Abstract
Herpes B virus (B virus [BV]) is a macaque herpesvirus that is occasionally transmitted to humans where it can cause rapidly ascending encephalitis that is often fatal. To understand the low susceptibility of BV to the acyclonucleosides, we have cloned, expressed, and characterized the BV thymidine kinase (TK), an enzyme that is expected to “activate” nucleoside analogs. This enzyme is similar in sequence and properties to the TK of herpes simplex virus (HSV), i.e., it has a broad substrate range and low enantioselectivity and is sensitive to inhibitors of HSV TKs. The BV enzyme phosphorylates some modified nucleosides and acyclonucleosides and l enantiomers of thymidine and related antiherpetic analogs. However, the potent anti-HSV drugs acyclovir (ACV), ganciclovir (GCV), and 5-bromovinyldeoxyuridine were poorly or not phosphorylated by the BV enzyme under the experimental conditions. The antiviral activities of a number of marketed antiherpes drugs and experimental compounds were compared against BV strains and, for comparison, HSV type 1 (HSV-1) in Vero cell cultures. For most compounds tested, BV was found to be about as sensitive as HSV-1 was. However, BV was less sensitive to ACV and GCV than HSV-1 was. The abilities of thymidine analogs and acyclonucleosides to inhibit replication of BV in Vero cell culture were not always proportional to their substrate properties for BV TK. Our studies characterize BV TK for the first time and suggest new lead compounds, e.g., 5-ethyldeoxyuridine and pencyclovir, which may be superior to ACV or GCV as treatment for this emerging infectious disease.
Herpes B virus (Cercopithecine herpesvirus 1) (BV) is an alphaherpesvirus that naturally infects members of the genus Macaca. In macaques, infection with BV is usually asymptomatic but can present as lesions on the face, in the mouth, or in the genital region. The lesions heal spontaneously but may appear again sporadically in the same way that oral and genital herpes simplex do in humans. However, when transmitted to humans, BV causes an acute ascending encephalitis that is usually fatal if not treated immediately (19). Transmission of BV to humans usually occurs through monkey bites or contact with infected monkey tissues or fluids. BV has been classified as a select agent by the U.S. Public Health Service (42CFR73.3).
Although BV is highly lethal in humans, the infrequency of human BV infections has not encouraged development of drugs specifically targeted to this virus. However, the extreme severity of BV infections makes this virus the primary zoonotic concern for persons working with macaques. Acyclovir (ACV), a drug widely used to treat herpes simplex virus (HSV) infections, is currently recommended for treatment of BV infections as well (3). However, BV is about 10 times less susceptible than HSV is to ACV and the related drug ganciclovir (GCV) (1, 3). No studies have examined the comparative sensitivity of BV to other antiherpes drugs, nor has the molecular basis of the lower susceptibility of BV to ACV or GCV been studied. With the increasing use of rhesus monkeys in biomedical research and the accompanying potential for more human BV infections, identification of antiviral drugs that are more effective in controlling BV infections is warranted.
Most of the antiherpes drugs used clinically or experimentally against human herpesviruses (HSV type 1 [HSV-1], HSV-2, varicella-zoster virus, and cytomegalovirus) are nucleoside analogs that are activated in infected cells by virus-encoded thymidine kinase (TK). Ultimately, the analogs are metabolized to the triphosphate form that is incorporated into the replicating viral DNA by the viral DNA polymerase, causing chain termination (reviewed in reference 20). Interestingly, HSV TKs are not stereoselective, being able to phosphorylate both enantiomers of thymidine (TdR) and other TdR analogs. Some inhibitors of the HSV TKs have been shown to block reactivation of latent HSV in animal models (21). Identification of drugs having a similar activity against BV would be of great value in treating human BV infections. This work was undertaken to study the properties of the key BV enzymes involved in DNA replication of the virus and to use their unique properties to find or design potential drugs to treat BV infections in humans. In this paper we focus on BV TK and report its relevance to the sensitivity of BV to various antiherpes drugs.
MATERIALS AND METHODS
Viruses and cells.
The standard E2490 laboratory strain of BV was used for initial studies, and three field isolates of BV (24105, 32425, and E90-136) were also used (15). The F strain of HSV-1 was used for comparison studies. All virus propagation and experimental assays were performed in Vero cells, and all work with infectious BV was conducted under biosafety level 3. Cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and maintained after experimental infection in DMEM containing 2% FBS.
Chemicals.
