Abstract
Bacteriolytic antibiotics cause the release of bacterial components that augment the host inflammatory response, which in turn contributes to the pathophysiology of brain injury in bacterial meningitis. In the present study, antibiotic therapy with nonbacteriolytic daptomycin was compared with that of bacteriolytic ceftriaxone in experimental pneumococcal meningitis, and the treatments were evaluated for their effects on inflammation and brain injury. Eleven-day-old rats were injected intracisternally with 1.3 × 104 ± 0.5 × 104 CFU of Streptococcus pneumoniae serotype 3 and randomized to therapy with ceftriaxone (100 mg/kg of body weight subcutaneously [s.c.]; n = 55) or daptomycin (50 mg/kg s.c.; n = 56) starting at 18 h after infection. The cerebrospinal fluid (CSF) was assessed for bacterial counts, matrix metalloproteinase-9 levels, and tumor necrosis factor alpha levels at different time intervals after infection. Cortical brain damage was evaluated at 40 h after infection. Daptomycin cleared the bacteria more efficiently from the CSF than ceftriaxone within 2 h after the initiation of therapy (log10 3.6 ± 1.0 and log10 6.3 ± 1.4 CFU/ml, respectively; P < 0.02); reduced the inflammatory host reaction, as assessed by the matrix metalloproteinase-9 concentration in CSF 40 h after infection (P < 0.005); and prevented the development of cortical injury (cortical injury present in 0/30 and 7/28 animals, respectively; P < 0.004). Compared to ceftriaxone, daptomycin cleared the bacteria from the CSF more rapidly and caused less CSF inflammation. This combined effect provides an explanation for the observation that daptomycin prevented the development of cortical brain injury in experimental pneumococcal meningitis. Further research is needed to investigate whether nonbacteriolytic antibiotic therapy with daptomycin represents an advantageous alternative over current bacteriolytic antibiotic therapies for the treatment of pneumococcal meningitis.
Bacterial meningitis causes high rates of mortality and morbidity, despite efficient antibiotic treatment. Long-term sequelae of the disease are common and include hearing loss, seizures, sensory-motor deficits, and impairment of learning and memory (15). Streptococcus pneumoniae is among the most common causes of bacterial meningitis (7, 59) and is associated with the highest incidence of sequelae in both children (40) and adults (55, 58). The inefficiency of the host immune system at controlling S. pneumoniae infections in the cerebrospinal fluid (CSF) allows the rapid growth of the bacteria. As a consequence, the proliferation and autolysis of the bacteria produce large quantities of proinflammatory bacterial components. These contribute to the intense inflammatory reaction that contributes to the development of damage to the brain (21, 33, 54). Bactericidal antibiotic regimens reduce the overall release of bacterial components, which would otherwise occur during unhindered replication and autolysis. Yet, cell wall-active antibacterials can temporarily enhance the liberation of bacterial components (38). This has been shown to cause a burst of meningeal inflammation during experimental meningitis (37, 52). In contrast, antibiotics acting on bacterial DNA, RNA, or protein synthesis may reduce the level of inflammation by limiting the release of bacterial components. Daptomycin, a new lipopeptide antibiotic, causes rapid bactericidal activity without cell lysis (48, 56). Its action results from depolarization of the bacterial cytoplasmic membrane, resulting in the cessation of RNA/protein synthesis (1). Daptomycin treatment could therefore provide an advantage by reducing the amounts of proinflammatory bacterial products released.
Recently, daptomycin was shown to be highly efficacious against penicillin- and quinolone-resistant pneumococci in an experimental rabbit model of meningitis (9). The high incidence of penicillin-nonsusceptible S. pneumoniae strains (10, 11, 30) and the observed increase in the incidence of cephalosporin-resistant and vancomycin-tolerant strains may jeopardize the use of cefotaxime/ceftriaxone combined with vancomycin for the treatment of pneumococcal meningitis (53). Daptomycin would therefore represent a valuable therapeutic option.
The aim of this study was to determine the potential beneficial effect of daptomycin therapy over ceftriaxone therapy in an infant rat model of meningitis in terms of inflammatory parameters and brain damage.
(Part of this work was presented at the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2005, Washington, DC).
MATERIALS AND METHODS
Animal model of meningitis.
All animal studies were approved by the Animal Care and Experimentation Committee of the Canton Bern, Bern, Switzerland, and followed National Institutes of Health guidelines for the performance of experiments with animals. A well-characterized model of infant rat pneumococcal meningitis was used (3, 18, 25, 29, 43). Eleven-day-old Wistar rats (n = 111; Charles River, Germany) were injected intracisternally with 10 μl of saline containing 1.3 × 104 ± 0.5 × 104 CFU of Streptococcus pneumoniae (a clinical isolate of a serotype 3 strain) or with 10 μl of sterile, pyrogen-free saline (n = 14) as controls. Eighteen hours later, CSF samples were obtained by puncture of the cisterna magna and cultured quantitatively on sheep blood agar plates to document that they had meningitis. The number of bacteria in the CSF was determined by plating serial dilutions of 10 μl of CSF on blood agar plates (34). The animals were randomized to receive daptomycin (n = 56; 50 mg/kg of body weight subcutaneously [s.c.]; Cubicin; kindly provided by Cubist Pharmaceuticals Inc., Lexington, MA) or ceftriaxone (n = 55; 100 mg/kg of body weight s.c.; Rocephine; Roche Pharma) (3, 18, 25, 29, 43). A second intracisternal puncture was performed at defined time points after the infection, i.e., 20 h (n = 16 for each treatment group), 24 h (n = 9 for each treatment group), and 40 h (n = 30 for daptomycin and n = 28 for ceftriaxone) after infection; and the bacterial titers in the CSF were determined. The withdrawal of CSF by intracisternal puncture did not consistently yield sufficient volumes for the assessment of all CSF parameters. Two animals treated with ceftriaxone and one animal treated with daptomycin died prematurely, and data for those animals were excluded from the evaluation of the study results.
