Abstract
Topical microbicides (cellulose acetate 1,2 benzene dicarboxylate [CAP], PRO 2000, SPL7013, and UC781) are being investigated to reduce the sexual transmission of human immunodeficiency virus type 1 (HIV-1). These products were shown to prevent the transfer of infectious HIV-1 from urogenital and colorectal epithelial cell lines to peripheral blood mononuclear cells. However, it was unclear if the topical microbicides rendered the virus noninfectious and/or reduced the binding to the epithelial cells. To test this, epithelial cells were cultured with HIV-1 in the presence or absence of topical microbicides or their placebos. The cells were washed, RNA lysates were made, and real-time PCR was performed for HIV-1. PRO 2000 and SPL7013 significantly (P < 0.0001) reduced the amount of bound HIV-1 to the colorectal epithelial cell line across clades A, B, C, and CRF01-AE. While none of the products reduced the binding of HIV-1 clades A and C to the urogenital cell line, CAP, PRO 2000, and SPL7013 significantly (P ≤ 0.002) reduced the binding of clades B and CRF01-AE. In general, PRO 2000 and SPL7013 placebos significantly (P < 0.0001) reduced the amount of bound HIV-1 but were less than the active products. UC781, its placebo, and hydroxyethyl cellulose (placebo for CAP) minimally affected the amount of bound HIV-1. These results suggest that rendering HIV-1 noninfectious may not correlate to the amount of HIV-1 bound to epithelial cells and possible shedding into mucosal secretions. Therefore, functional virological assays in addition to measuring viral RNA should be included when clinically evaluating topical microbicide use by infected persons.
Sexual transmission of human immunodeficiency virus type 1 (HIV-1) through mucosal surfaces is the most common mode of transmission worldwide, with women representing the majority of new infections (22). Despite evidence showing that latex condoms are effective at preventing the sexual transmission of HIV-1 (6, 13, 26, 29), their use is inconsistent. Moreover, many women lack the ability to negotiate condom use with their sexual partner (10, 15, 28). This highlights the need for products such as topical microbicides that could decrease HIV-1 transmission at mucosal sites and be used without partner consent. Although sexual transmission of HIV-1 is not fully understood, current data suggest that HIV-1 associates with but does not infect vaginal/cervical epithelial cells (8, 31). The duration of this association may impact the establishment of HIV-1 infection by providing an opportunity for the virus to interact with underlying immune cell targets. Potential anti-HIV-1 microbicides are designed to interfere with the HIV transmission cycle while maintaining the integrity of the epithelial barrier.
Several mechanisms of action exist for microbicide candidates. These strategies include (i) strengthening the innate immune defenses (e.g., antimicrobial peptides), (ii) killing or inactivating the pathogen (nonoxynol-9, cellulose acetate 1,2-benzene dicarboxylate [CAP]), (iii) inhibiting viral fusion and entry (e.g., napthphalene sulfonate polymers [PRO 2000], dendrimers [SPL7013], CAP), and (iv) inhibiting viral replication (e.g., reverse transcriptase inhibitors [UC781]). We previously have shown that microbicide candidates CAP, PRO 2000, SPL7013, and UC781 inhibit infectious virus transfer from epithelial cells to activated immune cells (9). However, it was unclear if the microbicides tested were inhibiting interactions between the virus and epithelial cells or rendering the virus noninfectious through other means. The purpose of this study was to investigate the association of HIV-1 with epithelial cells and determine whether the formulated microbicides affect the adherence of HIV-1 associated with these epithelial cells.
MATERIALS AND METHODS
Cells.
Epithelial cell lines were obtained from the American Type Culture Collection (Manassas, VA). Unless otherwise indicated, all tissue culture reagents were obtained from Invitrogen Corp., Carlsbad, CA. The urogenital cell line (A431) (12) and the colorectal cell line (Caco-2) (11) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.1 mM sodium bicarbonate. Cells were maintained at 37°C, 7% CO2. Normal human peripheral blood mononuclear cells (PBMCs) were obtained by leukophoresis from HIV-1-negative blood donors, purified by differential centrifugation, and stored in the gas phase of liquid nitrogen until needed. PBMCs were depleted of CD8 T cells by using anti-CD8-conjugated magnetic beads (Dynal, Lake Success, NY) according to the manufacturer's instructions. The CD8-depleted PBMCs were stimulated for 3 days at 37°C in 7% CO2 in complete RPMI 1640 medium (cRPMI with 10% fetal bovine serum, 100 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin) supplemented with 10% interleukin-2 and 0.5 μg/ml of phytohemagglutinin-P which showed optimal proliferation as measured by [3H]thymidine. After 3 days, the PBMCs were washed and suspended in cRPMI with 10% interleukin-2 (Roche Diagnostics, Indianapolis, IN).