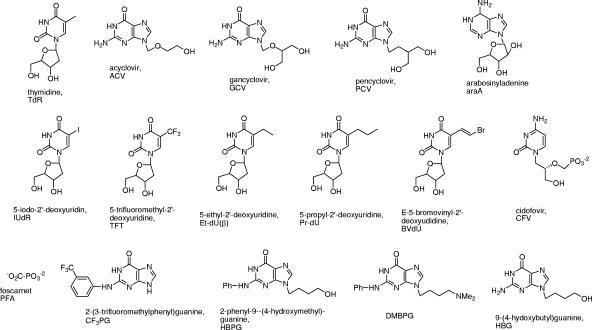
Analytical-grade reagents were used exclusively. Bacterial medium components were from Difco, and Ni-nitrilotriacetic acid (Ni-NTA) Superflow resin was from QIAGEN. Restriction and modification enzymes were from Promega, Sigma, or Roche. Isopropylthio-β-d-galactoside (IPTG) was from Sigma, and [3H]thymidine, 20 Ci/mmol, was from New England Nuclear. The acyclonucleosides ACV, GCV, and pencyclovir (PCV), the thymidine analogs 5-iodo-2′-deoxyuridine (IUdR), 5-trifluoromethyl-2′-deoxyuridine (TFT), and E-(5-bromovinyl)-2′-deoxyuridine (BVdU), and foscarnet (PFA) were available commercially. Cidofovir (CFV) was a gift from Gilead Sciences Inc. Authors contributed the following experimental compounds: 9-(4-hydroxybutyl)guanine (HBG), 9-(4-hydroxybutyl)-N2-phenylguanine (HBPG), and related TK inhibitors (G. E. Wright) (13, 22); 5-ethyl-2′-deoxyuridine (Et-dU, α and β anomers) and β-5-propyl-2′-deoxyuridine (Pr-dU) (D. Shugar) (5, 18); l-thymidine (l-TdR), l-5-iodo-2′-deoxyuridine (l-IUdR), and l-E-(5-bromovinyl)-2′-deoxyuridine) (l-BVdU) (F. Focher) (16, 17). Structures of compounds used in this work are shown in Fig. 1.
FIG. 1.
Structures of nucleosides and related compounds referred to in this work.
Construction of recombinant bacterial expression vector for BV TK.
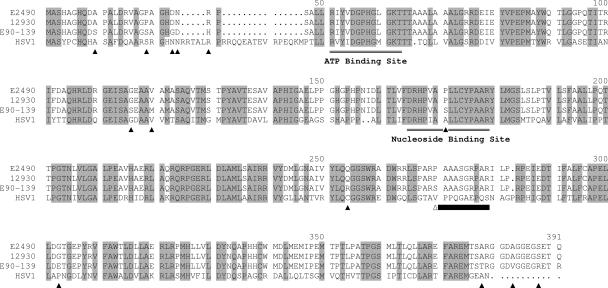
The TK gene of BV strain E2490 (GenBank accession number AB096160) was inserted in pTopo and amplified by PCR by using the following primers: 5′-GAACATGCTCGAGCACGCCGGCCATCAGGATGC-3′ (primer 1, sense) and 5′-ACGTCTGTTCGAACCATCTTTATTGCGCC-3′ (primer 2, antisense). The amplified product was digested with XhoI and Csp45I (present in the primers) and inserted into the multiple cloning site of pTrcHisA (Invitrogen), previously digested with the same restriction enzymes, to give the recombinant bacterial expression vector pHis-BV-TK, which contains the complete BV TK sequence with an N-terminal His6 tag, resulting in a protein of the amino acid sequence of Fig. 2. By using the same protocol, we also obtained a mutant BV TK gene lacking 30 bp (856 to 885); see the deleted sequence in Fig. 2.
FIG. 2.
Alignment of BV and HSV-1 TK sequences. Amino acids constituting the ATP and the nucleoside binding sites are indicated, and all residues that are conserved between HSV-1 and all BV isolates are highlighted in gray. Residues that differ between the cynomolgous and rhesus BV sequences are indicated by black triangles under the sequences. The 10 amino acids (residues 270 to 279) deleted in the mutant recombinant BV TK referred to in the text are indicated by a black bar under the sequences.
Expression and purification of recombinant BV TK from bacterial cells.
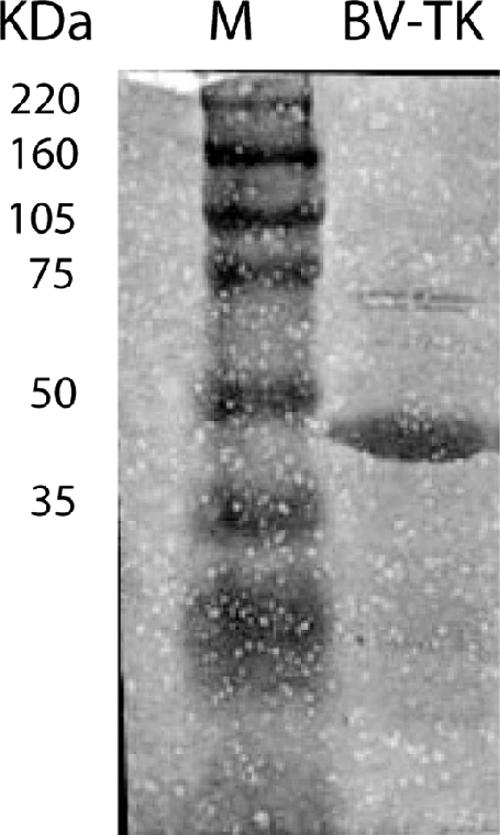
Expression and purification of the BV TK were carried out as described by the manufacturer of Ni-NTA Superflow resin (QIAGEN). Briefly, a fresh overnight saturated culture of Escherichia coli (JM109 strain) transformed with the recombinant pHis-BV-TK was diluted 1:100 in 1 liter of 2XYT broth (10 g/liter Bacto-yeast extract, 16 g/liter Bacto-tryptone, 5 g/liter NaCl; Gibco) containing ampicillin (60 μg/ml) and incubated at 37°C with shaking. At an optical density at 600 nm of 0.6, IPTG was added to a final concentration of 1 mM, and the culture was incubated for another 4 h at 37°C. After centrifugation, the bacterial cell pellet was resuspended in 4 volumes of lysis buffer (50 mM sodium phosphate [pH 8.0], 300 mM NaCl, 10 mM imidazole, 1 mM phenylmethanesulfonyl fluoride, and 1 mg/ml lysozyme) and incubated on ice for 30 min. Cells were then sonicated on ice, and the lysate was centrifuged at 10,000 × g for 30 min at 4°C. The supernatant was loaded on a Ni-NTA Superflow column (1 ml), and at a flow rate of 0.25 ml/min, the column was first washed with lysis buffer and then with 50 mM sodium phosphate buffer (pH 8.0) containing 300 mM NaCl and 20 mM imidazole. The protein was step eluted with 250 mM imidazole in 50 mM sodium phosphate buffer (pH 8.0), 300 mM NaCl, and 1 mg/ml bovine serum albumin. Fractions were collected for enzymatic activity analysis. The TK enzyme, collected from peak fractions, was dialyzed against 50 mM Tris-HCl (pH 7.5) containing 20% glycerol and 1 mM dithiothreitol (DTT) and frozen in liquid nitrogen until use. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (Fig. 3) showed a predominant protein band at 40 kDa of approximately 98% purity. We also expressed and purified the BV TK mutant lacking 30 bp (see above). Once eluted from the Ni-NTA column, this enzyme was found completely inactive in our assay conditions. This finding rules out the possibility that E. coli TK contaminates our wild-type BV TK preparation.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of the affinity-purified recombinant BV TK. Lanes: M, molecular mass markers; BV-TK, 6 μg of recombinant TK eluted from the Ni-NTA column (98% pure by densitometry [see Materials and Methods]).