MIC determination.
For MIC determination, blood agar plates were inoculated with the strain used in this study, and the MICs of ceftriaxone and daptomycin were determined by using antibiotic gradient strips (Etest; AB Biodisk, Solna, Sweden), according to the manufacturer's recommendations. Reference strain ATCC 49619 was tested in parallel.
Determination of TNF-α levels in CSF.
Harvested CSF samples were cleared of bacteria and leukocytes by centrifugation (13,000 rpm at 4°C) for 5 min. CSF samples were diluted 20-fold, and tumor necrosis factor alpha (TNF-α) levels were determined by enzyme-linked immunosorbent assay (ELISA), according to the instructions of the manufacturer. The interassay coefficient of variance is less than 10% for 20 independent assays, according to the manufacturer (detection limit, <5 pg/ml; Rat TNF-α Quantikine ELISA; R&D Systems, Abington, United Kingdom).
Quantification of MMP-9 in CSF.
The amount of matrix metalloprotease-9 (MMP-9) was assessed by gel zymography, as described previously (25). In brief, the proteins from 3 μl of CSF samples were separated by electrophoresis under nonreducing conditions in 10% polyacrylamide-sodium dodecyl sulfate gels containing 1% gelatin (Sigma). After separation of the proteins, the gels were incubated for 1 h in sodium dodecyl sulfate removal buffer (2.5% Triton X-100 in distilled water), followed by incubation in incubation buffer (50 mM NaCl, 10 mM CaCl2, 50 mM Tris, pH 7.6) for 18 h at 37°C. The gelatinolytic activity of MMP-9 was determined by densitometric quantification of the substrate lysis zones around 92 kDa (MMP-9) and 72 kDa (MMP-2) in gels stained with Coomassie blue. Since MMP-2 is constitutively present in CSF and changes in its levels in bacterial meningitis are 10- to 100-fold smaller than those of MMP-9 (47), it served as an internal control for the semiquantitative assessment of MMP-9. Therefore, the amount of MMP-9 was expressed as an MMP-9/MMP-2 index (25, 26, 32, 35).
Histology and morphometry.
For histopathological examination, the animals were killed by an overdose of pentobarbital (100 mg/kg intraperitoneally) and were subsequently perfused with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS). Their brains were removed and fixed in PFA and then cryopreserved in 18% sucrose in PBS at 4°C overnight. Twelve coronal brain sections per animal were evaluated for neuronal injury of the cortex and hippocampus, as previously described in detail (43). The area of cortical necrosis was expressed as the percentage of the total area of the cortex in each section, and the mean value per animal was calculated. For apoptosis, the numbers of cells with morphological changes compatible with apoptosis (fragmented cell bodies, condensed nuclei) were counted in three visual fields (×400) in each of the four blades of the dentate gyrus. The average number per animal was calculated from all sections evaluated. Histopathological evaluations were done by investigators who were unaware of the clinical, microbiologic, and treatment data for the animal.
Statistical analysis.
Statistical analyses were performed by using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Comparison of the data between two groups was done by using the unpaired Student t test. For data that were not normally distributed, the Mann-Whitney U test was used. For comparison of cortical injury, a value of 0 was assigned to the absence of cortical damage, and the Wilcoxon signed rank test was used to determine significance. For comparison of the differences in the numbers of animals with cortical damage, a contingency table was created, and significance was determined by the chi-square test. A P value <0.05 was considered statistically significant.
RESULTS
MICs of daptomycin and ceftriaxone.
The MIC of daptomycin for the S. pneumoniae strain (serotype 3) used in this study was determined to be 0.25 mg/liter. This corresponds to the value obtained for reference strain ATCC 49619 (0.38 mg/liter) and is in the range of values (between 0.06 and 0.5 mg/liter) which have been described for 346 other pneumococcal strains (41). The ceftriaxone MIC for the pneumococcal strain used in the present study was 0.023 mg/liter, and the MIC for reference strain ATCC 49619 was 0.094 mg/liter.
Kinetics of bacterial clearance from CSF by daptomycin or ceftriaxone.
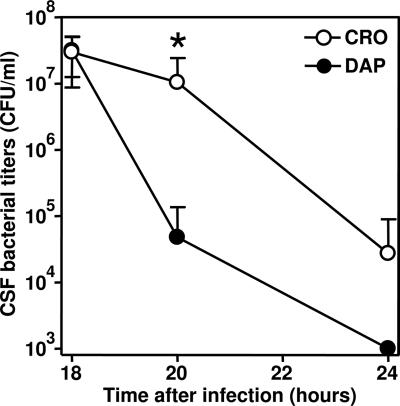
Daptomycin was more efficient than ceftriaxone at decreasing the bacterial titer 20 h after infection, i.e., 2 h after the initiation of the therapy (P < 0.015; n = 9 for both treatment groups). Four hours later, the CSF bacterial titers for all daptomycin-treated animals were below the detection limit (<103 CFU/ml) (Fig. 1). At 40 h after infection, none of the CSF samples of any of the animals showed bacterial growth.