Topical microbicides.
All microbicides tested were in formulations designed for human use (Table 1). Micronized CAP was provided in the formulation of Aquateric (containing approximately 13% CAP; FMC Corp., Philadelphia, PA) by Lindsey F. Kimball Research Institute (NewYork, NY) and Dow Pharmaceutical Sciences (Petaluma, CA). Hydroxyethyl cellulose was provided by the manufacturer and used as a placebo for CAP. PRO 2000 (4% formulation), a formulated naphthalene sulfonate polymer, and its placebo were provided by Indevus Pharmaceuticals, Inc., (Lexington, MA). SPL7013, a polylysine dendrimer formulated into a 5% gel, and its placebo were provided by Starpharma Pty. Ltd., Melbourne, Australia. UC781 (0.1% formulation), a formulated reverse transcriptase inhibitor, and its placebo were provided by Biosyn, Inc., Huntington Valley, PA. Each microbicide and its placebo were previously tested for toxicity against epithelial cell lines (9). Nontoxic dilutions in this study were created by diluting the formulated product in culture medium and were as follows: CAP, 1:50; HEC, 1:10; PRO 2000 and placebo, 1:25; SPL7013 and placebo, 1:25; UC781 and placebo, 1:10.
TABLE 1.
Topical microbicide products and placebos tested
| Product (reference) | Constituent | Placebo | Placebo constituent |
|---|---|---|---|
| CAP (21) | Pharmaceutical excipient; 13% polyanionic polymer with antimicrobial activity | Hydroxyethyl cellulose | 3.25% aqueous gel |
| PRO 2000 (24) | Naphthalene sulfonate polymer (4%) with antimicrobial activity | Cross-linked polyacrylic acid polymer-based aqueous gel | 1% gelling agent with pH buffering capacity |
| SPL7013 (5) | Lysine dendrimer (5%) in which the surface has been derivatized with sodium naphthalene disulfonate groups with antimicrobial activity | Cross-linked polyacrylic acid polymer-based aqueous gel | 5% gelling agent with pH buffering capacity |
| UC781 (3) | Tight binding (0.1%) nonnucleoside reverse transcriptase inhibitor; anti-HIV-1 activity only | Cross-linked polyacrylic acid polymer-based aqueous gel | 1% gelling agent with pH buffering capacity |
HIV stocks.
Laboratory-adapted subtype B, HIV-1BaL, was obtained from Advanced Biotechnologies, Inc.(Columbia, MD), and HIV-1LAI was obtained from the Centers for Disease Control and Prevention (Atlanta, GA) stocks. All primary isolates were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Health. (Bethesda, MD). The primary isolates utilized in this study include clade A (CCR5, 97USSN54 and CXCR4, 96USSN20), clade B (CRCX4, 93US143), clade C (CCR5, 96USNG58 and CXCR4, 96USNG31), and clade CRF01-AE (CCR5, 93TH078 and CXCR4, CMU08). The RNA copies of each viral stock were determined by the Amplicor HIV-1 monitor test, version 1.5 (Roche Molecular Systems, Alameda, CA).
HIV-1 transfer from epithelial cells to PBMCs.
The urogenital (A431) epithelial cell line was plated at 5 × 105 cells per well of 12-well plates and cultured overnight. Anti-HIV-1 efficacy was tested by adding a diluted concentration of the product while the cells were exposed to virus. The diluted concentrations of each microbicide and placebo were previously determined to be nontoxic to the epithelial cells and PBMCs (9). HIV-1BaL (100 TCID50/well) was mixed with the diluted microbicide or placebo and immediately added to the epithelial cultures in duplicate for 18 h. The cultures were washed three times with phosphate-buffered saline before 2.5 × 106 activated PBMCs were added. Half-volume medium changes were done every 3 to 4 days, and the supernatant was stored at −80°C to test for HIV-1 infection by using an HIV-1 p24gag protein enzyme-linked immunosorbent assay (Coulter Immunology, Hialeah, FL).
