Abstract
Recent studies indicated that sensitive parasites could increase in frequency in a population when drugs are removed, suggesting that the life span of affordable antimalarial drugs could be expanded. We studied 97 samples from Bolivar State, Venezuela, an area where sulfadoxine-pyrimethamine (SP) has not been used for 8 years due to its ineffectiveness. We characterized point mutations in two genes that have been implicated in resistance to SP, dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps). We also assayed neutral microsatellite markers around the dhfr (chromosome 4) and dhps (chromosome 8) genes and on chromosomes 2 and 3 to track the origin and spread of resistant alleles. We found that drug-resistant SP mutants are fixed in the population. Two genotypes were present in the samples, dhfr(50R/51I/108N) dhps(437G/540E/581G) (90.7%) and dhfr(51I/108N) dhps(437G/581G) (9.3%). We show a single microsatellite haplotype for all of the dhfr and dhps alleles, and the alleles at the microsatellite loci are different from those present in Africa. Thus, in these samples from Venezuela, there is a single origin for both dhfr and dhps SP-resistant alleles, and these alleles originated independently of those characterized from Africa. Furthermore, this is the first report of a “hitchhiking effect” on the genetic variation around dhps due to selection by SP using an extensive set of microsatellite markers. Our results indicate that, in areas where there is limited gene flow, the fixation of drug-resistant parasites in the population is stable, even after drug selection is relaxed.
The emergence of drug-resistant Plasmodium falciparum is a serious public health problem in many countries where malaria is endemic (7, 28). Sulfadoxine-pyrimethamine (SP) is an antifolate drug commonly used to treat P. falciparum infections because of its ease of administration, affordability, and efficacy. Unfortunately, resistance to SP has been documented in many parts of the world, compromising the use of this drug for treating uncomplicated P. falciparum malaria.
SP acts as an inhibitor of the P. falciparum folic acid pathway, and point mutations in the genes encoding dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps) have been implicated in SP resistance (9). The dhfr(S108N) point mutation and additional mutations at codons 50, 51, 59, and 164 act synergistically to increase resistance to pyrimethamine (4, 21). Similarly, mutations at dhps codons 436, 437, 540, 581, and 613 act synergistically to increase the level of resistance to sulfadoxine (7).
Recent studies following the frequency of chloroquine-resistant parasites over time showed that sensitive parasites could increase in frequency soon after the drug pressure on the parasite population was decreased (13). These results suggest that, with appropriate drug management, it may be possible to increase the life span of safe and affordable antimalarial drugs such as SP.
In the context of the Americas, SP resistance has been widely reported in the Amazon basin (28); however, the drug is still effective on the Pacific Coast of South America. Indeed, there is increasing concern that resistant alleles from the Amazon and Orinoco basins could be introduced to the Pacific Coast, hampering the usefulness of SP in those areas where it is still effective. Since SP is no longer in use in the Amazon and Orinoco basins, it is possible that, over time, sensitive parasites could increase in frequency in the absence of drug pressure, as has been observed in the case of chloroquine (13). If this is the case, the risk of introducing SP mutants from the Amazon into the Pacific Coast will be substantially reduced.
Determining whether SP-resistant mutants could be introduced in the Pacific Coast of South America from P. falciparum populations in the Amazon and Orinoco basins requires a clear understanding of the origin of SP-resistant genotypes circulating in the region. The importance of gene flow in parasite populations in relation to drug resistance has been highlighted by the presence of resistant genotypes against SP and chloroquine in Africa that could have originated in Southeast Asia (17, 23, 24, 32). In the specific case of SP resistance, a single origin was proposed for a highly resistant triple mutant (51I, 59R, 108N) genotype in Africa and Southeast Asia (17, 23, 24) using microsatellite loci around dhfr. However, this observation contrasted with the epidemiological information that suggested multiple origins for SP-resistant genotypes (7); we have indeed recently reported three additional haplotypes for the dhfr triple mutant genotype in western Kenya (16). There are not comparable studies in the Americas.
In the case of dhps, one previous study utilized just three microsatellite markers to show several haplotypes for the dhps double mutant allele in Africa (23); however, there is still limited information about the origin of dhps-resistant genotypes worldwide.
The most comprehensive molecular epidemiologic investigation on SP resistance in the Amazon basin utilized microsatellite markers on multiple chromosomes along with genotyping and sequencing (3). This investigation showed strong linkage across the genome, suggesting a clonal expansion of highly resistant parasites among several regions in the Amazon (3). It was evident from this study that at least two different dhfr triple mutant genotypes [dhfr(C50R/N51E/S108N) and dhfr(N51E/S108N/I164L)] with a likely common origin and a single dhps triple mutant genotype [dhps(A437G/K540E/A581G)] with a common origin had widely spread in the Amazon region. However, since this study utilized only two microsatellite markers closely located to the dhfr and dhps genes and very few parasite isolates from each region, the evolutionary origin and spread of SP-resistant parasites in the Americas requires further investigation. Such a study will also help to further the understanding of how these parasites are related to SP-resistant parasites from Asia and Africa.
In order to address these issues, we have characterized point mutations in the genes dhfr and dhps as well as studied the microsatellite loci surrounding these genes in P. falciparum isolates to further investigate the origin and dynamics of SP-resistant malaria. We have also characterized additional microsatellite markers on chromosomes 2 and 3 to assess the levels of genetic variation and linkage disequilibrium at putatively neutral regions of the genome. The results derived from these neutral markers have been contrasted with the observed patterns in the regions surrounding the genes dhfr and dhps.