FIG. 1.
Bacterial titers in CSF of daptomycin (DAP)- and ceftriaxone (CRO)-treated animals. Titers were determined before initiation of the therapy (18 h after infection; n = 18 for each group), 2 h later (20 h after infection; n = 9 per group), and 6 h later (24 h after infection; n = 9). The origin of the y axis was set to the bacterial titer detection limit of 103 CFU/ml. *, titers were significantly different at 20 h after infection (P < 0.015). At 24 h after infection, the titers for the daptomycin-treated animals were under the detection limit.
CSF TNF-α levels.
We measured the levels of the proinflammatory cytokine TNF-α in CSF 2 and 6 h after administration of the antibiotic. Although the concentration of TNF-α in the CSF was higher in the ceftriaxone-treated group (4,578 ± 928 versus 3,059 ± 576 pg/ml in the daptomycin-treated group), this difference did not reach statistical significance. At 24 h after infection, the TNF-α levels in CSF were markedly decreased and there were no significant differences in the TNF-α levels between the two groups (249 ± 73 pg/ml for the ceftriaxone-treated group versus 352 ± 145 pg/ml for daptomycin-treated group).
Assessment of MMP-9 concentrations by gel zymography.
MMP-9 was quantified by gel zymography at 20, 24, and 40 h after infection. At 20 h and 24 h, there were no statistically significant differences in the levels of MMP-9 between the ceftriaxone- and the daptomycin-treated groups. However, at 40 h after infection, while the amount of MMP-9 remained elevated in the CSF of the ceftriaxone-treated animals, the MMP-9 level in the CSF of the daptomycin-treated animals was significantly lower (Table 1). The CSF of all groups of infected animals displayed higher levels of MMP-9 than the CSF of control, uninfected animals (0.085 ± 0.124, n = 6).
TABLE 1.
MMP-9 levels in CSF of daptomycin- and ceftriaxone-treated animals 20, 24, and 40 h after infection, expressed as MMP-9/MMP-2 indices
| Time after infection | MMP-9/MMP-2 index with:
|
P valuea | |
|---|---|---|---|
| CRO treatment | DAP treatment | ||
| 20 h | 1.7 ± 1.2 (6) | 1.6 ± 1.0 (4) | NS |
| 24 h | 1.1 ± 0.6 (4) | 1.2 ± 0.9 (3) | NS |
| 40 h | 1.8 ± 1.4 (13) | 0.6 ± 0.5 (16) | <0.005 |
A significant difference (P < 0.005) between the two experimental groups was evident at 40 h after infection. P values were obtained by the Mann-Whitney test. NS, not significant.
Assessment of cortical brain damage.
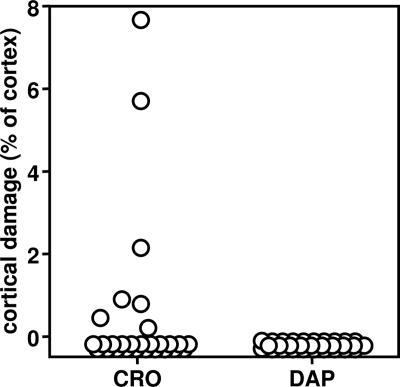
Cortical damage was assessed at 40 h after infection. Only the animals treated with ceftriaxone displayed cortical damage, ranging from 0.26% to 7.62% of the total cortical area (Fig. 2 and 3).
FIG. 2.
Differences in the extent of cerebral cortical damage 40 h after infection in animals with experimental pneumococcal meningitis treated with ceftriaxone (CRO) and that in animals with experimental pneumococcal meningitis treated with daptomycin (DAP). Only the animals treated with ceftriaxone showed cortical brain damage, ranging for 0.26% to 7.62% of the total cortex area. In contrast, the daptomycin-treated animals had no cortical damage (P < 0.02, Wilcoxon signed rank test).
FIG. 3.
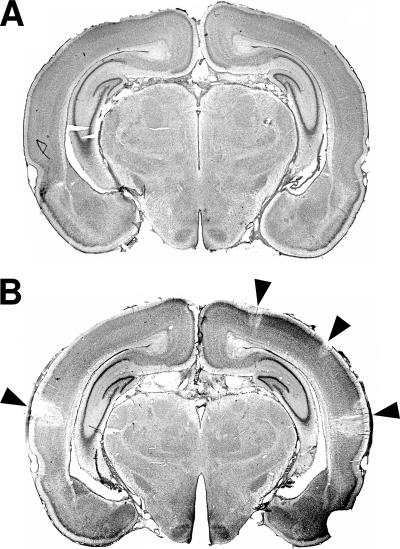
Representative histological sections of daptomycin-treated (A) or ceftriaxone-treated (B) animals stained with cresyl violet 40 h after infection. Regions of decreased neuronal density (arrowheads) were found only in the cortices of the ceftriaxone-treated animals.
The number of animals exhibiting morphological evidence of cortical damage (0/30 daptomycin-treated animals versus 7/28 ceftriaxone-treated animals) was significantly different (P < 0.004, chi-square test) between the two groups.
In the group treated with ceftriaxone, the level of MMP-9 in the CSF was significantly higher in animals that showed cortical brain injury than in those without cortical brain damage (1.3 ± 1.0 [n = 10] and 3.4 ± 1.3 [n = 3], respectively; P < 0.05).
Assessment of apoptotic brain injury in the dentate gyrus.