Determination of HIV-1 RNA bound to epithelial cells.
Urogenital (A431) and colorectal (Caco-2) epithelial cell lines were plated separately at 2.5 × 105 cells/well in 24-well plates and cultured overnight at 37°C, 7% CO2. To evaluate how much HIV-1 was bound to the epithelial cells, RNA equivalents of an HIV-1 isolate (100, 10, or 1) were added to the appropriate wells (virus controls). To test the effect that the formulated microbicides have on HIV-1 binding, nontoxic concentrations of formulated products were mixed with 100 RNA equivalents of virus (REV) and immediately added to the appropriate wells. Each treatment or virus control was performed in duplicate and cultured overnight at 37°C, 7% CO2.
RNA extraction.
All wells were washed three times with phosphate-buffered saline. The cell monolayer was observed for intactness before proceeding. Cells were lysed using RNeasy lysis buffer (QIAGEN, Valencia, CA), homogenized with the QIAGEN QIAshredder, and extracted using the RNeasy protocol with optional DNase digestion. RNeasy columns were washed with two 25-μl volumes of RNase-free H2O to yield a total volume of 50 μl RNA per sample.
Quantitative real-time PCR.
The amount of virus bound to epithelial cells was determined using an in-house real-time PCR (17). Forward and reverse primers were designed from a conserved HIV-1 sequence in the long terminal repeat (LTR) region. Each reaction contained 12.5 μl of master mix composed of 1 μl (each) of forward primer (0.2 μM), reverse primer (0.4 μM), and HIV probe (0.2 μM) mix, 1 μl of deoxynucleoside triphosphate mix, 1 μl of enzyme mix, 5 μl of 5× buffer (QIAGEN Onestep PCR kit), 0.2 μl of RNase inhibitor (Roche Diagnostics), and 1.5 μl of a 1:100 dilution of 100 μM Rox Standard II (Standard II; Synthegen, Houston, TX). Extracted DNase-digested RNA (12.5 μl) was added to each reaction mixture. Each sample was run in triplicate in a 96-well format using the ABI-prism 7000 sequence detection system.
Statistical analysis.
To measure the effect that cell type, viral subtype, and microbicide have on virus bound to epithelial cells, a mixed-effects model was used. Cell type, viral subtype, and microbicide were fixed effects, and the repeated measures on the wells and different experiments (or batches) were the random effects. Bonferroni adjustments were made to significance levels for multiple comparisons. All modeling was done using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
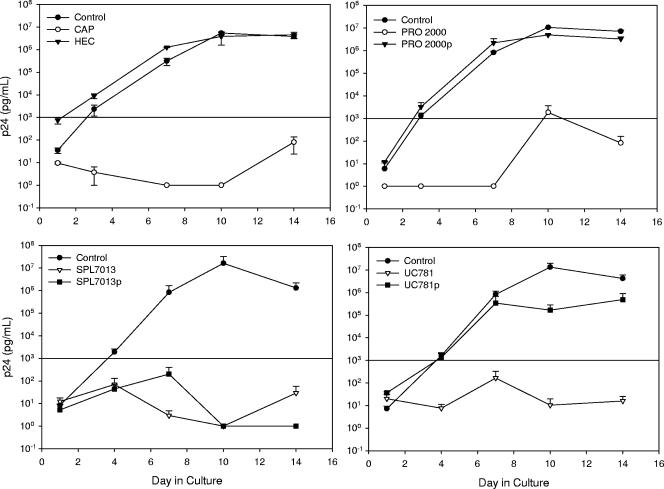
CAP, PRO 2000, SPL7013, and UC781 were previously shown to prevent the transfer of infectious HIV-1 from urogenital (HEC-1-A [uterine carcinoma] and ME-180 [cervical carcinoma]) and colorectal (Caco-2 and SW873) epithelial cells to PBMCs (9). Those findings have been extended using the A431 epithelial cell line, which has characteristics similar to vaginal epithelial cells. To determine the extent of microbicide effectiveness at reducing HIV-1 transfer, day 10 was analyzed because this is the day on which virus replication began to plateau in the control wells. On day 10, CAP, PRO 2000, SPL7013, and UC781 reduced infectious HIV-1BaL transfer by 6.1, 3.8, 7.2, and 6.1 log10, respectively (Fig. 1). HEC and PRO 2000 placebo were similar to the control and did not affect the transfer of infectious HIV-1BaL. UC781 placebo modestly reduced the transfer of HIV-1BaL by 1.9 log10, while SPL7013 placebo reduced the transfer of HIV-1BaL by 7.2 log10, similar to the SPL7013 gel. These data were consistent with our previous work showing that the formulated microbicides blocked transfer of infectious virus and the placebos, SPL7013 and UC781, but not PRO 2000, had modest reductions of infectious virus transfer (9). While these topical microbicides reduce the transfer of infectious HIV-1 to activated immune cells, it is unclear how these products affect this transfer, i.e., whether they inactivate the virus with or without affecting viral binding to the epithelial cells.