TK activity assay.
Recombinant BV TK was assayed at 37°C for 20 min in 25 μl of a reaction mixture containing 30 mM HEPES K+, pH 7.5, 6 mM MgCl2, 6 mM ATP, 0.5 mM DTT, and 1 μM of [methyl-3H]TdR (2,200 cpm/pmol). The reaction was terminated by spotting 20 μl of the reaction mixture on a 23-mm DEAE paper disks (DE-81; Whatman). The disks were washed twice in an excess of 1 mM ammonium formate (pH 5.6) in order to remove unconverted nucleoside and washed once in ethanol. Filters were dried, and radioactivity was counted in 1 ml of Betamax (ICN) scintillation fluid. One unit is defined as the amount of enzyme that phosphorylates 1 nmol of TdR to TMP in 1 hour at 37°C. Enzyme activity was linear up to 40 min. For inhibitor assays, stock solutions of inhibitors in dimethyl sulfoxide (DMSO) were serially diluted into enzyme assay mixtures. The enzyme was assayed with at least five inhibitor concentrations in duplicate to determine the inhibitor concentration reducing activity by 50% (IC50) in the above assay. When inhibitor Kis were measured, the assay was carried out at several concentrations of [3H]TdR.
TK substrate assay.
When nucleoside analogs were tested as possible substrates of BV TK, each compound (100 μM) was incubated at 37°C for 0, 10, 20, 30, and 40 min in a mixture (25 μl) containing 30 mM HEPES K+, pH 7.5, 6 mM MgCl2, 6 mM ATP, 0.5 mM DTT, and the required amount of enzyme. Samples were then heated at 100°C for 5 min and centrifuged for 15 min at 10,000 rpm in an Eppendorf bench centrifuge. Supernatants were transferred to a new tube for subsequent high-performance liquid chromatography (HPLC) analysis. The reverse-phase chromatography method used to separate nucleosides from nucleotides employed a Shimadzu HPLC system, consisting of an ALLTIMA C18-NUC 100A 5U column (4.6 mm × 25 cm) (Alltech) at room temperature under the following conditions: injection volume, 20 μl; detection, UV 260 nm; eluents of the linear gradient, buffer A (20 mM KH2PO4, pH 7.5) and buffer B (20 mM KH2PO4, pH 5.2, 60% methanol); elution time, 40 min from 0% to 100% buffer B; and flow rate, 0.5 ml/min. Two independent experiments were performed in which each point was run in duplicate. Enzyme activity was linear up to 40 min. Percentage values were calculated by analysis of the UV peak areas eluted from the column by using the Shimadzu class VP chromatography data system 4.3.
Antiviral assays.
Virus stocks were diluted in cold DMEM containing 2% FBS, and six-well trays containing confluent monolayers of Vero cells were infected with 200 μl/well of virus (to yield 150 to 200 PFU/well). Drugs were dissolved in DMSO at 10 mg/ml and diluted in overlay medium (DMEM containing 2% FBS and 0.25% methylcellulose). After 1 h of adsorption at 37°C, the viral inoculum was removed, and cells were overlaid with 2.5 ml of medium. Each drug concentration was tested in duplicate wells. Plates were incubated at 37°C for 36 to 48 h, and plaques were counted. The percent plaque reduction was calculated based on the number of plaques obtained in the absence of drugs.
Cytotoxicity assays.
Drugs were dissolved in DMSO and threefold serially diluted (200 to 0.1 μg/ml) in growth medium, DMEM supplemented with 10% fetal calf serum, containing 2.5 μCi/ml of [methyl-3H]TdR (25 Ci/mmol; Amersham). Each drug was assayed in three independent experiments. The 15% confluent Vero cells were incubated in 5% CO2/95% air at 37°C for 2 days, and cells were collected with an automated cell harvester onto fiberglass filters. Trichloroacetic acid (10%) was added to digest the cells, and trichloroacetic acid-precipitable counts were determined in a scintillation counter. Mean control incorporation into DNA was 3,720 ± 331 cpm.