Apoptosis in the hippocampal dentate gyrus was assessed at 20, 24, and 40 h after infection (Table 2). Two hours after the initiation of the antibiotic therapy, the numbers of apoptotic cells in the dentate gyrus were similar for both daptomycin- and ceftriaxone-treated animals. At 24 h after infection, the amount of hippocampal apoptotic damage increased in both groups, peaking at between 24 and 36 h after infection, in accordance with previous observations (14). At 40 h after infection, the number of apoptotic cells returned to the level found at 20 h. The clearance of apoptotic cells by phagocytic cells, including macrophages and microglia, contributes to the regression of apoptosis over time (42). For all time points tested, the level of apoptosis in the dentate gyri in both daptomycin- and ceftriaxone-treated animals was higher than that in the dentate gyri of the uninfected control animals (Table 2).
TABLE 2.
Apoptotic cell numbers in dentate gyrus of animals with experimental pneumococcal meningitis treated with ceftriaxone compared with those in animals treat with daptomycin
| Time (h) after infection | No. (%) of apoptotic cells in dentate gyrus witha:
|
|
|---|---|---|
| CRO treatment | DAP treatment | |
| 20 | 5.6 ± 3.8 (9) | 5.5 ± 2.4 (9) |
| 24 | 18.4 ± 7.6 (9) | 14.9 ± 11.5 (9) |
| 40 | 9.0 ± 9.6 (28) | 6.1 ± 10.2 (30) |
Apoptotic cells were more frequent in the ceftriaxone-treated animals at all time points tested, but the differences between all treatment groups did not reach statistical significance. CRO, ceftriaxone; DAP, daptomycin.
DISCUSSION
Daptomycin belongs to a new class of bactericidal antibiotics called lipopeptides which are able to rapidly kill clinically relevant gram-positive bacteria. The present study demonstrates a beneficial effect of therapy with daptomycin over that with ceftriaxone in an infant rat model of experimental pneumococcal meningitis. This was reflected by the more rapid decrease in the CSF bacterial titers, the decreased concentration of MMP-9 in the CSF, and the prevention of cortical injury.
The mechanisms of brain injury have been investigated in patients who have died from bacterial meningitis and in corresponding experimental disease models (21). Once the bacteria have gained access to the CSF, they multiply and, by releasing bacterial products such as cell wall fragments, induce an inflammatory response that is intensified by the bacteriolytic effects of antibiotics (5, 13, 39, 51). The inflammatory reaction in the subarachnoid and ventricular spaces induces cerebral vasculitis and brain edema, leading to cerebral ischemic injury, preferentially in the cerebral cortex (21). In the hippocampal dentate gyrus, neurons undergo programmed cell death, characterized by caspase-dependent apoptosis (14). The damage to the hippocampus likely represents the histopathological correlate to the learning and memory deficits frequently observed in children who have survived bacterial meningitis (15, 23).
Dexamethasone is currently the only therapy used clinically that has been reported to be effective in improving the survival in patients with pneumococcal meningitis. To be effective, however, adjunctive dexamethasone therapy must be started prior to or simultaneously with the antibiotic treatment, a therapeutic regimen that may be difficult to implement in emergency situations. This was impressively demonstrated in a recent prospective, multicenter, observational study of 156 consecutive adults hospitalized for pneumococcal meningitis in which adjunctive steroids were given to 65 patients (42%), but only 16 received corticosteroids concomitantly or within 30 min of administration of the first antibiotic dose (2). Equally important is the fact that although adjunctive dexamethasone therapy reduces the mortality rate, a significant beneficial effect on neurological sequelae has not been conclusively demonstrated to date. Recent investigations of the long-term outcomes for adults with bacterial meningitis showed no significant differences between patients treated with dexamethasone and those treated with a placebo. The proportions of patients with persisting neurological sequelae or hearing loss were similar in the dexamethasone and the placebo groups. The overall rate of cognitive dysfunction did not differ significantly between the patients and the control subjects (57). Moreover, experimental studies suggest that therapy with dexamethasone may even aggravate brain damage and the corresponding long-term deficits (24, 49, 60).
The beneficial effect of daptomycin on brain injury is likely due to its multiple effects, including its rapid killing and its attenuation of the inflammation. First, daptomycin was more rapid than ceftriaxone at clearing pneumococci from the CSF. It is well documented that a rapid sterilization of the CSF is a prognostic factor for a favorable outcome in patients with bacterial meningitis (22). In this study, we used a daptomycin dosage of 50 mg/kg administered s.c. This is in accord with the findings of a study with adult rats, in which a similar dosage (40 mg/kg administered s.c.) resulted in a maximum concentration in serum and an area under the concentration-time curve from 0 to 24 h for serum comparable to those obtained in humans administered 6 mg/kg intravenously (45). The dosage used in the rabbit experimental model (15 mg/kg) also achieved similar peak levels in serum (9). Higher dosages up to 12 mg/kg (once daily for 14 days) have been shown to be well tolerated (4). However, such a high dosage is likely unnecessary, since it has been shown that higher doses do not increase the antibacterial efficacy, since sufficient CSF level/MIC and area under the concentration-time curve for CSF/MIC ratios can be obtained with lower dosages (9). No data on the pharmacokinetics of daptomycin in infants are currently available, but the pharmacokinetics of daptomycin in infants are likely to be different from those in adults. Nevertheless, the dosage used in the present study was sufficient to clear the CSF of bacteria at a rate faster than that achieved with ceftriaxone.
