414Tb ± SD |
|---|---|
| 0 | 0.06 ± 0.02 |
| 0.10 | 0.16 ± 0.04 |
| 0.20 | 0.35 ± 0.11 |
| 0.40 | 0.56 ± 0.13 |
| 0.60 | 0.66 ± 0.16 |
| 0.80 | 0.75 ± 0.20 |
| 1.0 | 1.19 ± 0.66 |
| 100 | 99.82 ± 0.14 |
Synthesized M414T mutant RNA was added to wild-type RNA to reach the final percentages shown in the table. M414T percentages were measured in first-round PCR products.
%M414T = 100 × M414T copies/(M414T copies + wild-type copies). Data are shown as the means from four different experiments. The limit of detection of the M414T mutant/wild-type ratio in DNA samples is >0.1%.
The results obtained by allele-specific real-time PCR were also confirmed by a traditional TA clonal sequencing assay. The first-round PCR products generated from several samples were used for both allele-specific real-time PCR and clonal sequencing. As shown in Table 2, results obtained with both assays are in very good agreement; however, the traditional TA cloning assay was much more time-consuming and labor-intensive.
TABLE 2.
Comparison of M414T proportions obtained by allele-specific real-time PCR with a conventional clonal sequencing method
| Sampleb | Mean %M414Ta ± SD
|
|
|---|---|---|
| Real-time PCR | Clonal sequencing | |
| 1 | 7.50 ± 1.46 | 6.25 (1/16) |
| 2 | 9.65 ± 3.73 | 6.25 (1/16) |
| 3 | 22.95 ± 10.81 | 25 (4/16) |
| 4 | 47.56 ± 3.38 | 50 (8/16) |
Real-time PCR %M414T = 100 × M414T copies/(M414T copies + wild-type copies). Data are shown as the means of three different experiments. Clonal sequencing was calculated as the %M414T = 100 × M414T clones/total clones.
Samples were derived from the HCV 1b-N strain replicon cell line treated with polymerase inhibitor A-782759.
Detection of preexisting M414T mutation in HCV replicon cell lines and HCV-containing sera.
Two HCV 1b subgenomic replicon cell lines derived from two different strains (con1 and N) were evaluated for preexistence of the M414T mutation. These cell lines have been maintained and continually passaged in our laboratory for more than 3 months. A minor population of the M414T variant was detected from both con1 and N strain replicon cells at 0.22 and 0.18% of the total species, respectively (Table 3), indicating the preexistence of resistance mutation M414T in the treatment-naive replicon population.
TABLE 3.
Detection of M414T mutant from 1b replicon cell lines and HCV subtype 1b-infected subjects
| Replicon or subject no. | Sample | Mean %M414Ta ± SD |
|---|---|---|
| Replicon | 1b-conl | 0.22 ± 0.04 |
| 1b-N | 0.18 ± 0.05 | |
| Subject no. | 0712 | <0.1 |
| 1025 | <0.1 | |
| 1182 | <0.1 | |
| 1694 | 0.17 ± 0.05 | |
| 1934 | 0.19 ± 0.06 | |
| 4374 | 0.11 ± 0.04 | |
| 4376 | 0.60 ± 0.17 | |
| 4377 | 0.23 ± 0.06 | |
| 4393 | <0.1 | |
| 4409 | <0.1 | |
| 4410 | <0.1 | |
| 4411 | <0.1 | |
| 4413 | 0.12 ± 0.04 | |
| 4428 | <0.1 | |
| 4430 | <0.1 |
The %M414T = 100 × M414T copies/(M414T copies + wild-type copies). Data are shown as the means from three different experiments. The limit of detection of the M414T mutant is >0.1% in the population.
The assay for detection of the M414T mutation was also performed on samples from 20 treatment-naive individuals infected with HCV subtype 1b. We were unable to obtain first-round PCR products from five of the samples possibly due to RT or PCR primer mismatch because of the high level of sequence diversity of HCV genomes. M414T mutant/wild-type ratios above the discriminatory limit of detection for the assay were found in 6 of the remaining 15 samples ranging from 0.11 to 0.60% (Table 3). The M414T mutant levels in the other subjects were below the limit of detection for the assay (0.1%). The M414T mutation was not detected by population sequencing of RT-PCR products containing the entire NS5B gene from any of the 20 RNA samples.