Bolivar State in Venezuela has a substantial number of reported cases of malaria (60% of the nationally reported cases) (2), mainly among miners, agricultural workers, and indigenous groups. SP was widely used in Venezuela as an alternative to chloroquine in the 1970s and 1980s, although in vitro SP resistance was noted in 1977. Unfortunately, clinical resistance to SP resulted in the discontinued use of the drug in 1998 (2). We expect an origin for SP mutants in this population independent from that of SP mutants found in Africa and Southeast Asia. Given that SP is no longer being used in this area, it may be possible that SP-sensitive parasites could have a selective advantage over the mutants in the absence of drug pressure.
MATERIALS AND METHODS
Study subjects.
We analyzed 97 blood samples collected from adults from a surveillance study in Sifontes municipality in Bolivar State, Venezuela, between June 2003 and May 2004. Any patient testing positive for malaria by microscopy was invited to participate in the study. This border area, close to Guyana, is the epicenter of multidrug-resistant P. falciparum malaria in Venezuela. All patients were treated according to national guidelines. Venous whole-blood samples were collected before treatment. In 2003, P. falciparum was treated with quinine (10 mg/kg of body weight every 8 h for 7 days) plus primaquine (0.5 mg/kg body weight for 2 days). By mid-2004, P. falciparum was treated with mefloquine (25 mg/kg body weight in two doses of 15 and 10 mg/kg) plus artesunate (4 mg/kg body weight daily for 3 days). The study protocol was approved by the bioethics commission of the Instituto de Altos Estudios Dr. Arnoldo Gabaldon in Venezuela.
DNA isolation and genotyping methods.
DNA was isolated from whole blood using the QIAamp DNA mini kit (QIAGEN, Valencia, CA). All samples were genotyped for P. falciparum mutations at dhfr codons 50, 51, 59, 108, and 164 and dhps codons 436, 437, 540, 581, and 613 by pyrosequencing. No multiple-strain infections were detected by pyrosequencing of the mutations.
The sequences of primers used for the pyrosequencing of dhfr and dhps are provided in the supplemental material. The primary and nested PCRs consisted of the following in a total volume of 100 μl: 1 μl of the template, a 16 μM concentration of the deoxynucleoside triphosphates, 0.5 μM primF, 0.5 μM primR, 1× PCR buffer with 15 mM MgCl2 (Applied Biosystems, Foster City, CA), 0.75 mM MgCl2 (dhfr), or 1.0 mM MgCl2 (dhps), and 2.5 units Taq DNA polymerase (Promega, Madison, WI). Thermal cycling conditions for the primary dhfr reaction were as follows: 95°C for 3 min; 45 cycles of 92°C for 30 s, 45°C for 45 s, and 72°C for 45 s; and 72°C for 3 min. Thermal cycling conditions for the primary dhps reaction were as follows: 95°C for 5 min; 45 cycles of 92°C for 30 s, 45°C for 30 s, and 65°C for 45 s; and 72°C for 15 min. Thermal cycling conditions for the nested reaction for dhfr codons 50, 51, and 59 and for dhps codons 436, 437, 540, and 581 were as follows: 95°C for 5 min; 25 cycles of 92°C for 30 s, 45°C for 30 s, and 65°C for 30 s; and 72°C for 15 min. Thermal cycling conditions for the nested reaction for dhfr codons 108 and 164 and for dhps codon 613 were as follows: 95°C for 5 min; 25 cycles of 92°C for 30 s, 42°C for 30 s, and 65°C for 45 s; and 72°C for 15 min.
Microsatellite analysis.
Microsatellite analysis was conducted on all samples. Samples were assayed for 26 microsatellite loci that span 700 kb on chromosome 4 around dhfr (17, 23, 24), 23 loci that span 698 kb on chromosome 8 around dhps (23), 4 loci on chromosome 2 that span 78 kb, and 3 loci on chromosome 3 that span 94 kb (27). Microsatellite PCR primer sequences are provided in the supplemental material. Single-reaction PCR and thermal cycling conditions are detailed by Nair et al. (17), and nested PCRs and thermal cycling conditions are detailed by Roper et al. (23). PCR products were separated on Applied Biosystems 3100 and 3130xl capillary sequencers and scored using GeneMapper software v3.7 (Applied Biosystems, Foster City, CA). For all analyses, if multiple peaks (alleles) were detected at any locus, the value of the highest peak was used for the analysis, as done previously by Machado et al. (14). Multiple alleles were not detected for any of the loci used for haplotype determination (Tables 1 and 2). Haplotypes were classified as being different if they contained more than one different allele across the loci. Missing data (no amplifications) are reported but not considered for defining haplotypes.