A second attribute of daptomycin which makes it attractive for the therapy of bacterial meningitis is that it acts in a nonbacteriolytic way. The effect of daptomycin has been proposed to result from a calcium-dependent depolarization of the cytoplasmic membrane (1). The resulting collapse in the electrochemical gradient may then be responsible for the observed cessation in the biosynthesis of proteins, RNA, DNA, peptidoglycans, lipoteichoic acids (LTAs), and lipids. Other mechanisms could also be involved, since it has been demonstrated that in nongrowing cells treated with daptomycin, membrane depolarization occurred after cell death (17). In the CSF of rabbits with experimental pneumococcal meningitis, therapy with daptomycin led to an approximately 10-fold lower release of 3H-labeled choline, assessed as an index of bacterial cell wall lysis, compared to that achieved by therapy with ceftriaxone (49a).
The release of proinflammatory components of bacterial origin (LTA and peptidoglycans for gram-positive bacteria) has been shown to be one of the first steps in the pathophysiology of bacterial meningitis. LTA is able to activate the innate immune response through activation of the Toll-like receptor TLR2 (12, 19, 20, 28). Furthermore, LTA concentrations in CSF have been shown to be associated with neurological sequelae and mortality in patients with pneumococcal meningitis (46). Peptidoglycans from gram-positive bacteria contain highly inflammatory substructures, in the form of trimers or more complex structures, which might be revealed in vivo during endogenous bacterial wall remodeling or bacterial hydrolysis caused by an exogenous host lysozyme or antibiotics (36). We postulate that daptomycin treatment results in the attenuated release of such proinflammatory cell wall components. We did not measure the concentration of LTA- or peptidoglycan-derived constituents in the CSF in the present study, but the attenuated release of membrane- or cell wall-bound [3H]choline in the CSF has been observed in the rabbit model, as well as in in vitro experiments, when daptomycin and ceftriaxone antibiotic treatments were compared (49a).
The use of nonbacteriolytic antibiotics has been shown to exert potentially beneficial effects in the treatment of bacterial meningitis. In in vitro experiments, the release of LTA/teichoic acid during the treatment of S. pneumoniae infections with different antibiotics that inhibit bacterial protein synthesis (rifampin, rifabutin, quinupristin-dalfopristin, or clindamycin) was attenuated compared to that observed when ceftriaxone or meropenem treatment was used (16, 31, 50). Similarly, group B streptococci exposed to rifampin or clindamycin (as opposed to ampicillin or cefotaxime) reduced the levels of production of inflammatory mediators by murine macrophages upon stimulation (6). In an experimental model of meningitis, the use of clindamycin, rifampin, and quinupristin-dalfopristin has also been proven to be beneficial in terms of LTA release, neurological damage, and/or mortality (5, 13, 39, 51).
Assessment of the TNF-α concentration in the CSF 2 and 6 h after the initiation of antibiotic therapy with either ceftriaxone or daptomycin did not document significant differences. However, at 40 h after infection, we demonstrated a statistically significantly lower concentration of MMP-9 in the CSF of animals treated with daptomycin compared with that in the CSF of animals treated with ceftriaxone. Brain damage starts to evolve at about 20 h after infection in the experimental meningitis model. However, the processes that underlie brain damage persist for several days after the initiation of antibiotic therapy. Recent experimental and clinical evidence supports this concept. In experimental pneumococcal meningitis, assessment of mRNA expression in brain regions prone to injury revealed that the genes relevant for brain damage have not yet reached their peak expression even at 40 h after infection, i.e., 22 h after the initiation of antibiotic therapy (8). One of these genes encodes the endogenous tissue inhibitor of MMP-9 (TIMP-1) (47). TIMP-1 primarily complexes with MMP-9 and thus inhibits the biological effect of excess MMP-9. In a patient with pneumococcal meningitis, increasing levels of MMP-9 in CSF were associated with persisting vasculitis and delayed brain injury beyond 4 days after the initiation of antibiotic therapy (44). MMP-9 has been identified as an important contributor to brain damage in patients with bacterial meningitis (25). In children with meningitis, the amount of MMP-9 in the CSF represents a risk factor for an adverse outcome (26). In situ zymography experiments detected increased gelatinolytic activity within the meninges, the subarachnoid space, the penetrating vessels, and the perivascular space. An excess of MMP-9 has been found to contribute to the development of cortical lesions in patients with bacterial meningitis and in experimental models (44, 47). In children with bacterial meningitis, high levels of MMP-9 were identified as a risk factor for persisting neurological sequelae (27). This clinical observation falls in line with the present data, which showed significantly higher MMP-9 levels in ceftriaxone-treated animals with cortical brain injury than in those with no brain damage. MMP inhibition resulted in a decrease in cortical damage in experimental pneumococcal meningitis (23, 35). In accordance with these findings, we observed a decrease in the MMP-9 level in association with an attenuation of cortical damage in daptomycin-treated animals.
In conclusion, using a infant rat experimental model of pneumococcal meningitis, we compared daptomycin therapy with ceftriaxone therapy. The more rapid clearance of bacteria in the CSF in association with less inflammation and cortical damage identifies treatment with daptomycin as a new, potentially valuable approach for further research into the treatment of pneumococcal meningitis.
Acknowledgments
We thank Judith Steenbergen (Cubist Pharmaceuticals Inc.) for sharing data and for critical reading of the manuscript and Sara Droz, Ursula Ackermann, and Jürg Kummer (Institute for Infectious Diseases; University of Bern) for excellent technical support.