RESULTS
Recombinant BV thymidine kinase.
The DNA sequence of the BV TK gene from the standard laboratory strain E2490 has been published (14). Because E2490 is an attenuated vaccine strain of BV, it was necessary to determine whether the E2490 TK was representative of nonattenuated field isolates of BV. We therefore sequenced the TK gene of two field isolates of BV, one from a rhesus macaque (strain 12930; GenBank accession number EF426677) and one from a cynomolgous macaque (strain E90-136; GenBank accession number EF426678). The TK genes were amplified by PCR, and the PCR products were purified and sequenced without cloning. The amino acid sequences (Fig. 2) of the rhesus BV isolates E2490 and 12930 were identical, and the cynomolgous E90-136 BV sequence differed by only 14 amino acids (3.6%), 11 of which were conservative substitutions. Amino acid sequence homology between the BV and HSV TKs was considerably less (approximately 55% identical and 65% similar). However, amino acid residues of the HSV TK that have been identified as part of the ATP and nucleoside binding sites or otherwise critical to TK activity were all conserved in the BV TK polypeptide (Fig. 2).
Kinetic properties of recombinant BV TK.
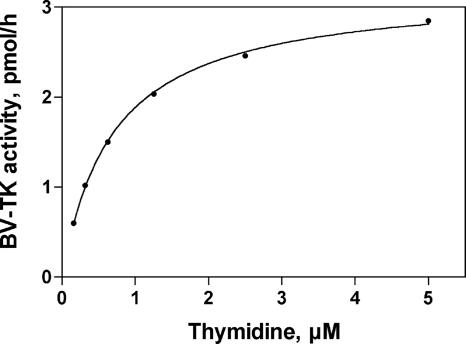
In order to determine the Km and Vmax of BV TK, we assayed a limiting amount of enzyme with several concentrations of [3H]TdR and the reaction velocity V was determined at each substrate concentration. Figure 4 shows the kinetics of TdR phosphorylation by recombinant BV TK. Analysis revealed that the enzyme had Km and Vmax values for TdR of 0.7 μM and 0.2 nmol/h/μg, respectively (see also Table 3). The Km value of 1 μM for recombinant HSV-1 TK was similar (13), but the specific activity of the HSV-1 enzyme was higher (17 nmol/h/μg; F. Focher, unpublished results) than the value for BV TK.
FIG. 4.
Kinetics of BV TK activity in the presence of increasing concentrations of [3H]TdR. Under the assay conditions described in Materials and Methods, enzyme velocity of 2,200 cpm/20 min corresponds to 0.2 nmol of TdR transformed to TMP in 1 h by 1 μg of enzyme.
TABLE 3.
Kinetic properties of phosphorylation of substrates by BV TKa
| Substrate | Km (μM) (mean ± SD)b | Vmax (nmol h−1 μg−1) (mean ± SD)b | Vmax/Km |
|---|---|---|---|
| TdR | 0.70 ± 0.05 | 0.20 ± 0.005 | 0.28 |
| HBPG | 7.40 ± 0.047 | 3.90 ± 0.1 | 0.52 |
| l-TdR | 0.65 ± 0.15 | 0.15 ± 0.008 | 0.23 |
Assayed at variable concentrations of substrate.
The values are the means from two independent experiments in which each concentration was tested in duplicate.
Inhibition of BV TK by nucleosides and related compounds.
We first tested several nucleoside analogs for their ability to inhibit the phosphorylation of [3H]TdR catalyzed by BV TK, and Table 1 shows the IC50 values of the compounds tested. Certain analogs of TdR, e.g., 5-trifluoromethyl (TFT), 5-iodo (IUdR), and 5-ethyl (Et-dU) derivatives, were potent inhibitors, but the E-5-bromovinyl derivative, BVdU, was a weak inhibitor. The latter result contrasts with the reported high affinity of BVdU for HSV-1 TK, a Ki of 0.24 μM (2). The acyclonucleoside antiherpes drugs ACV and GCV weakly inhibited BV TK activity; IC50 values of ca. 1 mM could only be estimated at the highest concentrations of ACV and GCV tested (Table 1). The weak inhibition by ACV and GCV seems unexpected, but in fact these acycloguanosines do not have high affinity even for the HSV TK enzymes (9). For example, the IC50 values for ACV against recombinant HSV-1 and HSV-2 TKs were 122 and 200 μM, respectively (F. Focher, unpublished observations). In contrast, the “methylene” isosteres of ACV and GCV, viz., HBG and PCV, respectively, were at least 10-fold more active as inhibitors of BV TK than the acyclonucleosides themselves (Table 1).
TABLE 1.