TABLE 1.
dhfr haplotypes by genotype and haplotype grouping
| Positions of mutations | Groupa |
dhfr microsatellite haplotype at kbb:
|
nc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −7.55 | −5.3 | −4.49 | −4.4 | −3.87 | −1.22 | −0.3 | 0.2 | 0.52 | 1.48 | 5.87 | |||
| 50/51/108 | 1 | 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 113 | 29 |
| 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 10 | |||
| 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 9 | ||
| 145 | 199 | 195 | 206 | 221 | 95 | 157 | 97 | 200 | 3 | ||||
| 145 | 195 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 2 | ||||
| 145 | 220 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 1 | |||
| 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 1 | ||||
| 139 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 113 | 1 | ||
| 199 | 195 | 206 | 221 | 95 | 157 | 97 | 200 | 113 | 1 | ||||
| 199 | 195 | 178 | 221 | 95 | 157 | 97 | 200 | 1 | |||||
| 147 | 199 | 195 | 221 | 95 | 157 | 97 | 200 | 1 | |||||
| 139 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 1 | ||||
| 145 | 199 | 195 | 178 | 208 | 221 | 95 | 157 | 97 | 200 | 113 | 2 | ||
| 145 | 199 | 195 | 208 | 221 | 95 | 157 | 97 | 200 | 1 | ||||
| 2 | 145 | 199 | 195 | 178 | 208 | 221 | 95 | 157 | 97 | 200 | 117 | 1 | |
| 3 | 139 | 220 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 10 | |
| 139 | 220 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 113 | 9 | ||
| 139 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 2 | ||
| 139 | 220 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 1 | |||
| 139 | 220 | 195 | 206 | 221 | 95 | 157 | 97 | 200 | 1 | ||||
| 139 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 1 | |||
| 51/108 | 1 | 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 113 | 5 |
| 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 117 | 2 | ||
| 145 | 199 | 195 | 178 | 206 | 221 | 95 | 157 | 97 | 200 | 2 | |||
Haplotype groups were classified as being different if they contained two or more different alleles across loci, with missing data ignored.
Locations of the microsatellite loci with respect to dhfr in kilobases, where negative positions are 5′ to dhfr and nonnegative positions are 3′ to dhfr. Alleles are given as PCR product sizes. Empty cells represent loci that did not amplify.
Number of samples with the specific pattern.
TABLE 2.
dhps haplotypes by genotype and haplotype grouping
| Positions of mutations | Groupa |
dhps microsatellite haplotype at kbb:
|
nc | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| −7.4 | −2.5 | −1.6 | −0.8 | 0.06 | 0.144 | 1.59 | 6.19 | 9.8 | |||
| 437/540/581 | 1 | 307 | 263 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 61 |
| 309 | 263 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 4 | ||
| 307 | 265 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 3 | ||
| 307 | 263 | 138 | 122 | 132 | 170 | 178 | 229 | 10 | |||
| 263 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 2 | |||
| 307 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 1 | |||
| 263 | 138 | 122 | 132 | 170 | 178 | 229 | 3 | ||||
| 309 | 138 | 122 | 132 | 170 | 178 | 229 | 1 | ||||
| 307 | 263 | 138 | 122 | 170 | 195 | 178 | 229 | 2 | |||
| 307 | 263 | 138 | 122 | 170 | 178 | 229 | 1 | ||||
| 437/581 | 1 | 307 | 263 | 138 | 122 | 132 | 170 | 195 | 178 | 229 | 4 |
| 307 | 263 | 138 | 122 | 132 | 170 | 178 | 229 | 3 | |||
| 263 | 138 | 122 | 132 | 170 | 178 | 229 | 2 | ||||
Haplotype groups were classified as being different if they contained two or more different alleles across loci, with missing data ignored.
Locations of the microsatellite loci with respect to dhfr in kilobases, where negative positions are 5′ to dhfr and nonnegative positions are 3′ to dhfr. Alleles are given as PCR product sizes. Empty cells represent loci that did not amplify.
Number of samples with the specific pattern.
Statistical analysis.
Selection due to SP is expected to generate two specific signatures in the genetic makeup of parasite populations (5): (i) the genetic diversity around dhps and dhfr decreasing at the population level due to an increase in frequency of the resistant mutants favored by drug pressure or a selective sweep and (ii) linkage disequilibrium between dhps and dhfr, that is, an association between the dhps and dhfr alleles. It is worth noting that linkage disequilibrium could be the result of inbreeding rather than drug selection. Thus, the baseline levels of neutral genetic diversity and linkage disequilibrium in the population were obtained from the microsatellite markers on chromosomes 2 and 3.
The genetic diversity for each microsatellite locus was measured by calculating the expected heterozygosities (He) and the number of alleles per locus (A). He was calculated for each locus with the formula He = [n/(n − 1)][1 − ∑pi2], where n is the number of isolates sampled and pi is the frequency of the ith allele. The sampling variance for He was calculated as 2(n − 1)/n3[2(n − 2)][∑pi3 − (∑pi2)2] (18). The Excel microsatellite tool kit was used to compute allele frequencies and A (19).
The pattern of baseline linkage disequilibrium was determined by significant associations among microsatellite loci using an exact test of linkage disequilibrium (22). Loci between kb −89 and kb 90 on chromosome 4, those between kb −37 and kb 71 on chromosome 8, and those on chromosomes 2 and 3 were used for the analysis. Only polymorphic loci could be used in this analysis. Associations were tested between pairs of loci on different chromosomes by using 10,000 Monte Carlo steps in Arlequin version 3.01 (6). The Excel microsatellite tool kit was used to format data for use in Arlequin (19). The null hypothesis is that no linkage disequilibrium should be observed between loci on different chromosomes in a population with random mating; thus, we cannot reject testing for associations in each pair of loci independently. We considered an ad hoc global test, where we expected up to 5% of loci on independent chromosomes to show significant associations by chance in a population with random mating. This global criterion was applied with and without correcting the P value of the specific pair of locus comparisons using a Bonferroni correction.