This work was supported by grants from the Swiss National Science Foundation (grant 632-66057.01) and NIH (grant 2P50NS035902-06). Additional support was obtained through a grant from Cubist Pharmaceuticals Inc.
No financial conflicts are reported by the authors.
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Allen, N. E., W. E. Alborn, Jr., and J. N. Hobbs, Jr. 1991. Inhibition of membrane potential-dependent amino acid transport by daptomycin. Antimicrob. Agents Chemother. 35:2639-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auburtin, M., M. Wolff, J. Charpentier, E. Varon, Y. Le Tulzo, C. Girault, I. Mohammedi, B. Renard, B. Mourvillier, F. Bruneel, J. D. Ricard, and J. F. Timsit. 2006. Detrimental role of delayed antibiotic administration and penicillin-nonsusceptible strains in adult intensive care unit patients with pneumococcal meningitis: the PNEUMOREA prospective multicenter study. Crit. Care Med. 34:2758-2765. [DOI] [PubMed] [Google Scholar]
- 3.Auer, M., L. A. Pfister, D. Leppert, M. G. Täuber, and S. L. Leib. 2000. Effects of clinically used antioxidants in experimental pneumococcal meningitis. J. Infect. Dis. 182:347-350. [DOI] [PubMed] [Google Scholar]
- 4.Benvenuto, M., D. P. Benziger, S. Yankelev, and G. Vigliani. 2006. Pharmacokinetics and tolerability of daptomycin at doses up to 12 milligrams per kilogram of body weight once daily in healthy volunteers. Antimicrob. Agents Chemother. 50:3245-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bottcher, T., H. Ren, M. Goiny, J. Gerber, J. Lykkesfeldt, U. Kuhnt, M. Lotz, S. Bunkowski, C. Werner, I. Schau, A. Spreer, S. Christen, and R. Nau. 2004. Clindamycin is neuroprotective in experimental Streptococcus pneumoniae meningitis compared with ceftriaxone. J. Neurochem. 91:1450-1460. [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann, K. C., A. J. Talati, R. E. Akbari, E. A. Meals, and B. K. English. 2005. Group B streptococci exposed to rifampin or clindamycin (versus ampicillin or cefotaxime) stimulate reduced production of inflammatory mediators by murine macrophages. Pediatr. Res. 57:419-423. [DOI] [PubMed] [Google Scholar]
- 7.Chavez-Bueno, S., and G. H. McCracken, Jr. 2005. Bacterial meningitis in children. Pediatr. Clin. N. Am. 52:795-810, vii. [DOI] [PubMed] [Google Scholar]
- 8.Coimbra, R. S., V. Voisin, A. B. de Saizieu, R. L. Lindberg, M. Wittwer, D. Leppert, and S. L. Leib. 2006. Gene expression in cortex and hippocampus during acute pneumococcal meningitis. BMC Biol. 4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottagnoud, P., M. Pfister, F. Acosta, M. Cottagnoud, L. Flatz, F. Kuhn, H. P. Muller, and A. Stucki. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doern, G. V., S. S. Richter, A. Miller, N. Miller, C. Rice, K. Heilmann, and S. Beekmann. 2005. Antimicrobial resistance among Streptococcus pneumoniae in the United States: have we begun to turn the corner on resistance to certain antimicrobial classes? Clin. Infect. Dis. 41:139-148. [DOI] [PubMed] [Google Scholar]
- 12.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 13.Gerber, J., K. Pohl, V. Sander, S. Bunkowski, and R. Nau. 2003. Rifampin followed by ceftriaxone for experimental meningitis decreases lipoteichoic acid concentrations in cerebrospinal fluid and reduces neuronal damage in comparison to ceftriaxone alone. Antimicrob. Agents Chemother. 47:1313-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gianinazzi, C., D. Grandgirard, H. Imboden, L. Egger, D. N. Meli, Y. D. Bifrare, P. C. Joss, M. G. Täuber, C. Borner, and S. L. Leib. 2003. Caspase-3 mediates hippocampal apoptosis in pneumococcal meningitis. Acta Neuropathol. (Berlin) 105:499-507. [DOI] [PubMed] [Google Scholar]
- 15.Grimwood, K., V. A. Anderson, L. Bond, C. Catroppa, R. L. Hore, E. H. Keir, T. Nolan, and D. M. Roberton. 1995. Adverse outcomes of bacterial meningitis in school-age survivors. Pediatrics 95:646-656. [PubMed] [Google Scholar]
- 16.Heer, C., K. Stuertz, R. R. Reinert, M. Mader, and R. Nau. 2000. Release of teichoic and lipoteichoic acids from 30 different strains of Streptococcus pneumoniae during exposure to ceftriaxone, meropenem, quinupristin/dalfopristin, rifampicin and trovafloxacin. Infection 28:13-20. [DOI] [PubMed] [Google Scholar]
- 17.Jung, D., A. Rozek, M. Okon, and R. E. Hancock. 2004. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem. Biol. 11:949-957. [DOI] [PubMed] [Google Scholar]
- 18.Kim, Y. S., R. A. Sheldon, B. R. Elliott, Q. Liu, D. M. Ferriero, and M. G. Täuber. 1995. Brain injury in experimental neonatal meningitis due to group B streptococci. J. Neuropathol. Exp. Neurol. 54:531-539. [DOI] [PubMed] [Google Scholar]