Effects of nucleoside analogs on BV TK activity (IC50)a
| Nucleoside analog | Acronym | IC50 (μM) (mean ± SD)b |
|---|---|---|
| Thymidine analogs | ||
| 5-Trifluoromethyl-2′-deoxyuridine | TFT | 2.0 ± 0.1 |
| l-Thymidine | l-TdR | 4.1 ± 0.02 |
| E-5-(Bromovinyl)deoxyuridine | BVdU | 32.3 ± 3.5 |
| l-5-(Bromovinyl)deoxyuridine | l-BVdU | 15.0 ± 2.0 |
| 5-Iododeoxyuridine | IUdR | 1.5 ± 0.2 |
| l-5-Iodo-2′-deoxyuridine | l-IUdR | 2.0 ± 0.2 |
| 5-Ethyl-2′-deoxyuridine | Et-dU | 0.15 ± 0.01 |
| α-5-Ethyl-2′-deoxyuridine | α-Et-dU | 52 ± 5.0 |
| 5-Propyl-2′-deoxyuridine | Pr-dU | 4.2 ± 0.2 |
| Acyclonucleosides | ||
| Acyclovir | ACV | 1,000 ± 210 |
| Ganciclovir | GCV | 800 ± 112 |
| Cidofovir | CFV | 900 ± 155 |
| Penciclovir | PCV | 84.4 ± 5.5 |
| 2-Amino-9-(4-hydroxybutyl)-6-oxopurine | HBG | 177 ± 20 |
| HSV TK inhibitors | ||
| N2-(3-Trifluoromethylphenyl)guanine | CF3PG | 3.5 ± 0.15 |
| 2-Phenylamino-9-(4-hydroxy-butyl)-6-oxopurine | HBPG | 11.5 ± 1.0 |
| 2-Phenylamino-9-(N,N-dimethylaminobutyl)-6-oxopurine | DMBPG | 1.2 ± 0.15 |
Enzyme assays contained 1 μM [3H]TdR and were run in duplicate with at least five concentrations of inhibitor.
The values are the means for two independent experiments in which each concentration was tested in duplicate.
The HSV TK inhibitors HBPG and several 2-phenylguanines were moderately potent as inhibitors of BV TK but ca. 10-fold weaker than their activity against HSV TKs (13). Miscellaneous nucleoside analogs, e.g., arabinosyladenine (araA), lyxofuranosyluracil, and 6-methyluridine, and the α anomer of Et-dU, did not inhibit BV TK at 100 μM (not shown).
Substrate properties of nucleosides and analogs.
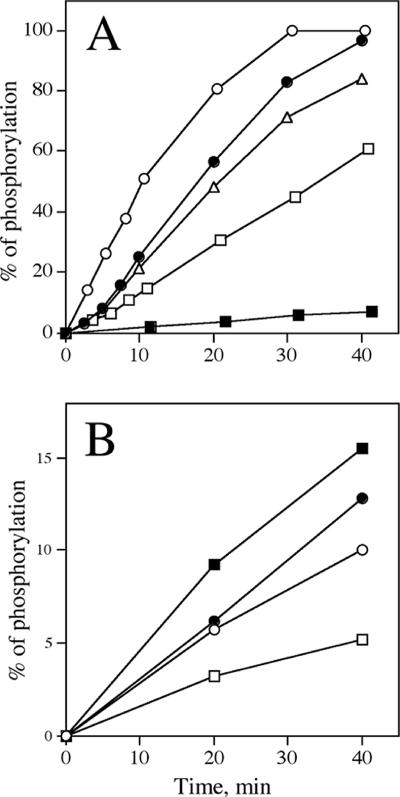
The recombinant BV TK enzyme was also used to evaluate whether it was able to phosphorylate various nucleoside derivatives and acyclonucleosides (Table 2), which could then be considered possible antiviral drugs. The possible substrates ACV, GCV, CFV, PCV, TFT, IUdR, BVdU, and HBPG were incubated at both 0.1 and 1 mM with excess BV TK. Reaction mixtures were analyzed by reverse-phase HPLC, and UV peak areas of starting compound and putative phosphorylated product were used to determine the kinetics of phosphorylation, if any. Under assay conditions which resulted in the complete phosphorylation of TdR to its monophosphate TMP, the compounds TFT, PCV, IUdR, BVdU, and HBPG were phosphorylated with different efficiencies (Fig. 5), but ACV, GCV, and CFV were very poorly or not phosphorylated under these conditions (data not shown). Interestingly, the HSV TK inhibitor HBPG was very efficiently phosphorylated with a Km of 7.4 μM and a Vmax of 3.9 nmol/h/μg. Table 3 shows the kinetic properties of HBPG compared with those of the natural substrate TdR. Comparison of Vmax/Km ratios, which estimate the efficiency of phosphorylation, indicate that BV TK phosphorylated HBPG with an efficiency 1.8 times greater than for TdR.
TABLE 2.
Phosphorylation of nucleoside analogs (100 μM) by BV TKa
| Analog | Phosphorylation (%)b (mean ± SD) |
|---|---|
| TdR | 100 |
| l-TdR | 130 ± 8.0 |
| TFT | 55 ± 4.2 |
| BVdU | 0 |
| l-BVdU | 0 |
| IUdR | 58 ± 2.5 |
| l-IUdR | 94 ± 4.3 |
| Et-dU | 82 ± 4.1 |
| Pr-dU | 31 ± 2.5 |
| ACV | 0 |
| GCV | 0 |
| CFV | 0 |
| PCV | 72 ± 5.1 |
| HBPG | 200 ± 25 |
| HBG | 94 ± 7.2 |
Determined by HPLC analysis of reaction mixtures containing BV TK after 30 min of incubation.
Relative to the mean of TdR, 0.5 nmol TMP produced in the assay reaction conditions (see Materials and Methods). Zero values indicate no detectable monophosphate at 100 μM or 1 mM.
FIG. 5.