FIG. 1.
Ability of microbicide product and placebo formulations to block clade B HIV-1BaL transfer from A431 epithelial cell lines to activated PBMCs. Epithelial cell lines were cultured overnight with virus and nontoxic epithelial concentrations of product or placebo. The cells were washed, and activated PBMCs were added. Virus infection of the PBMCs was monitored using the HIV-1 p24gag enzyme-linked immunosorbent assay. The data presented are the averages ± standard errors of the means from at least two independent experiments. Cultures that were positive for HIV infection had HIV-1 p24 levels of >1 ng/ml, as indicated by the horizontal line. Product dilutions were 1:50 for CAP, 1:10 for HEC, 1:25 for PRO 2000 and placebo, 1:25 for SPL7013 and placebo, and 1:10 for UC781 and placebo (9). The formulated microbicide is listed along with its placebo designated by the small “p.”
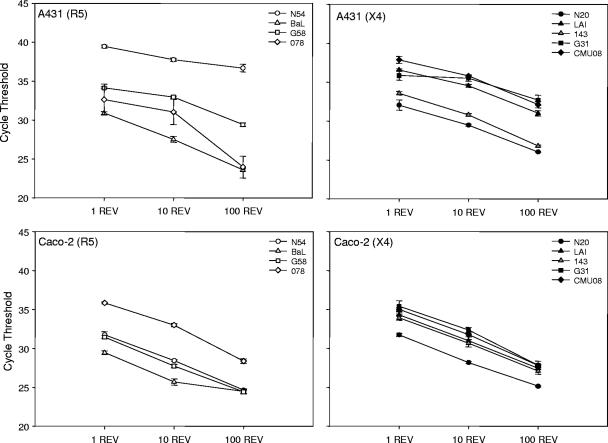
Since little is known about the association between HIV-1 and epithelial cells, differences in binding based on HIV-1 clade and coreceptor usage was investigated. A431 or Caco-2 epithelial monolayers were cultured with 100, 10, or 1 REV from representative primary HIV-1 isolates from clades A, B, C, and CRF01-AE as well as laboratory adapted isolates, HIV-1BaL and HIV-1LAI, and the relative amount of HIV-1 bound was determined using quantitative real-time PCR. Although the HIV-1 isolates tested showed evidence that the amount of epithelial-associated viral RNA varied, as represented by the cycle threshold, these differences did not group by viral clade (Fig. 2). Overall, less HIV-1 was bound to the A431 cell line than the Caco-2 cell line, as shown by the higher cycle threshold values (P ≤ 0.004). Further analysis showed no significant differences in HIV-1 clade binding to the A431 cell line. Clades B, C, and CRF01-AE bound to similar levels to the Caco-2 cell line, but CRF01-AE bound less than clade A (P = 0.001). When grouped by coreceptor usage, CXCR4-using isolates did not bind as well as CCR5-using isolates to the Caco-2 cell line (P < 0.05), but there was no significant difference for the A431 cell line.
FIG. 2.
Ability of HIV-1 viral isolates to bind to epithelial cell lines. Monolayers of 2.5 × 105 Caco-2 or A431 cells were incubated with 1, 10, and 100 REV of an HIV-1 isolate. Cells were washed and lysed, and RNA was extracted. Real-time PCR was used to amplify RNA from a conserved HIV-1 LTR region, and the average cycle threshold ± standard error of the mean is graphed. These data represent results from at least two independent experiments performed in triplicate. All P values were determined with respect to a mixed-effects model of virus bound to epithelial cells. Binding of HIV-1 isolates to the A431 cell line are presented in the upper panels. HIV-1 isolates binding to the Caco-2 cell line are presented in the lower panels. Clade A (N20, N54); clade B (BaL, LAI, 143); clade C (G31, G58); CRF01-AE (078, CMU08). R5 (CCR5) and X4 (CXCR4) represent coreceptor usage by the virus.