- 19.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 20.Koedel, U., T. Rupprecht, B. Angele, J. Heesemann, H. Wagner, H. W. Pfister, and C. J. Kirschning. 2004. MyD88 is required for mounting a robust host immune response to Streptococcus pneumoniae in the CNS. Brain 127:1437-1445. [DOI] [PubMed] [Google Scholar]
- 21.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 22.Lebel, M. H. 1992. Adverse outcome of bacterial meningitis due to delayed sterilization of cerebrospinal fluid. Antibiot. Chemother. 45:226-238. [DOI] [PubMed] [Google Scholar]
- 23.Leib, S. L., J. M. Clements, R. L. Lindberg, C. Heimgartner, J. M. Loeffler, L. A. Pfister, M. G. Täuber, and D. Leppert. 2001. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734-1742. [DOI] [PubMed] [Google Scholar]
- 24.Leib, S. L., C. Heimgartner, Y. D. Bifrare, J. M. Loeffler, and M. G. Täuber. 2003. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr. Res. 54:353-357. [DOI] [PubMed] [Google Scholar]
- 25.Leib, S. L., D. Leppert, J. Clements, and M. G. Täuber. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leppert, D., S. L. Leib, C. Grygar, K. M. Miller, U. B. Schaad, and G. A. Hollander. 2000. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 31:80-84. [DOI] [PubMed] [Google Scholar]
- 27.Leppert, D., R. L. Lindberg, L. Kappos, and S. L. Leib. 2001. Matrix metalloproteinases: multifunctional effectors of inflammation in multiple sclerosis and bacterial meningitis. Brain Res. Brain Res. Rev. 36:249-257. [DOI] [PubMed] [Google Scholar]
- 28.Letiembre, M., H. Echchannaoui, F. Ferracin, S. Rivest, and R. Landmann. 2005. Toll-like receptor-2 deficiency is associated with enhanced brain TNF gene expression during pneumococcal meningitis. J. Neuroimmunol. 168:21-33. [DOI] [PubMed] [Google Scholar]
- 29.Loeffler, J. M., R. Ringer, M. Hablutzel, M. G. Täuber, and S. L. Leib. 2001. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J. Infect. Dis. 183:247-252. [DOI] [PubMed] [Google Scholar]
- 30.Lynch, J. P., III, and G. G. Zhanel. 2005. Escalation of antimicrobial resistance among Streptococcus pneumoniae: implications for therapy. Semin. Respir. Crit. Care Med. 26:575-616. [DOI] [PubMed] [Google Scholar]
- 31.Mattie, H., K. Stuertz, R. Nau, and J. T. van Dissel. 2005. Pharmacodynamics of antibiotics with respect to bacterial killing of and release of lipoteichoic acid by Streptococcus pneumoniae. J. Antimicrob. Chemother. 56:154-159. [DOI] [PubMed] [Google Scholar]
- 32.Meli, D. N., S. Christen, and S. L. Leib. 2003. Matrix metalloproteinase-9 in pneumococcal meningitis: activation via an oxidative pathway. J. Infect. Dis. 187:1411-1415. [DOI] [PubMed] [Google Scholar]
- 33.Meli, D. N., S. Christen, S. L. Leib, and M. G. Täuber. 2002. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr. Opin. Infect. Dis. 15:253-257. [DOI] [PubMed] [Google Scholar]
- 34.Meli, D. N., R. S. Coimbra, D. G. Erhart, G. Loquet, C. L. Bellac, M. G. Täuber, U. Neumann, and S. L. Leib. 2006. Doxycycline reduces mortality and injury to the brain and cochlea in experimental pneumococcal meningitis. Infect. Immun. 74:3890-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meli, D. N., J. M. Loeffler, P. Baumann, U. Neumann, T. Buhl, D. Leppert, and S. L. Leib. 2004. In pneumococcal meningitis a novel water-soluble inhibitor of matrix metalloproteinases and TNF-alpha converting enzyme attenuates seizures and injury of the cerebral cortex. J. Neuroimmunol. 151:6-11. [DOI] [PubMed] [Google Scholar]
- 36.Moreillon, P., and P. A. Majcherczyk. 2003. Proinflammatory activity of cell-wall constituents from gram-positive bacteria. Scand. J. Infect. Dis. 35:632-641. [DOI] [PubMed] [Google Scholar]
- 37.Mustafa, M. M., J. Mertsola, O. Ramilo, X. Saez-Llorens, R. C. Risser, and G. H. McCracken, Jr. 1989. Increased endotoxin and interleukin-1 beta concentrations in cerebrospinal fluid of infants with coliform meningitis and ventriculitis associated with intraventricular gentamicin therapy. J. Infect. Dis. 160:891-895. [DOI] [PubMed] [Google Scholar]
- 38.Nau, R., and H. Eiffert. 2005. Minimizing the release of proinflammatory and toxic bacterial products within the host: a promising approach to improve outcome in life-threatening infections. FEMS Immunol. Med. Microbiol. 44:1-16. [DOI] [PubMed] [Google Scholar]
- 39.Nau, R., A. Wellmer, A. Soto, K. Koch, O. Schneider, H. Schmidt, J. Gerber, U. Michel, and W. Bruck. 1999. Rifampin reduces early mortality in experimental Streptococcus pneumoniae meningitis. J. Infect. Dis. 179:1557-1560. [DOI] [PubMed] [Google Scholar]
- 40.Oostenbrink, R., K. G. Moons, G. Derksen-Lubsen, D. E. Grobbee, and H. A. Moll. 2002. Early prediction of neurological sequelae or death after bacterial meningitis. Acta Paediatr. 91:391-398. [DOI] [PubMed] [Google Scholar]