RESULTS
Pyrosequencing of mutations in dhfr and dhps known to be important for SP resistance revealed two multilocus genotypes present in our sample: dhfr(50R/51I/108N) linked to dhps(437G/540E/581G) (hereinafter referred to as a sextuple mutant), and dhfr(51I/108N) linked to dhps(437G/581G) (quadruple mutant). The sextuple mutant was present in 90.7% of the samples, and the quadruple mutant was present in 9.3% of the samples. Although the sample size is small for the samples containing quadruple mutant parasites (n = 9), the data clearly show a strong linkage between the two loci that are found in two different chromosomes (dhfr on chromosome 4 and dhps on chromosome 8). Specifically, the dhfr mutation 50R is always found in parasites with the dhps mutation 540E. All of the mutations found here have been documented in Bolivar State in previous studies (2, 30). The samples did not contain the highly resistant dhfr(164L) mutation, nor did they have the mutation dhfr(59R) or dhps(436F), all of which have previously been documented at several sites in South America (20, 26, 31). It has been noted that the mutations 50R and 59R are mutually exclusive in field samples, and our results are consistent with these previous findings (7). It is also important to note that none of the samples contained wild-type parasite alleles, which, based on other studies, seem to have been lost in the population somewhat recently. One study noted that 4% of 54 samples from 1995 had wild-type codons 51 and 108; it is unclear whether all of the dhfr codons were wild type on a given chromosome. That same study did not find any wild-type dhps alleles (30). Another study using samples from 1998 to 2000 did not have any wild-type dhfr or dhps alleles (2).
We conducted a microsatellite analysis to gain additional insight into the dhfr and dhps genotypes in Bolivar State, Venezuela. To determine microsatellite haplotypes, and hence genetic lineages, we chose chromosomal regions close to both genes spanning approximately 13.5 kb around dhfr on chromosome 4 and 17 kb around dhps on chromosome 8. We did not detect multiple “strain” infections at these microsatellite loci or for any point mutation from the genotyping; therefore, haplotype reconstruction is possible for the regions surrounding these two genes. Tables 1 and 2 show the dhfr and dhps haplotypes, respectively. Empty cells represent loci that did not amplify. The dhfr(50R/51I/108N) genotype has three microsatellite haplotypes, but the sample set is predominated by two of the haplotypes, haplotypes 1 and 3 (see groups 1 and 3 in Table 1). Haplotype 3 is characterized by alleles at the kb −7.55 and kb −5.3 loci. The criteria for defining haplotypes allowed for only one allelic difference, and as a result, haplotype 2 is distinct; however, we believe that this haplotype is closely related to haplotype 1. Therefore, we can conservatively suggest that there are at least two haplotypes for the dhfr(50R/51I/108N) genotype in these samples. Interestingly, the dhfr(51I/108N) genotype has a single haplotype, haplotype 1, which is also the predominant haplotype present for the dhfr(50R/51I/108N) genotype.
The dhps(437G/540E/581G) genotype has one microsatellite haplotype (Table 2). Similarly to the data for dhfr, the less frequent dhps(437G/581G) genotype has a single haplotype, haplotype 1, which is the same that is seen for the dhps(437G/540E/581G) genotype.
Selection due to drug pressure could generate the observed linkage disequilibrium between dhps and dhfr. However, linkage could be partially explained by a clonal expansion in a population with low transmission, a situation that favors the origin of inbred lineages. Inbreeding would affect not only dhfr and dhps but also the complete parasite genome. Clonal population expansion has been reported previously in study areas with similar transmission intensities (29). Therefore, in order to determine whether the linkage observed at these two loci is also noted at loci in other regions of the genome, we analyzed additional neutral microsatellite loci on chromosomes 2 and 3 (chromosomes that are not known to contain functional genes associated with antimalarial drug resistance) to study the association between loci on independent chromosomes. We employed an ad hoc global test where we expected up to 5% of loci on independent chromosomes to show significant associations by chance in a population with random mating. If we consider the association among 269 pairs of loci on independent chromosomes (chromosomes 2, 3, 4, and 8 [see Fig. S1 in the supplemental material]), 135 (50%) showed significant association (P < 0.05), a number that is clearly above the 14 significant associations expected by chance using our ad hoc global test. We corrected the P values by using the Bonferroni method (25), and we found 80 pairs showing significant association (30%), clearly above the 14 expected by chance (see Fig. S2 in the supplemental material). If we consider only the loci in chromosomes 2 and 3, we found significant associations in all pairs (P < 0.05) and in six out of eight pairs using the Bonferroni method. Overall, our data indicate that there is strong linkage disequilibrium in this population, even when considering only the markers that are expected to segregate independently from dhfr and dhps.