Levels of the M414T mutants in replicon cells upon and after treatment with HCV polymerase inhibitor A-782759.
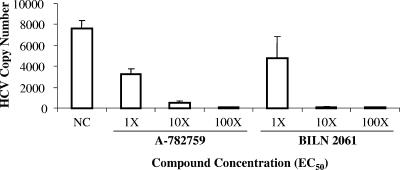
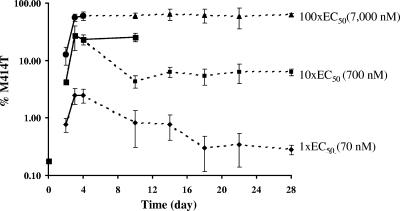
Since the M414T mutation confers high-level resistance to A-782759, it is of interest to understand its proportional change during and after treatment of replicon cells with A-782759. 1b-N replicon cells were treated with A-782759 at levels 1×, 10×, or 100× its EC50 for 4 days. The drug was then removed, and the cells were cultured under normal conditions for another 24 days (28 days total), with the exception of a portion of the 10× EC50 A-782759-treated cells that were continually cultured with the same concentration of inhibitor for up to 10 days. Replicon HCV RNA copy numbers were measured by TaqMan quantitative real-time PCR. At day 4, replicon HCV copy numbers were inhibited 58, 93, or 99% by 1×, 10×, or 100× the EC50 for A-782759, and 37, 99, or 99% by 1×, 10×, or 100× the EC50 for BILN-2061, respectively (Fig. 3). The proportion of M414T in the replicon population was monitored by allele-specific real-time PCR. As shown in Fig. 4, treatment with A-782759 rapidly increased the percentage of M414T mutants in the population. Treatment of replicon cells with a higher inhibitor concentration resulted in a higher prevalence of M414T mutants. At day 4, 2.5, 26, or 60% of the population was the M414T mutant upon treatment with A-782759 at 1×, 10×, or 100× its EC50, respectively (Fig. 4). From Fig. 4, we can see that with A-782759 treatment at 1×, 10×, or 100× its EC50, the percentage of M414T increased from a baseline level of 0.18 to 0.8, 4, or 12.6% at day 2 and of 2.5, 28, or 56% at day 3, respectively (Fig. 4, solid lines). The proportion of M414T mutant in each case reached a plateau after 3 days of treatment. We did not observe significant changes in M414T percentages between days 3 and 4 with inhibitor treatment at all concentrations (Fig. 4, solid lines). Furthermore, replicon cells continuously cultured with A-782759 at 10× its EC50 maintained the same percentage level of M414T in the population up to day 10 (Fig. 4, 10× EC50, solid line).
FIG. 3.
HCV copy numbers as measured by TaqMan quantitative real-time PCR in 1b-N replicon cells after compound treatment for 4 days. HCV RNA copy number is per 1 pg total RNA. NC, no compound control.
FIG. 4.
M414T mutant proportional changes in replicon cells treated with various concentrations of A-782759. At day 4, the inhibitor was removed from the replicon cells, except for where the solid line indicates that an 10× EC50 of A-782759 still applied to cells until day 10. Error bars represent the standard deviation from three different experiments.
Interestingly, M414T copy numbers obtained from the same numbers of cells treated for the same period of time were very similar between 1×, 10×, or 100× the EC50 of A-782759 treatment. Therefore, the difference in the prevalence of M414T mutants resulting from treatment with different concentrations of A-782759 was largely not due to changes in absolute M414T copy number but rather was due to the reduction of wild-type replicons and/or other non-M414T mutants. As a control, the protease inhibitor BILN 2061 did not change the level of M414T mutants at any drug concentrations (data not show).