Kinetics of phosphorylation of nucleoside analogs by BV TK. Panel A shows 100 μM TdR (•), HBPG (○), IUdR (□), BVdU (▪), and Et-dU (▵). Panel B shows 1 mM TdR (•), PCV (○), HBG (▪), and Pr-dU (□). Each nucleoside analog was incubated for the indicated time in the assay mixture described in Materials and Methods, and the products of the reaction were resolved by HPLC as described in Materials and Methods (100% corresponds to 2.5 nmol of substrate converted to monophosphate).
BV TK is not enantioselective.
When BV TK was incubated with l-TdR under conditions that resulted in the formation of the 5′-monophosphate of the natural substrate d-TdR, the phosphorylation rate was similar to that of the natural substrate. BV TK displayed Km and Vmax values of 0.65 μM and 0.15 nmol/h/μg, respectively, for l-TdR (Table 3). The lack of enantioselectivity of BV TK was also confirmed by similar inhibition by the d and l enantiomers of BVdU and IUdR (Table 1).
Antiviral activity in Vero cell cultures.
Plaque reduction assays were conducted in Vero cells infected with several strains of BV and, for comparison, with HSV-1. Strains of BV included 24105 and E90-136, both neuropathogenic in mice, and 32425, a strain that is nonpathogenic in mice, and the F strain of HSV-1. Duplicate cell cultures were infected with 150 to 200 PFU of virus for 1 h and then overlaid with medium containing various concentrations of the drug (or vehicle). The plaque morphology of BV varied with the drug concentration, making the quantitation of drug sensitivity difficult to assess. With increasing drug concentrations, the first effect noted was a decrease in plaque size. At higher drug concentrations, the characteristic syncytial activity of BV was inhibited, resulting in small clumps of rounded cells. For the purpose of these studies, foci of viral cytopathic effect were counted as plaques only if there was an area of clearing (cell lysis); the size of the plaque was not considered. Percent reduction of virus plaque formation was determined after 36 to 48 h of incubation at 37°C. For comparison, cytotoxicity in uninfected Vero cells was determined by measuring [3H]TdR incorporation by the DNA of proliferating cells. Approximate values of antiviral 50% effective concentration (EC50) and cytotoxic concentration of drug that reduced cellular DNA synthesis by 50% (CC50) of tested compounds are listed in Table 4.
TABLE 4.
Plaque reduction in BV-infected and HSV1-infected Vero cells and cytotoxicity in uninfected Vero cells by nucleoside analogs
| Nucleoside analog | Antiviral EC50 (μM)a (mean ± SD)
|
Cytotoxicity (CC50e [μM] [mean ± SD]) | |||
|---|---|---|---|---|---|
| BV 24105 | BV 32425 | BV E90-136 | HSV-1 F | ||
| ACV | 112 ± 65b | 118 ± 71c | 47.5 ± 24b | 1.4 ± 0.13d | >890 |
| GCV | 14.5 ± 12c | 23.5 ± 23.9d | 19.2 ± 13.7c | 0.7 ± 0.23d | 666 ± 106 |
| PCV | 17.0 ± 8.7b | 25.3 ± 13.8d | 17.3 ± 11.4c | 4.7 ± 2.1d | >790 |
| IUdR | 11.2 | 8.5 | NT | 2.8 | 6.8 ± 2.5 |
| TFT | 1.7 | 3.4 | NT | <1.7 | 50.6 ± 9.8 |
| BVdU | ≫300 | ≫300 | NT | 0.12 | >600 |
| Et-dU | 37.4 ± 20.3c | 48.0 ± 26c | 13.3 ± 14.8b | 2.1 ± 0.8d | >780 |
| CFV | 28.9 | 21.6 | NT | 14.4 | >720 |
| PFA | >500 | ca. 250 | NT | 250 | >1,000 |
| araA | 28 | 35 | NT | 7 | 38 ± 7.72 |
| HBPG | >330 | >330 | NT | 330 | >660 |
From single plaque reduction experiments (n = 1) run in duplicate, unless indicated otherwise. NT, not tested.
n = 5.
n = 4.
n = 3.
CC50, cytotoxic concentration of drug that reduced the viable cell number by 50%. Data were from [3H] TdR incorporation studies of subconfluent Vero cells in triplicate.
Anti-BV potency generally followed the ability of the compounds to be substrates for BV TK. The most potent compound was TFT, with EC50 values of 1.7 and 3.4 μM against two BV strains, nearly as potent as its activity against HSV-1. The other TdR analogs were also active, with the exception of BVdU. The estimated EC50 values of BVdU of >330 μM contrasts with its potent activity against HSV-1 (EC50 of 0.12 μM; see also reference 6). IUdR and Et-dU were active, although somewhat weaker than their activity against HSV-1.
The acyclonucleosides ACV and GCV were about 10-fold weaker against BV than against HSV-1, as previously reported (1, 3). Their weaker activity is consistent with, although not completely explained by, their lack of observed substrate sensitivity toward BV TK (Table 3). PCV, the methylene isostere of GCV (see structures in Fig. 1), was only three- to fourfold weaker against the BV strains than against HSV-1 (Table 4), an observation consistent with the efficient phosphorylation of PCV by BV TK (Table 3). PCV was reported to have an affinity to HSV-1 TK similar to that of TdR, although it was phosphorylated at only 9% of the rate for TdR at 250 μM (11). Does this simple change in structure give rise to this difference in properties of O-containing and CH2-containing 9-substituted guanines? A similar parallel at the level of TK was observed between HBG, the methylene isostere of ACV, and ACV itself. HBG, a compound with weak anti-HSV activity and a weak substrate for HSV-1 TK (10), was an efficient substrate for BV TK (Table 3).