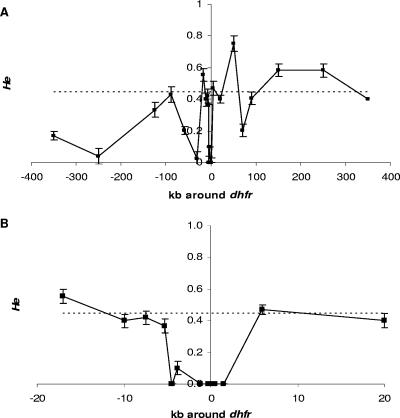
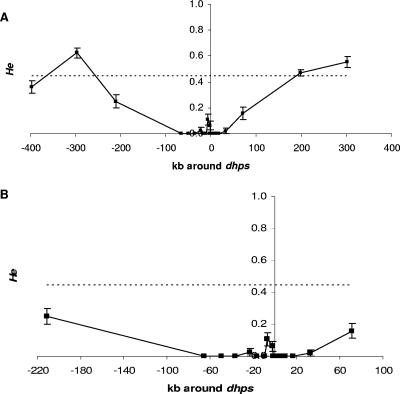
In addition to linkage disequilibrium between dhfr and dhps, we also expected a reduction of the genetic diversity around these loci (dhps and dhfr) due to an increase in the frequency of the resistant alleles favored by drug pressure (5). The microsatellite markers on chromosomes 2 and 3 were used as representative of the expected neutral variation in this population. The loci surrounding dhfr and dhps have fewer alleles and lower heterozygosities, basic measures of genetic diversity, than those on chromosomes 2 and 3, as evidenced in Fig. 1 and 2, respectively (see also the supplemental material). In particular, the region from kb −4.5 to kb 1.48 on chromosome 4 around dhfr has a notable decrease in the amount of variation compared to what is seen in the loci on chromosomes 2 and 3 (Fig. 1B). For dhps, the larger region from kb −66 to kb 33 has dramatically low heterozygosity (Fig. 2). The differences in the extents of the regions with low heterozygosity could be the result of several factors, including differences in selective pressures, the ages of the mutations, and the amounts of recombination. We indeed observed recombination events in the region 5′ to the dhfr gene (data not shown). The mean heterozygosities that we find for the loci on chromosomes 2 and 3 are consistent with those from a previous study using other neutral loci in an area of low transmission (1). The lack of alleles and lower heterozygosity in the regions directly surrounding dhfr and dhps, notably lower than the mean produced from the loci on chromosomes 2 and 3, indicate that the observed alleles on these two loci have undergone positive selection due to drug pressure.
FIG. 1.
Graphical display of He ±1 standard deviation across chromosome 4 around dhfr. Shown are the entire region assayed with microsatellite markers (A) and a close-up of the smaller region of the noted low heterozygosity (B). The dashed line in each graph is the mean He calculated from loci on chromosomes 2 and 3. On the x axis, negative numbers are positions 5′ to the gene and positive numbers are positions 3′ to the gene. The error bars show 1 standard deviation.
FIG. 2.
Graphical display of He (±1 standard deviation) across chromosome 8 around dhps. Shown are the entire region assayed with microsatellite markers (A) and a close-up of the smaller region of the noted low heterozygosity (B). The dashed line in each graph is the mean He calculated from loci on chromosomes 2 and 3. On the x axis, negative numbers are positions 5′ to the gene and positive numbers are positions 3′ to the gene. The error bars show 1 standard deviation.
DISCUSSION
Here we present the first study to utilize an extensive set of microsatellite markers surrounding dhfr and dhps and on chromosomes 2 and 3 to genetically characterize and understand the origin and spread of SP resistance in a P. falciparum population from South America. Two major observations can be made from this population that are of relevance to public health: dhfr and dhps SP-resistant alleles originated each as single events, and mutants are still fixed in this population for both genes, regardless of the fact that the drug is no longer used as per the national policy.
In terms of the origin, the dhfr(50R/51I/108N) genotype was represented by two similar haplotypes, each differing by alleles at two loci at the 5′ end of the haplotype, at kb −7.55 and −5.3, for the majority of the samples. Furthermore, the minor haplotype (3) is characterized by additional differences at loci at kb −10 and kb −17 (data not shown). These results suggest that a possible recombination event in this region of chromosome 4 has created these two different yet very similar haplotypes for the dhfr(50R/51I/108N) genotype.
The haplotype for the dhfr(51I/108N) genotype is the same as a haplotype for the dhfr(50R/51I/108N) genotype, thereby implying that these two genotypes are from the same chromosome 4 ancestral lineage. Likewise, the genotypes dhps(437G/581G) and dhps(437G/540E/581G) are represented by a single haplotype common to both, suggesting that these two genotypes are from the same lineage. The presence of the two different genotypes for the same haplotype can be explained by a gain or a loss of point mutations in codon 50 for dhfr or codon 540 for dhps. It is important to note that every sample that contained a mutation at codon 50 also had a mutation at codon 540, suggesting that these two mutations in concert may confer a greater benefit to the parasite than either mutation alone.
The dhfr(51I/108N) dhps(437G/581G) quadruple mutant was present in only nine samples analyzed. However, we did observe noticeably minor variation in the loci on chromosomes 2 and 3 for these samples (data not shown). This is suggestive of clonal expansion of this lineage in this population.