To examine whether the HCV NS5B amino acid position 414 wild type will rebound or outgrow M414T mutants after treatment, we removed the inhibitor at day 4 and continuously cultured cells without A-782759 for another 24 days. Reductions in the percentage of M414T mutants were observed in both replicon cell lines treated with 1× or 10× the EC50 of A-782759 (Fig. 4). At day 10, the M414T percentage decreased from 2.5 to 0.9% in cells treated with 1× the EC50 of A-782759. The wild-type replicon continued to outgrow the M414T variants, and the M414T proportion was reduced to ∼0.3% of the total population at day 18. The M414T mutants were maintained at this level up to day 28, indicating that a balance between position 414 wild-type and mutant replicons had been reached. In replicon cells treated with 10× the EC50 of A-782759, the removal of drug pressure reduced the M414T variants more significantly, from ∼30% ∼4% at day 10. However, no further reduction was observed thereafter up to day 28. Four days of treatment with 100× the EC50 of A-782759 elevated the level of M414T mutants to ∼60% of the replicon population. It was interesting that withdrawal of inhibitor did not result in wild-type viral rebound in these cells. The M414T mutants were maintained at the same level up to day 28, indicating that all of the surviving replicons, including those containing the M414T mutation, could replicate at similar rates.
In a previous study, we had reported that the M414T mutation impaired viral growth by 94% (or retained 6% of wild-type replication capacity) (16). To confirm the high prevalence of the M414T mutation as measured by allele-specific real-time PCRs, we amplified the entire NS5B gene from replicon cells treated with 100× the EC50 of A-782759 at day 28. The PCR products were sequenced and cloned into a TA cloning vector. M414T was the only mutation to be found from the population sequencing of the PCR products. However, up to nine mutations were found in each individual clone (Table 4). Of the 15 TA clones that were sequenced, 8 were found to contain the mutation M414T (53% of the population) (Table 4). This number was in agreement with the results obtained by using real-time PCR (∼60%). Since the M414T mutation alone greatly reduced viral replication capacity (16), other coexisting mutations found in the same clone may help the replicon gain replication capacity. Mutations Y448H/C and G554D were found in four and two clones, respectively. These mutations had previously been identified as conferring resistance to benzothiadiazine HCV polymerase inhibitors (12, 16). Thus, only one TA clone (clone 1) did not contain an identifiable benzothiadiazine resistance mutation.
TABLE 4.
NS5B mutations in TA clonesa
| TA clone | NS5B mutations |
|---|---|
| 1 | Y296C, A334V |
| 2 | M71I, S113R, Q148R, A252V, C366R, I392T, M414T, L459P, A553T |
| 3 | M414T, Y586C |
| 4 | R280C, E361V, G554D |
| 5 | M414T, Q514R |
| 6 | R32C, A75V, Q241R, V281V, M414T |
| 7 | K270R, Y448H, P495L |
| 8 | Y448H, P495L |
| 9 | K270R, Y448H, P495L |
| 10 | G378D, M414T, L525P |
| 11 | Y448C, Y586C |
| 12 | M414T, Y586C |
| 13 | I23T, G351E, M414T |
| 14 | L60P, V67A, M414T, S513P, N590S |
| 15 | C146R, C445R, S540T, G554D |
TA clones contained NS5B genes amplified by RT-PCR. RNA was extracted from replicon cells that had been treated with 100× above the EC50 (7,000 nM) of A-782759 for 4 days and then continually cultured without drug for another 24 days. Mutation M414T is indicated in boldface.
It was also found that the higher prevalence of M414T mutants in replicon cells correlated with a lower susceptibility to A-782759. Compared to wild-type replicon cells, cells treated with concentrations 1×, 10×, or 100× the EC50 of A-782759 exhibited 1.6-, 5.7-, or >100-fold losses in susceptibility to A-782759, respectively, at day 28, whereas they were still sensitive to the protease inhibitor BILN 2061.
DISCUSSION
We describe here the development of sensitive and quantitative real-time PCR assays to detect the mutation M414T that confers high-level resistance to the potent benzothiadiazine HCV polymerase inhibitor A-782759. The allele-specific real-time PCR assays are based on fluorescent reactions of SYBR green with DNA and use gene-specific primers to discriminate mutants with single nucleotide changes. We have successfully used these assays to detect and monitor the preexisting resistance mutation M414T in cells containing subgenomic HCV replicons and in sera from HCV genotype 1b-infected individuals. Compared to traditional mutation detection assays, including direct population sequencing, clonal sequencing, or hybridization to allele-specific oligonucleotides, the real-time PCR assays developed here are easy to use and cost-efficient and therefore suitable for large-scale operation. However, these assays can only be used for detection of the specific mutation amplified by the allele-specific primers. For each additional mutation, new assays need to be designed and validated. Another drawback of the assays is that they do not allow the determination of whether other mutations coexist in the viral strain that could help to restore the fitness of the resistant mutant virus.