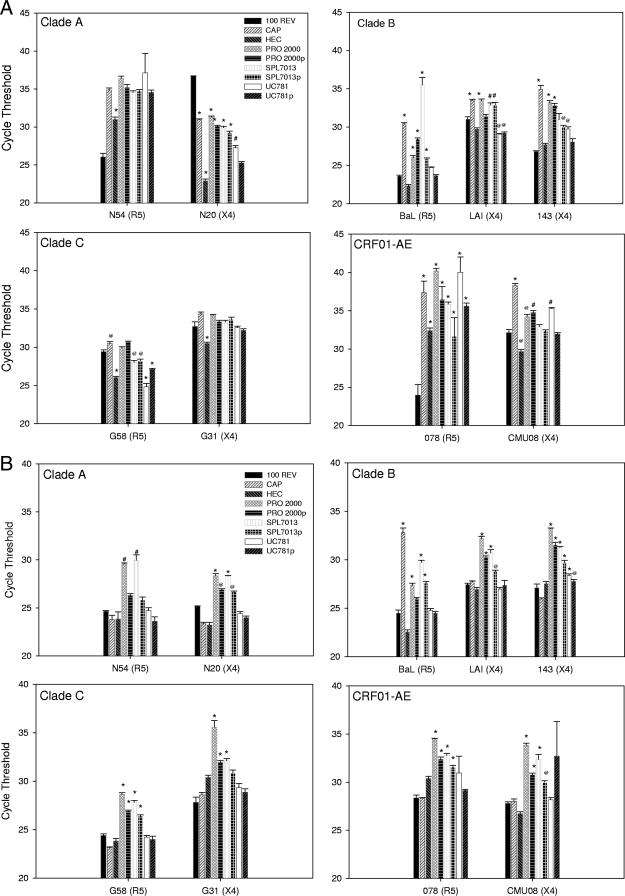
Next, the topical microbicide products were tested to determine whether or not they affect the binding of HIV-1 to the epithelial cells. When nontoxic dilutions of formulated products or their placebos were incubated with HIV-1BaL or HIV-1LAI, CAP, PRO 2000, and SPL7013 significantly inhibited the association of both viruses with the A431 cell line compared to the untreated 100-REV control (P ≤ 0.002) (Fig. 3A). Interestingly, the placebos for PRO 2000 and SPL7013 inhibited binding of HIV-1BaL (P ≤ 0.001) and SPL7013 placebo also inhibited HIV-1LAI (P ≤ 0.001). UC781 and its placebo as well as the placebo for CAP (HEC) did not reduce the binding of the laboratory-adapted HIV-1 to the A431 cell line. Using the Caco-2 cell line, similar results were obtained, with the exception of CAP not affecting HIV-1LAI binding (Fig. 3B).
FIG. 3.
Effect of formulated microbicide products on HIV-1 binding on the A431 (A) and Caco-2 (B) cell lines. Monolayers of 2.5 × 105 Caco-2 or A431 cells were incubated with 100 REV of an HIV-1 isolate in the absence or presence of the diluted formulated microbicide or placebo. Cells were washed and lysed, and RNA was extracted. Real-time PCR was used to amplify RNA from a conserved HIV-1 LTR region, and the average cycle threshold ± standard error of the mean is graphed. These data represent results from at least two independent experiments performed in triplicate. All P values were determined with respect to a mixed-effects model of virus bound to epithelial cells. @, P ≤ 0.01; #, P ≤ 0.001; *, P ≤ 0.0001 (relative to the 100-REV control [no microbicide or placebo]). The formulated microbicide is listed along with its placebo designated by the small “p.”