SP resistance arises by simple point mutations in one or two genes and spreads rapidly after introduction; therefore, there is an expectation that there will be multiple origins for resistance across the world where mutations arose locally and have been affected by drug selection (selection-mutation balance) (7, 8, 20). In this study, the microsatellite haplotype for the dhfr(51I/108N) genotype in Venezuela is different at six out of nine loci from the haplotype identified in samples from western Kenya (16). Furthermore, the dhfr(50R/51I/108N) lineage is different at nine out of nine loci from the haplotypes identified for the 51I/59R/108N lineages from western Kenya and is also different from the 51I/59R/108N/164L lineages in western Kenya and Southeast Asia (16, 17). This demonstrates that the drug-resistant parasites in Venezuela have arisen de novo as expected under a selection-mutation balance and differ dramatically from the multiple lineages in Africa and Southeast Asia (16, 17, 23, 24). Although we have shown other dhfr genotypes in Africa (16), we did not show that they were sweeping through the population, given their low frequency. This study provides further evidence that drug selection has acted on dhfr, since resistance phenotypes (mutations causing amino acid changes) are convergent from different genetic backgrounds, and these genotypes have increased in frequency in the population (5, 32). We also found strong evidence of selection on dhps mutations in this population, as evidenced by a selective sweep of resistant mutants, an observation that has not been made elsewhere to the best of our knowledge. These findings emphasize that comparative approaches among different populations are needed to understand the origin and spread of pyrimethamine resistance.
The second major observation is that the mutants remain fixed in the population and that no wild-type parasites were observed. The fixation of dhfr mutant alleles has also been seen in samples from Cambodia, where artesunate-mefloquine has been the first-line treatment for 5 years (12). These observations appear to be inconsistent with previous work, which suggests that, in the absence of drug pressure, wild-type mutants should increase in frequency (13). However, natural selection requires genetic variation to operate; if a mutation is fixed in a population, then there are no wild-type mutants to compete with the drug-resistant mutants in the absence of the drug. This implies that, in areas with low genetic diversity and low migration, a fixed drug-resistant mutant represents a “definitive” event, rendering that drug permanently ineffective as a means of malaria control, at least until the local ecology changes to allow the reintroduction of wild types by migration or reverse point mutations take place, the latter being a very unlikely event. This observation underlines the need to consider the local evolutionary history and ecology in order to understand the dynamics of mutations associated with drug resistance.
It is also important to point out that the maintenance of highly SP-resistant alleles could be due to the use of other drugs that may also impart selective pressure on dhfr and dhps. Cotrimoxazole (CTX [trimethoprim-sulfamethoxazole]) is a drug used to treat acute respiratory infections, and cross-resistance between trimethoprim, a component of CTX, and pyrimethamine has been demonstrated in vitro (10, 11). Similarly, cross-resistance between sulfamethoxazole and sulfadoxine has also been demonstrated (10). CTX is used to treat a minority of pediatric respiratory infections in our study area. The samples used in this study were taken from adults, mostly miners 15 years of age and older. No patient that contributed a sample for this study reported CTX use. This, together with the limited use of CTX in the population and massive past use of SP to treat malaria, supports our assertion that the genetic patterns that we see here are a result of SP use in the region.
We also observed strong linkage disequilibrium among markers on chromosomes 2 and 3, an expected observation in an area with low transmission intensity where there is a high level of inbreeding (29). These results contrast with a study using a different set of markers in Brazil, where the authors found various amounts of linkage disequilibrium across different sites (14). These contrasting results could be explained by differences in regional transmission intensities. As there is still more genetic variation on chromosomes 2 and 3 than is seen around dhfr or dhps, it is likely that selection by drug pressure also played a role in the observed pattern of linkage disequilibrium by shaping this P. falciparum population, favoring the expansion of a few lineages. Nevertheless, we cannot definitively separate the contribution of selection by drug pressure from inbreeding on the observed pattern of linkage disequilibrium.
We also observed a larger region of lowered variation around dhps (99 kb) than around dhfr (6 kb). We can hypothesize that the region of lowered variation around dhfr has been broken up and narrowed over time by recombination. By contrast, dhps mutations may have arisen after those found in the dhfr gene, so the region on chromosome 8 surrounding dhps has not had enough time for recombination to break apart this linkage. Thus, the simplest conclusion is that the dhfr alleles are older than the dhps alleles in this population. This conclusion is consistent with reports from Africa which note an increase in pyrimethamine resistance several years before sulfadoxine resistance was noted (15, 23).
Researchers are exploring the use of microsatellite markers to track the spread of drug-resistant parasites in populations. This study confirms that there are different SP-resistant alleles between continents (16). Our results indicate that tracking these particular lineages is possible in South America, as is possible at sites in Southeast Asia (17). However, we acknowledge that the lineages present in Venezuela are not representative of parasites for the entire continent of South America. Further investigation is needed to understand the dynamics of mutations associated with SP resistance in South America.
There is concern that drug-resistant parasites present in the Amazon and Orinoco basins may be introduced to the Pacific Coast, causing a serious problem with SP resistance in the currently drug-sensitive region. Thus, gene flow between the Pacific Coast and the Amazon and Orinoco basin populations needs to be assessed. In addition, the scenario of an independent origin of SP resistance on the Pacific Coast of South America cannot be disregarded.
In summary, we found that SP-resistant mutant genotypes present in the Bolivar region of Venezuela originated independently from others found in Asia and Africa and that these genotypes have been fixed in the population. The fact that only a few lineages may be involved in SP resistance makes a molecular surveillance system based on a few microsatellite markers viable. However, given that SP mutants can emerge de novo on the Pacific Coast, surveillance systems need to be complemented by more-comprehensive population studies and cannot rely simply on a few genetic markers.