Other anti-HSV drugs tested—CFV, PFA, and araA—had similar activity against the BV strains and HSV-1. The TK inhibitor/substrate HBPG was inactive against both viruses.
Cytotoxicity was noted with thymidine analogs that are expected to be phosphorylated by the host TK (TFT and IUdR) and by araA (Table 4). Other TdR analogs and acyclonuceosides had little cytotoxic effect between 600 and 1,000 μM (Table 4), including the close substrate relative Et-dU.
DISCUSSION
We have expressed and assayed the BV thymidine kinase and have established a BV-infected cell culture system. Although the BV and HSV TKs show similar Km values for the natural substrate, screening of various antiherpes drugs, experimental compounds, and TK inhibitors/substrates has revealed different responses of the BV and HSV enzymes (Tables 1 and 2). Most notably, the acyclonucleosides ACV and GCV that are the mainstay of anti-HSV drugs bind weakly to and are not detectably phosphorylated (“activated”) by BV TK under the conditions used. However, the methylene isostere of GCV, i.e., PCV, is a reasonable substrate. Thymidine analogs, such as the anti-HSV drug IUdR and 5-alkyldeoxyuridines, are well phosphorylated, but surprisingly, the potent anti-HSV drug BVdU is not. N2-Phenylguanines act as inhibitors of BV TK but are less potent than against HSV TKs. The lead anti-HSV TK compound HBPG inhibited and was readily phosphorylated by BV TK. The l enantiomers of nucleosides were phosphorylated at rates similar to those of their d counterparts, showing a lack of stereoselectivity of BV TK similar to that reported for the HSV enzymes (8, 12, 16, 17).
Results of anti-BV testing generally paralleled the results of BV TK testing (Table 4). The thymidine analogs IUdR, TFT, and Et-dU were especially potent anti-BV compounds, although the first two compounds were also cytotoxic. Nucleoside analogs that were not phosphorylated had weaker activity (ACV and GCV) or lacked anti-BV activity (BVdU) compared to their potency against HSV-1. One exception was the phosphonate CFV, which was a moderately potent antiviral, probably because it bypasses the phosphorylation step. PFA and HBPG were weak anti-BV compounds, weaker even than their activity against HSV.
The most potent anti-BV compounds iododeoxyuridine (IUdR) and TFT are not suitable development candidates for BV infections. IUdR and TFT have been used topically for HSV keratitis but are too toxic for systemic use because they are nonselectively phosphorylated by viral and host cell TKs and have significant cytotoxicity (Table 4). araA is both a potent antiviral and cytotoxic compound. CFV, although also a potent antiviral drug with a broad spectrum of activity, has limitations; it is available only as an intravenous formulation and can be nephrotoxic if multiple administrations are needed for therapeutic use.
The most promising anti-BV compounds that we have identified are Et-dU and PCV. Et-dU is equal to or more potent than the currently recommended drug ACV, and its potency is comparable to the potency of GCV (Table 4). Et-dU has been marketed in the past (in Europe and Canada) as a topical treatment for ocular herpes infections. A summary of its clinical pharmacology suggests that it is a relatively nontoxic but highly efficacious antiherpes drug (4, 7). Its high water solubility may make it a prime candidate for testing against BV encephalitis, and it may also warrant a second look as treatment for HSV encephalitis. PCV is currently marketed as a topical drug, although its diacetylated, 6-deoxy prodrug form famciclovir is available for systemic use.
Acknowledgments
This work was supported by grants AI055128 (G.E.W.) and RR07849 (R.E.) from the National Institutes of Health and by MURST-FIRB grant RBAU1LSR4_001 (to F.F.). We thank Gilead Sciences Inc. for a gift of cidofovir.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Boulter, E. A., B. Thornton, F. J. Bauer, and A. Bye. 1980. Successful treatment of experimental B virus (Herpesvirus simiae) infection with acyclovir. Br. Med. J. 280:681-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng, Y.-C., G. Dutschman, E. DeClercq, E. A. Jones, S. G. Rahim, G. Verhelst, and R. T. Walker. 1981. Differential affinities of 5-(2-halogenovinyl)-2′-deoxyuridines for deoxythymidine kinases of various origins. Mol. Pharmacol. 20:230-233. [PubMed] [Google Scholar]
- 3.Cohen, J. I., J. S. Davenport, J. A. Stewart, S. Deitchmann, J. K. Hilliard, L. E. Chapman, and the B Virus Working Group. 2002. Recommendations for prevention of and therapy for exposure to B virus (Cercopithecine Herpesvirus 1). Clin. Infect. Dis. 35:1191-1203. [DOI] [PubMed] [Google Scholar]