- 41.Pankuch, G. A., M. R. Jacobs, and P. C. Appelbaum. 2003. Bactericidal activity of daptomycin against Streptococcus pneumoniae compared with eight other antimicrobials. J. Antimicrob. Chemother. 51:443-446. [DOI] [PubMed] [Google Scholar]
- 42.Parnaik, R., M. C. Raff, and J. Scholes. 2000. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10:857-860. [DOI] [PubMed] [Google Scholar]
- 43.Pfister, L.-A., J. H. Tureen, S. Shaw, S. Christen, D. M. Ferriero, M. G. Täuber, and S. L. Leib. 2000. Endothelin inhibition improves cerebral blood flow and is neuroprotective in pneumococcal meningitis. Ann. Neurol. 47:329-335. [PubMed] [Google Scholar]
- 44.Pugin, D., J. C. Copin, M. C. Goodyear, T. Landis, and Y. Gasche. 2006. Persisting vasculitis after pneumococcal meningitis. Neurocrit. Care 4:237-240. [DOI] [PubMed] [Google Scholar]
- 45.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schneider, O., U. Michel, G. Zysk, O. Dubuis, and R. Nau. 1999. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53:1584-1587. [DOI] [PubMed] [Google Scholar]
- 47.Sellner, J., and S. L. Leib. 2006. In bacterial meningitis cortical brain damage is associated with changes in parenchymal MMP-9/TIMP-1 ratio and increased collagen type IV degradation. Neurobiol. Dis. 21:647-656. [DOI] [PubMed] [Google Scholar]
- 48.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spreer, A., J. Gerber, M. Hanssen, S. Schindler, C. Hermann, P. Lange, H. Eiffert, and R. Nau. 2006. Dexamethasone increases hippocampal neuronal apoptosis in a rabbit model of Escherichia coli meningitis. Pediatr. Res. 60:210-215. [DOI] [PubMed] [Google Scholar]
- 49a.Stucki, A., M. Cottagnoud, V. Winkelmann, T. Schaffner, and P. Cottagnoud. 2007. Daptomycin produces an enhanced bactericidal activity compared to ceftriaxone, measured by [3H] choline release in the cerebrospinal fluid, in experimental meningitis due to a penicillin-resistant pneumococcal strain without lysing its cell wall. Antimicrob. Agents Chemother. 51:2249-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuertz, K., H. Schmidt, H. Eiffert, P. Schwartz, M. Mader, and R. Nau. 1998. Differential release of lipoteichoic and teichoic acids from Streptococcus pneumoniae as a result of exposure to beta-lactam antibiotics, rifamycins, trovafloxacin, and quinupristin-dalfopristin. Antimicrob. Agents Chemother. 42:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stuertz, K., H. Schmidt, F. Trostdorf, H. Eiffert, M. Mader, and R. Nau. 1999. Lower lipoteichoic and teichoic acid CSF concentrations during treatment of pneumococcal meningitis with non-bacteriolytic antibiotics than with ceftriaxone. Scand. J. Infect. Dis. 31:367-370. [DOI] [PubMed] [Google Scholar]
- 52.Täuber, M. G., A. M. Shibl, C. J. Hackbarth, J. W. Larrick, and M. A. Sande. 1987. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J. Infect. Dis. 156:456-462. [DOI] [PubMed] [Google Scholar]
- 53.Tunkel, A. R., B. J. Hartman, S. L. Kaplan, B. A. Kaufman, K. L. Roos, W. M. Scheld, and R. J. Whitley. 2004. Practice guidelines for the management of bacterial meningitis. Clin. Infect. Dis. 39:1267-1284. [DOI] [PubMed] [Google Scholar]
- 54.Tuomanen, E., H. Liu, B. Hengstler, O. Zak, and A. Tomasz. 1985. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 151:859-868. [DOI] [PubMed] [Google Scholar]
- 55.van de Beek, D., J. de Gans, L. Spanjaard, M. Weisfelt, J. B. Reitsma, and M. Vermeulen. 2004. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351:1849-1859. [DOI] [PubMed] [Google Scholar]
- 56.Wale, L. J., A. P. Shelton, and D. Greenwood. 1989. Scanning electron microscopy of Staphylococcus aureus and Enterococcus faecalis exposed to daptomycin. J. Med. Microbiol. 30:45-49. [DOI] [PubMed] [Google Scholar]
- 57.Weisfelt, M., M. Hoogman, D. van de Beek, J. de Gans, W. A. Dreschler, and B. A. Schmand. 2006. Dexamethasone and long-term outcome in adults with bacterial meningitis. Ann. Neurol. 60:456-468. [DOI] [PubMed] [Google Scholar]
- 58.Weisfelt, M., D. van de Beek, L. Spanjaard, J. B. Reitsma, and J. de Gans. 2006. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol. 5:123-129. [DOI] [PubMed] [Google Scholar]
- 59.Yogev, R., and J. Guzman-Cottrill. 2005. Bacterial meningitis in children: critical review of current concepts. Drugs 65:1097-1112. [DOI] [PubMed] [Google Scholar]
- 60.Zysk, G., W. Bruck, J. Gerber, Y. Bruck, H. W. Prange, and R. Nau. 1996. Anti-inflammatory treatment influences neuronal apoptotic cell death in the dentate gyrus in experimental pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 55:722-728. [DOI] [PubMed] [Google Scholar]