HIV-1BaL and HIV-1LAI are clade B laboratory-adapted viruses that utilize CCR5 or CXCR4 coreceptor, respectively, to initiate infection. Although laboratory-adapted isolates did not appear to bind differently than primary isolates, it may be possible that microbicides differentially affect the binding of primary HIV-1 isolates to epithelial cells. Primary HIV-1 isolates that represent several different HIV-1 clades were included to evaluate viral adherence to epithelial cells (Fig. 3A and B). While CAP significantly (P ≤ 0.01) reduced the binding of 5 of 7 primary HIV-1 isolates to the A431 cell line, it had no effect on the binding of the primary isolates to the Caco-2 cell line. The placebo HEC had minimal effect on the reduction of HIV-1 binding to either cell line. Compared to HEC, CAP significantly (P ≤ 0.01) inhibited more virus binding to the A431 cell line but not to the Caco-2 cell line. PRO 2000 significantly (P ≤ 0.01) reduced the binding of 4 of 7 primary HIV-1 isolates to the A431 cell line and of 7 of 7 primary HIV-1 isolates to the Caco-2 cell line. The PRO 2000 placebo had similar results to PRO 2000. Compared to PRO 2000 placebo, PRO 2000 significantly (P ≤ 0.01) inhibited more virus binding to the Caco-2 cell line but not to the A431 cell line. SPL7013 and SPL7013 placebo behaved in a manner similar to that of PRO 2000 and PRO 2000 placebo for both cell lines. UC781 significantly (P ≤ 0.01) reduced the binding of 4 of 7 primary HIV-1 isolates to the A431 cell line and of 1 of 7 primary HIV-1 isolates to the Caco-2 cell line. The UC781 placebo reduced the binding of 2 of 7 primary HIV-1 isolates to the A431 cell line and 1 of 7 primary isolates to the Caco-2 cell line. As a result, there was no statistical difference between UC781 and UC781 placebo in the binding of HIV-1 to the epithelial cell lines.
DISCUSSION
HIV-1 has the ability to bind to epithelial cells, remain infectious, and be transferred to activated immune cells (8, 31). HIV-1 binding to epithelial cells is believed to occur through a nonspecific interaction with heparan sulfate proteoglycans (19). Topical microbicide products may or may not affect the interaction between HIV-1 and the epithelial cell depending on their mode of action. The data presented here show that, overall, there was no quantitative difference between HIV-1 clades binding to A431 and Caco-2 epithelial cell lines, with the exception of CRF01-AE binding less than clade A to the Caco-2 cell line. When stratified based on coreceptor usage, CCR5-using HIV-1 bound better to the epithelial cells. The envelope of CCR5-using viruses is less basic than CXCR4-using viruses (14) and should bind less well to heparan sulfate proteoglycans. It is unknown why the CCR5-using HIV-1 bound better to the epithelial cell lines but suggests that additional factors/receptors, such as galactosyl ceramide, may play a role in virus binding (2).
Inhibiting infectious virus transfer from epithelial cells to PBMCs can be the result of several different mechanisms. Determining the extent of HIV-1 binding to epithelial cells in the presence of candidate microbicides can provide information on whether the microbicide is rendering the virus noninfectious by limiting its access to epithelial binding sites and/or by disrupting the viral transmission cycle once the virus has adhered to the epithelial layer. PRO 2000 inhibits infectious virus transfer by binding to gp120 (24, 25). PRO 2000 was shown to bind the gp120 of CCR5- and CXCR4-using viruses equally well (25). The large structure of the microbicide inhibits virus/cell interactions, effectively producing a matrix which “traps” HIV-1 so access to the cell is limited. The activity for SPL7013, an acidic lysine dendrimer, is similar. The negative charge attracts and hinders virus from accessing target cells (30). Both PRO 2000 and SPL7013 reduced the binding of HIV-1 to the epithelial cells. Similarly, CAP binds to the gp120 and inhibits the reactivity to CCR5 and CXCR4, thus acting as a fusion inhibitor (20, 21). CAP, which has been shown to reduce infectious virus transfer from several epithelial cell lines to activated PBMCs (9), surprisingly only inhibited binding of HIV-1 to the A431 cell line. UC781, which directly targets HIV-1 by inhibiting the reverse transcriptase, was shown not to affect the association of HIV-1IIIB (a laboratory-adapted virus) with an epithelial cell layer (4). This is in agreement with our data showing a minimal effect on HIV-1BaL and HIV-1LAI binding to the epithelial cell lines but preventing HIV-1 replication (9). It was unexpected that UC781 inhibited binding of 4 of 7 primary isolates to A431 cells and 1 of 7 primary isolates to Caco-2 cells. The formulated product may contain ingredients that affect virus binding, as the UC781 placebo affected HIV-1 binding to a similar level.