- 4.Davis, W. B., J. E. Oakes, J. P. Vacik, R. R. Rebert, and J. A. Taylor. 1979. 5-Ethyl-2′-deoxyuridine as a systemic agent for treatment of herpes simplex virus encephalitis. Comparison of effects in a normal and immunosuppressed murine model, p. 140-150. In K. K. Gauri (ed.), Anti-herpesvirus chemotherapy: experimental and clinical aspects. Karger, Basel, Switzerland. [PubMed]
- 5.De Clercq, E., J. Descamps, and D. Shugar. 1978. 5-Propyl-2′-deoxyuridine: a specific anti-herpes agent. Antimicrob. Agents Chemother. 13:545-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeClercq, E. 1984. Biochemical aspects of the selective antiherpes activity of nucleoside analogues. Biochem. Pharmacol. 33:2159-2169. [DOI] [PubMed] [Google Scholar]
- 7.Elze, K.-L. 1979. Ten years of clinical experiences with ethyldeoxyuridine, p. 134-139. In K. K. Gauri (ed.), Anti-herpesvirus chemotherapy: experimental and clinical aspects. Karger, Basel, Switzerland. [PubMed]
- 8.Focher, F., S. Spadari, and G. Maga. 2003. Antivirals at the mirror: the lack of stereospecificity of some viral and human enzymes offers novel opportunities in antiviral drug development. Curr. Drug Targets Infect. Disord. 3:41-53. [DOI] [PubMed] [Google Scholar]
- 9.Keller, P. M., J. A. Fyfe, L. Beauchamp, C. M. Lubbers, P. A. Furman, H. J. Schaeffer, and G. B. Elion. 1981. Enzymatic phosphorylation of acyclic nucleoside analogs and correlations with antiherpetic activities. Biochem. Pharmacol. 30:3071-3076. [DOI] [PubMed] [Google Scholar]
- 10.Larsson, A., S. Alenius, N. G. Johansson, and B. Öberg. 1983. Antiherpetic activity and mechanism of action of 9-(4-hydroxybutyl)guanine. Antivir. Res. 3:77-86. [DOI] [PubMed] [Google Scholar]
- 11.Larsson, A., K. Stenberg, A.-C. Ericson, U. Hagland, W.-A. Yisak, N. G. Johansson, B. Öberg, and R. Datema. 1986. Mode of action, toxicity, pharmacokinetics, and efficacy of some new antiherpesvirus guanosine analogs related to buciclovir. Antimicrob. Agents Chemother. 30:598-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maga, G., A. Verri, W. Ponti, L. Bonizzi, G. Poli, A. Garbesi, D. Niccolai, S. Spadari, and F. Focher. 1993. Lack of stereospecificity of suid pseudorabies virus (PRV) thymidine kinase. Biochem. J. 294:381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manikowski, A., A. Verri, A. Lossani, B. M. Gebhardt, J. Gambino, F. Focher, S. Spadari, and G. E. Wright. 2005. Inhibition of herpes simplex virus thymidine kinases by 2-phenylamino-6-oxopurines and related compounds: structure-activity relationships and antiherpetic activity in vivo. J. Med. Chem. 48:3919-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohsawa, K., D. H. Black, H. Sato, K. Rogers, and R. Eberle. 2003. Sequence and genetic arrangement of the UL region of the monkey B virus (Cercopithecine herpesvirus 1) genome and comparison with the UL region of other primate herpesviruses. Arch. Virol. 148:989-997. [DOI] [PubMed] [Google Scholar]
- 15.Ritchey, J. W., M. E. Payton, and R. Eberle. 2005. Clinicopathological characterization of monkey B virus (Cercopithecine herpesvirus 1) infection in mice. Comp. Med. 55:246-250. [DOI] [PubMed] [Google Scholar]
- 16.Spadari, S., G. Maga, F. Focher, G. Ciarrocchi, R. Manservigi, F. Arcamone, M. Capobianco, A. Carcuro, F. Colonna, S. Iotti, and A. Garbesi. 1992. l-Thymidine is phosphorylated by herpes simplex virus type 1 thymidine kinase and inhibits viral growth. J. Med. Chem. 35:4214-4220. [DOI] [PubMed] [Google Scholar]
- 17.Spadari, S., G. Ciarrocchi, F. Focher, A. Verri, G. Maga, F. Arcamone, E. Iafrate, S. Manzini, A. Garbesi, and L. Tondelli. 1995. 5-Iodo-2′-deoxy-L-uridine (L-IdU) and (E)-5-(2-bromovinyl)-2′-deoxy-l-uridine (L-BVdU): selective phosphorylation by herpes simplex virus type 1 thymidine kinase, antiherpetic activity and cytotoxicity studies. Mol. Pharmacol. 47:1231-1238. [PubMed] [Google Scholar]
- 18.Świerkowski, M., and D. Shugar. 1969. A nonmutagenic thymidine analog with antiviral activity: 5-ethyldeoxyuridine. J. Med. Chem. 12:533-534. [DOI] [PubMed] [Google Scholar]
- 19.Whitley, R. J. 1996. Cercopithecine herpes virus 1 (B virus), p. 2623-2635. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott Raven, Philadelphia, PA.
- 20.Wright, G. E., and N. C. Brown. 1990. Deoxyribonucleotide analogs as inhibitors and substrates of DNA polymerases. Pharm. Ther. 47:447-497. [DOI] [PubMed] [Google Scholar]
- 21.Wright, G. E., J. J. Gambino, H. Sun, and B. M. Gebhardt. 1999. Status of inhibitors of herpes simplex thymidine kinase. Curr. Opin. Anti-infect. Drugs 1:541-546. [Google Scholar]
- 22.Xu, H., G. Maga, F. Focher, E. R. Smith, S. Spadari, J. Gambino, and G. E. Wright. 1995. Synthesis, properties and pharmacokinetic studies of N2-phenylguanine derivatives as inhibitors of herpes simplex virus thymidine kinases. J. Med. Chem. 38:87-94. [DOI] [PubMed] [Google Scholar]