The allele-specific real-time PCR for the M414T mutant had a discriminatory limit of 0.1% mutant relative to the wild type. RT and first-round PCR did not change the M414T mutant/wild-type ratios in synthesized RNA samples since allele-specific real-time PCR detected the same mutant/wild-type ratios in the first-round PCR products as in the RNA samples. This is not surprising, since the primers used for RT and first-round PCR exactly match the sequences of these synthesized RNAs. It is expected that RT and/or first-round PCR should have the same efficiency to amplify both M414T mutant and wild type in RNA samples extracted from patient sera if both the mutant and the wild type have the same sequences in the regions of primer binding for RT and first-round PCR. However, if in a particular case gene sequence changes associated with the M414T mutation happened concomitantly in the primer regions as well, the RT reaction and/or first-round PCR could artificially change the M414T mutant/wild-type ratio in the first-round PCR products that would be used as a template in the allele-specific real-time PCR. In the present study, 9 of 15 samples had no detectable M414T mutant level (<0.1% of the wild type), and in these cases we cannot rule out the unlikely possibility that lack of detection is due to concomitant sequence changes in the regions of primer binding.
It is widely accepted that drug-resistant viruses are likely to preexist within the quasispecies and are selected upon the application of drug pressure (2, 22, 24, 30). However, this is the first report demonstrating the direct detection of the preexistence of HCV drug-resistant species in treatment-naive replicon cells or sera from HCV-infected individuals. Previously, we have estimated the frequency of HCV 1b-N replicons resistant to A-782759 and other inhibitors by selecting resistant colonies from cell populations in the presence of both HCV inhibitor and G418 for 3 to 4 weeks under conditions in which cells do not undergo splitting (11, 16). In a recent study, we reported a 0.62% resistance frequency to 10× the EC50 of A-782759 in 1b-N replicon cells (16), which indicated that 0.62% of the total replicon RNA species in the population could survive under the treatment. Here we determined that ∼30% of surviving replicons contained M414T in 1b-N replicons treated with the same concentration of A-782759. From the combination of these two independent studies, we conclude that ∼0.19% (0.62% × 30%) of the initial 1b-N replicon population contained the M414T mutation. This number was in agreement with the direct measurement of preexisting M414T mutant in treatment-naive 1b-N replicon cells by allele-specific real-time PCRs, which was 0.18%.
The levels of M414T-containing virus were variable among the HCV-infected subjects at baseline in the present study. This variation may indicate the influence of genetic background on the prevalence of a specific mutation. A higher level of drug-resistant variants at baseline could result in the faster accumulation of resistant mutants under drug pressure and in turn could indicate a faster loss of susceptibility to the drug. Therefore, the determination of the prevalence of key preexisting resistance mutations will be meaningful for predicting the development of resistance to a drug. It is expected that there will be large differences in the level of preexisting M414T mutants among HCV-infected individuals. The use of A-782759 monotherapy should be avoided in patients with a high level of preexisting M414T in their viral quasispecies.
Previously, we had observed that the M414T mutation greatly reduced replicon fitness when the single mutation was introduced by site-directed mutagenesis into a replicon vector (16). In the present study, after the withdrawal of 1× or 10× the EC50 of A-782759, we observed a rapid and significant decrease in the level of M414T mutant in replicon cells before a plateau was reached. This phenomenon indicated that other species, either wild-type and/or mutants containing other resistance mutations besides M414T, could outgrow the M414T mutants. However, it appears that different M414T strains exhibit different replication capacities, since some of the M414T variants were continually present in the population. These results demonstrate that HCV can overcome the loss of fitness induced by a mutation, possibly by acquiring additional compensatory mutations. In contrast to the results seen with 1× or 10× the EC50 of A-782759, there was no reduction in the percentage of M414T mutants after withdrawal of the inhibitor in replicon cells pretreated with A-782759 at 100× its EC50. In this case, it is possible that after selection with a very high level of inhibitor, the wild type was greatly reduced or eliminated and that mutants resistant to A-782759 were the most fit variants. Indeed, mutations were identified from all of the 15 sequenced clones, and 14 of them carried previously identified resistance mutations (M414T, Y448H/C, or G554D) (12, 16). It is interesting that multiple mutations were observed in each clone. Some of these mutations might be necessary for reversing the loss of replication fitness caused by certain unfit mutations such as M414T.