Supplementary Material
Acknowledgments
We thank the anonymous reviewers, whose comments improved the manuscript. We are grateful to Vincent Hill, Parasitic Diseases Branch, Division of Parasitic Diseases, NCZVED, CDC, for use of the PSQ MA96 machine and vacuum tool station for pyrosequencing.
The work was funded by the Antimicrobial Drug Resistance Working Group, Centers for Disease Control and Prevention (CDC), and the Amazon Malaria Initiative of the U.S. Agency for International Development. In addition, the Atlanta Research and Education Foundation, Atlanta VA Medical Center, helped support this work. A. A. Escalante was supported by NIH grant R01 GM60740. K. Mueller was supported by an Emerging Infectious Diseases Fellowship from the Association of Public Health Laboratories sponsored by the CDC.
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Published ahead of print on 5 February 2007.
REFERENCES
- 1.Anderson, T. J., B. Haubold, J. T. Williams, J. G. Estrada-Franco, L. Richardson, R. Mollinedo, M. Bockarie, J. Mokili, S. Mharakurwa, N. French, J. Whitworth, I. D. Velez, A. H. Brockman, F. Nosten, M. U. Ferreira, and K. P. Day. 2000. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 17:1467-1482. [DOI] [PubMed] [Google Scholar]
- 2.Contreras, C. E., J. F. Cortese, A. Caraballo, and C. V. Plowe. 2002. Genetics of drug-resistant Plasmodium falciparum malaria in the Venezuelan state of Bolivar. Am. J. Trop. Med. Hyg. 67:400-405. [DOI] [PubMed] [Google Scholar]
- 3.Cortese, J. F., A. Caraballo, C. E. Contreras, and C. V. Plowe. 2002. Origin and dissemination of Plasmodium falciparum drug-resistance mutations in South America. J. Infect. Dis. 186:999-1006. [DOI] [PubMed] [Google Scholar]
- 4.Cortese, J. F., and C. V. Plowe. 1998. Antifolate resistance due to new and known Plasmodium falciparum dihydrofolate reductase mutations expressed in yeast. Mol. Biochem. Parasitol. 94:205-214. [DOI] [PubMed] [Google Scholar]
- 5.Escalante, A. A., O. E. Cornejo, A. Rojas, V. Udhayakumar, and A. A. Lal. 2004. Assessing the effect of natural selection in malaria parasites. Trends Parasitol. 20:388-395. [DOI] [PubMed] [Google Scholar]
- 6.Excoffier, L., G. Laval, and S. Schneider. 2005. Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol. Bioinform. Online 1:47-50. [PMC free article] [PubMed] [Google Scholar]
- 7.Gregson, A., and C. V. Plowe. 2005. Mechanisms of resistance of malaria parasites to antifolates. Pharmacol. Rev. 57:117-145. [DOI] [PubMed] [Google Scholar]
- 8.Hastings, I. M. 2004. The origins of antimalarial drug resistance. Trends Parasitol. 20:512-518. [DOI] [PubMed] [Google Scholar]
- 9.Hayton, K., and X. Z. Su. 2004. Genetic and biochemical aspects of drug resistance in malaria parasites. Curr. Drug Targets Infect. Disord. 4:1-10. [DOI] [PubMed] [Google Scholar]
- 10.Iyer, J. K., W. K. Milhous, J. F. Cortese, J. G. Kublin, and C. V. Plowe. 2001. Plasmodium falciparum cross-resistance between trimethoprim and pyrimethamine. Lancet 358:1066-1067. [DOI] [PubMed] [Google Scholar]
- 11.Khalil, I., A. M. Ronn, M. Alifrangis, H. A. Gabar, G. M. Satti, and I. C. Bygbjerg. 2003. Dihydrofolate reductase and dihydropteroate synthase genotypes associated with in vitro resistance of Plasmodium falciparum to pyrimethamine, trimethoprim, sulfadoxine, and sulfamethoxazole. Am. J. Trop. Med. Hyg. 68:586-589. [DOI] [PubMed] [Google Scholar]
- 12.Khim, N., C. Bouchier, M.-T. Ekala, S. Incardona, P. Lim, E. Legrand, R. Jambou, S. Doung, O. M. Puijalon, and T. Fandeur. 2005. Countrywide survey shows very high prevalence of Plasmodium falciparum multilocus resistance genotypes in Cambodia. Antimicrob. Agents Chemother. 49:3147-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kublin, J. G., J. F. Cortese, E. M. Njunju, R. A. Mukadam, J. J. Wirima, P. N. Kazembe, A. A. Djimde, B. Kouriba, T. E. Taylor, and C. V. Plowe. 2003. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 187:1870-1875. [DOI] [PubMed] [Google Scholar]
- 14.Machado, R. L., M. M. Povoa, V. S. Calvosa, M. U. Ferreira, A. R. Rossit, E. J. dos Santos, and D. J. Conway. 2004. Genetic structure of Plasmodium falciparum populations in the Brazilian Amazon region. J. Infect. Dis. 190:1547-1555. [DOI] [PubMed] [Google Scholar]