Clinical trials ongoing using small molecular inhibitors against HCV infection indicate that resistance mutations found in vivo correlate well with findings in vitro using HCV replicon systems (25). Viral rebound was observed from patients under monotherapy with protease inhibitor VX-950 as early as day 3 (23). In the present study, we observed rapid elevation in the level of M414T during a relatively short exposure to inhibitor and the continued presence of the mutation at a higher level after removal of inhibitor in vitro by using an HCV replicon system. Although it has been estimated that 1012 virions may be produced and cleared per day in an HCV-infected individual, the rate of virus production in a human liver cell may be significantly lower than that in a replicon cell (5, 17). Therefore, drug resistance arising from the selection of a dominant mutation may not accumulate as quickly as in replicon cultures, where high levels of RNA replication are supported in every cell harboring replicons. How quickly mutations conferring resistance to A-782759 accumulate in HCV-infected patients is still unknown. However, our results indicate that monotherapy using a benzothiadiazine polymerase inhibitor could result in fast viral breakthrough and permanent genotypic and phenotypic changes in patients. Whether or not this is true for other classes of HCV inhibitors remains to be seen.
In conclusion, we have developed sensitive allele-specific real-time PCR assays for quantitatively detecting the key NS5B mutation M414T. Our results indicate that resistant mutants preexist as a minor population in replicon cells and are selected within days of treatment with inhibitor. The findings from the present study suggest that early application of combination therapy of an HCV-specific inhibitor with interferon-based regimens or other classes of available inhibitors will be necessary to avoid quick viral rebound or treatment failure.
Acknowledgments
We thank our coworkers Tim Middleton and Chih-Ming Chen in Antiviral Research, Abbott Laboratories, for helpful discussions.
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Cohen, J. 1999. The scientific challenge of hepatitis C. Science 285:26-30. [DOI] [PubMed] [Google Scholar]
- 2.Domingo, E., L. Menendez-Arias, and J. J. Holland. 1997. RNA virus fitness. Rev. Med. Virol. 7:87-96. [DOI] [PubMed] [Google Scholar]
- 3.Grahovac, B., J. Bingulac-Popovic, B. Vucelic, I. Hrstic, R. Ostojic, V. Drazic, M. Balija, and D. Grgicevic. 2001. Dynamics of serum hepatitis C virus load and quasispecies complexity during antiviral therapy in patients with chronic hepatitis C. J. Clin. Virol. 20:85-89. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann, E., J. H. Lee, G. Marinos, M. Modi, and S. Zeuzem. 2003. Effect of ribavirin on hepatitis C viral kinetics in patients treated with pegylated interferon. Hepatology 37:1351-1358. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann, E., and S. Zeuzem. 2006. The kinetics of hepatitis C virus. Eur. J. Gastroenterol. Hepatol. 18:339-342. [DOI] [PubMed] [Google Scholar]
- 6.Lavanchy, D., and B. McMahon. 2000. Worldwide prevalence and prevention of hepatitis C, p. 185-202. In J. T. Liang and J. H. Hoofnagle (ed.), Hepatitis C. Academic Press, Inc., San Diego, CA.