- 15.Mberu, E. K., M. K. Mosobo, A. M. Nzila, G. O. Kokwaro, C. H. Sibley, and W. M. Watkins. 2000. The changing in vitro susceptibility pattern to pyrimethamine/sulfadoxine in Plasmodium falciparum field isolates from Kilifi, Kenya. Am. J. Trop. Med. Hyg. 62:396-401. [DOI] [PubMed] [Google Scholar]
- 16.McCollum, A. M., A. C. Poe, M. Hamel, C. Huber, Z. Zhou, Y. P. Shi, P. Ouma, J. Vulule, P. Bloland, L. Slutsker, J. W. Barnwell, V. Udhayakumar, and A. A. Escalante. 2006. Antifolate resistance in Plasmodium falciparum: multiple origins and identification of novel dhfr alleles. J. Infect. Dis. 194:189-197. [DOI] [PubMed] [Google Scholar]
- 17.Nair, S., J. T. Williams, A. Brockman, L. Paiphun, M. Mayxay, P. N. Newton, J. P. Guthmann, F. M. Smithuis, T. T. Hien, N. J. White, F. Nosten, and T. J. Anderson. 2003. A selective sweep driven by pyrimethamine treatment in southeast Asian malaria parasites. Mol. Biol. Evol. 20:1526-1536. [DOI] [PubMed] [Google Scholar]
- 18.Nash, D., S. Nair, M. Mayxay, P. N. Newton, J. P. Guthmann, F. Nosten, and T. J. Anderson. 2005. Selection strength and hitchhiking around two anti-malarial resistance genes. Proc. Biol. Sci. 272:1153-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park, S. D. E. 2001. Trypanotolerance in West African cattle and the population genetic effects of selection. Ph.D. thesis. University of Dublin, Dublin, Ireland.
- 20.Plowe, C. V., J. F. Cortese, A. Djimde, O. C. Nwanyanwu, W. M. Watkins, P. A. Winstanley, J. G. Estrada-Franco, R. E. Mollinedo, J. C. Avila, J. L. Cespedes, D. Carter, and O. K. Doumbo. 1997. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine-sulfadoxine use and resistance. J. Infect. Dis. 176:1590-1596. [DOI] [PubMed] [Google Scholar]
- 21.Plowe, C. V., J. G. Kublin, and O. Doumbo. 1998. P. falciparum dihydrofolate reductase and dihydropteroate synthase mutations: epidemiology and role in clinical resistance to antifolates. Drug Resist. Updates 1:389-396. [DOI] [PubMed] [Google Scholar]
- 22.Raymond, M., and F. Rousset. 1995. An exact test for population differentiation. Evolution 49:1280-1283. [DOI] [PubMed] [Google Scholar]
- 23.Roper, C., R. Pearce, B. Bredenkamp, J. Gumede, C. Drakeley, F. Mosha, D. Chandramohan, and B. Sharp. 2003. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet 361:1174-1181. [DOI] [PubMed] [Google Scholar]
- 24.Roper, C., R. Pearce, S. Nair, B. Sharp, F. Nosten, and T. Anderson. 2004. Intercontinental spread of pyrimethamine-resistant malaria. Science 305:1124. [DOI] [PubMed] [Google Scholar]
- 25.Sankoh, A. J., M. F. Huque, and S. D. Dubey. 1997. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat. Med. 16:2529-2542. [DOI] [PubMed] [Google Scholar]
- 26.Schmider, N., G. Peyerl-Hoffmann, M. Restrepo, and T. Jelinek. 2003. Short communication: point mutations in the dihydrofolate reductase and dihydropteroate synthase genes of Plasmodium falciparum isolates from Colombia. Trop. Med. Int. Health 8:129-132. [DOI] [PubMed] [Google Scholar]
- 27.Su, X., M. T. Ferdig, Y. Huang, C. Q. Huynh, A. Liu, J. You, J. C. Wootton, and T. E. Wellems. 1999. A genetic map and recombination parameters of the human malaria parasite Plasmodium falciparum. Science 286:1351-1353. [DOI] [PubMed] [Google Scholar]
- 28.Talisuna, A. O., P. Bloland, and U. D'Alessandro. 2004. History, dynamics, and public health importance of malaria parasite resistance. Clin. Microbiol. Rev. 17:235-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urdaneta, L., A. Lal, C. Barnabe, B. Oury, I. Goldman, F. J. Ayala, and M. Tibayrenc. 2001. Evidence for clonal propagation in natural isolates of Plasmodium falciparum from Venezuela. Proc. Natl. Acad. Sci. USA 98:6725-6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urdaneta, L., C. Plowe, I. Goldman, and A. A. Lal. 1999. Point mutations in dihydrofolate reductase and dihydropteroate synthase genes of Plasmodium falciparum isolates from Venezuela. Am. J. Trop. Med. Hyg. 61:457-462. [DOI] [PubMed] [Google Scholar]
- 31.Vasconcelos, K. F., C. V. Plowe, C. J. Fontes, D. Kyle, D. F. Wirth, L. H. Pereira da Silva, and M. G. Zalis. 2000. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase of isolates from the Amazon region of Brazil. Mem. Inst. Oswaldo Cruz 95:721-728. [DOI] [PubMed] [Google Scholar]
- 32.Wootton, J. C., X. Feng, M. T. Ferdig, R. A. Cooper, J. Mu, D. I. Baruch, A. J. Magill, and X. Z. Su. 2002. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418:320-323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.