- 7.Lech, W. J., G. Wang, Y. L. Yang, Y. Chee, K. Dorman, D. McCrae, L. C. Lazzeroni, J. W. Erickson, J. S. Sinsheimer, and A. H. Kaplan. 1996. In vivo sequence diversity of the protease of human immunodeficiency virus type 1: presence of protease inhibitor-resistant variants in untreated subjects. J. Virol. 70:2038-2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Pogam, S., H. Kang, S. F. Harris, V. Leveque, A. M. Giannetti, S. Ali, W. R. Jiang, S. Rajyaguru, G. Tavares, C. Oshiro, T. Hendricks, K. Klumpp, J. Symons, M. F. Browner, N. Cammack, and I. Najera. 2006. Selection and characterization of replicon variants dually resistant to thumb- and palm-binding nonnucleoside polymerase inhibitors of the hepatitis C virus. J. Virol. 80:6146-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin, C., K. Lin, Y. P. Luong, B. G. Rao, Y. Y. Wei, D. L. Brennan, J. R. Fulghum, H. M. Hsiao, S. Ma, J. P. Maxwell, K. M. Cottrell, R. B. Perni, C. A. Gates, and A. D. Kwong. 2004. In vitro resistance studies of hepatitis C virus serine protease inhibitors, VX-950 and BILN 2061: structural analysis indicates different resistance mechanisms. J. Biol. Chem. 279:17508-17514. [DOI] [PubMed] [Google Scholar]
- 10.Loubser, S., P. Balfe, G. Sherman, S. Hammer, L. Kuhn, and L. Morris. 2006. Decay of K103N mutants in cellular DNA and plasma RNA after single-dose nevirapine to reduce mother-to-child HIV transmission. AIDS 20:995-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu, L., T. Pilot-Matias, K. D. Stewart, J. Randolph, R. Pithawalla, W. He, P. Huang, L. Klein, H. Mo, and A. Molla. 2004. Mutations conferring resistance to a potent hepatitis C virus serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 48:2260-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu, L., P. Krishman, R. Pithawalla, T. Dekhtyar, T. Ng, W. He, T. Pilot-Matias, D. Larson, T. Bosse, R. Wagner, D. Kempf, A. Molla, and H. Mo. 2006. Selection and characterization of hepatitis C virus replicons resistant to a potent polymerase inhibitor A-837093. Hepatology. 44(Suppl. 1):351A. [Google Scholar]
- 13.Martell, M., J. I. Esteban, J. Quer, J. Genesca, A. Weiner, R. Esteban, J. Guardia, and J. Gomez. 1992. Hepatitis C virus (HCV) circulates as a population of different but closely related genomes: quasispecies nature of HCV genome distribution. J. Virol. 66:3225-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metzner, K. J., S. Bonhoeffer, M. Fischer, R. Karanicolas, K. Allers, B. Joos, R. Weber, B. Hirschel, L. G. Kostrikis, H. F. Gunthard, et al. 2003. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. J. Infect. Dis. 188:1433-1443. [DOI] [PubMed] [Google Scholar]
- 15.Migliaccio, G., J. E. Tomassini, S. S. Carroll, L. Tomei, S. Altamura, B. Bhat, L. Bartholomew, M. R. Bosserman, A. Ceccacci, L. F. Colwell, R. Cortese, R. De Francesco, A. B. Eldrup, K. L. Getty, X. S. Hou, R. L. LaFemina, S. W. Ludmerer, M. MacCoss, D. R. McMasters, M. W. Stahlhut, D. B. Olsen, D. J. Hazuda, and O. A. Flores. 2003. Characterization of resistance to non-obligate chain-terminating ribonucleoside analogs that inhibit hepatitis C virus replication in vitro. J. Biol. Chem. 278:49164-49170. [DOI] [PubMed] [Google Scholar]
- 16.Mo, H., L. Lu, T. Pilot-Matias, R. Pithawalla, R. Mondal, S. Masse, T. Dekhtyar, T. Ng, G. Koev, V. Stoll, K. D. Stewart, J. Pratt, P. Donner, T. Rockway, C. Maring, and A. Molla. 2005. Mutations conferring resistance to a hepatitis C virus (HCV) RNA-dependent RNA polymerase inhibitor alone or in combination with an HCV serine protease inhibitor in vitro. Antimicrob. Agents Chemother. 49:4305-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann, A. U., N. P. Lam, H. Dahari, D. R. Gretch, T. E. Wiley, T. J. Layden, and A. S. Perelson. 1998. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 282:103-107. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen, T. T., A. T. Gates, L. L. Gutshall, V. K. Johnston, B. Gu, K. J. Duffy, and R. T. Sarisky. 2003. Resistance profile of a hepatitis C virus RNA-dependent RNA polymerase benzothiadiazine inhibitor. Antimicrob. Agents Chemother. 47:3525-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer, S., V. Boltz, F. Maldarelli, M. Kearney, E. K. Halvas, D. Rock, J. Falloon, R. T. Davey, Jr., R. L. Dewar, J. A. Metcalf, J. W. Mellors, and J. M. Coffin. 2006. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. AIDS 20:701-710. [DOI] [PubMed] [Google Scholar]
- 20.Paolucci, S., F. Baldanti, G. Campanini, M. Zavattoni, E. Cattaneo, L. Dossena, and G. Gerna. 2001. Analysis of HIV drug-resistant quasispecies in plasma, peripheral blood mononuclear cells and viral isolates from treatment-naive and HAART patients. J. Med. Virol. 65:207-217. [DOI] [PubMed] [Google Scholar]
- 21.Pawlotsky, J. M. 2000. Hepatitis C virus resistance to antiviral therapy. Hepatology 32:889-896. [DOI] [PubMed] [Google Scholar]
- 22.Pawlotsky, J. M. 2006. Therapy of hepatitis C: from empiricism to eradication. Hepatology 43:S207-S220. [DOI] [PubMed] [Google Scholar]
- 23.Reesink, H. W., S. Zeuzem, C. J. Weegink, N. Forestier, A. Van Vliet, J. Van de Wetering de Rooij, L. McNair, S. Purdy, R. Kauffman, J. Alam, and P. L. M. Jansen. 2006. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase 1b, placebo-controlled, randomized study. Gastroenterology 131:997-1002. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro, R. M., and S. Bonhoeffer. 2000. Production of resistant HIV mutants during antiretroviral therapy. Proc. Natl. Acad. Sci. USA 97:7681-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarrazin, C., T. Kieffer, D. Bartels, B. Hanzelka, U. Muh, M. Welker, D. Wincheringer, C. Lin, T. Grossman, S. Purdy, C. Weegink, H. Reesink, S. Zeuzem, and A. Kwong. 2005. Characterization of viral variants in the HCV NS3 protease domain of genotype 1 patients that are selected during 14 days of dosing with VX-950. Hepatology 42(Suppl. 1):537A. [Google Scholar]
- 26.Sumpter, R., Jr., C. Wang, E. Foy, Y. M. Loo, and M. Gale, Jr. 2004. Viral evolution and interferon resistance of hepatitis C virus RNA replication in a cell culture model. J. Virol. 78:11591-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomei, L., S. Altamura, L. Bartholomew, M. Bisbocci, C. Bailey, M. Bosserman, A. Cellucci, E. Forte, I. Incitti, L. Orsatti, U. Koch, R. De Francesco, D. B. Olsen, S. S. Carroll, and G. Migliaccio. 2004. Characterization of the inhibition of hepatitis C virus RNA replication by nonnucleosides. J. Virol. 78:938-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong, X., R. Chase, A. Skelton, T. Chen, J. Wright-Minogue, and B. A. Malcolm. 2006. Identification and analysis of fitness of resistance mutations against the HCV protease inhibitor SCH 503034. Antivir. Res. 70:28-38. [DOI] [PubMed] [Google Scholar]
- 29.Tong, X., Z. Guo, J. Wright-Minogue, E. Xia, A. Prongay, V. Madison, P. Qiu, S. Venkatraman, F. Velazquez, F. G. Njoroge, and B. A. Malcolm. 2006. Impact of naturally occurring variants of HCV protease on the binding of different classes of protease inhibitors. Biochemistry 7:1353-1361. [DOI] [PubMed] [Google Scholar]
- 30.Trozzi, C., L. Bartholomew, A. Ceccacci, G. Biasiol, L. Pacini, S. Altamura, F. Narjes, E. Muraglia, G. Paonessa, U. Koch, R. De Francesco, C. Steinkuhler, and G. Migliaccio. 2003. In vitro selection and characterization of hepatitis C virus serine protease variants resistant to an active-site peptide inhibitor. J. Virol. 77:3669-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 32.Wright, M., J. Main, and H. C. Thomas. 2001. Treatment of chronic viral hepatitis. Antivir. Chem. Chemother. 12:201-212. [DOI] [PubMed] [Google Scholar]