The differences noted in binding of CCR5-using and CXCR4-using HIV-1 to the two epithelial cell lines and the effects that the products have on this binding are difficult to interpret. Many factors may be involved. The epithelial cells lack the CD4 receptor; however, they do express galactosyl ceramide as noted above that would aid in virus-cell adhesion. Further, differential levels of receptors that nonspecifically help in virus adhesion, such as heparan sulfate proteoglycans, would influence virus binding. How these molecules behave in the presence of these formulated topical microbicides has yet to be determined. It has been suggested that epithelial cells could be one of the “gatekeepers” that restricts HIV-1 entry (18). The epithelial cells lining the genital tract are different from those lining the gastrointestinal tract in terms of function, cell receptor expression, and structure. Clearly, additional cells and cell lines need to be evaluated to determine if these differences in virus binding and the effects of the products could be attributed to the genital and gastrointestinal tracts.
HEC has been proposed to be the “universal” placebo for clinical trials (27). The reasons for this are its stability, safety, and lack of any anti-HIV-1 activity in vitro and in vivo. The results presented here and elsewhere (9) show that HEC does not reduce HIV-1 binding to epithelial cells or the transfer of infectious HIV-1 from epithelial cells to immune cells. The placebos for PRO 2000 and SPL7013 did reduce the binding of HIV-1 to the epithelial cells. However, the PRO 2000 placebo did not reduce the transfer of infectious HIV-1, while the SPL7013 placebo and, to a lesser extent, the UC781 placebo did, as was shown here and elsewhere (9). These placebos contain cross-linked polyacrylic acid polymer-based aqueous gel (Carbopol) which has anti-HIV-1 activity in vitro (1, 9, 23). The overall ability of these placebos (formulations) to affect the binding of HIV-1 to the epithelial cells and the transfer of infectious virus may be the result of proprietary ingredients added to the formulations. The data suggest that a reduction in binding of HIV-1 to the epithelial cells does not equate to a reduction of infectious virus; an active ingredient is needed to prevent transfer of infectious HIV-1. It should be noted that, overall, less HIV-1 was bound to the epithelial cells when the active ingredient was present.
Topical microbicides will be used by HIV-1-infected women either because they are unaware of their infection status or to protect their sexual partner. The work presented here suggests that an effective microbicide may not affect the viral RNA levels, as measured in cervicovaginal secretions. For instance, cervicovaginal secretions taken from an HIV-1-infected person using UC781 could contain similar amounts of viral RNA (although noninfectious) as without microbicide use. Conversely, CAP, PRO 2000, or SPL7013 could inhibit cell-free HIV-1 from maintaining an association with epithelial cells and, potentially, the immune cell targets. A reduction of viral RNA levels could be possible in cervicovaginal secretions from a person using those products. Therefore, measuring only HIV-1 RNA levels may not be enough to determine microbicide efficacy. To address this issue, functional virological assays (7, 16), in addition to measurement of HIV-1 RNA, should be used when determining the effectiveness of a microbicide in clinical trials with HIV-1-infected participants.
Acknowledgments
The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Abner, S. R., P. C. Guenthner, J. Guarner, K. A. Hancock, J. E. Cummins, Jr., A. Fink, G. T. Gilmore, C. Staley, A. Ward, O. Ali, S. Binderow, S. Cohen, L. A. Grohskopf, L. Paxton, C. E. Hart, and C. S. Dezzutti. 2005. A human colorectal explant culture to evaluate topical microbicides for the prevention of HIV infection. J. Infect. Dis. 192:1545-1556. [DOI] [PubMed] [Google Scholar]
- 2.Bomsel, M., and A. Alfsen. 2003. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat. Rev. Mol. Cell Biol. 4:57-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borkow, G., D. Arion, M. A. Wainberg, and M. A. Parniak. 1999. The thiocarboxanilide nonnucleoside inhibitor UC781 restores antiviral activity of 3′-azido-3′-deoxythymidine (AZT) against AZT-resistant human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43:259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borkow, G., J. Barnard, T. M. Nguyen, A. Belmonte, M. A. Wainberg, and M. A. Parniak. 1997. Chemical barriers to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourne, N., L. R. Stanberry, E. R. Kern, G. Holan, B. Matthews, and D. I. Bernstein. 2000. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob. Agents Chemother. 44:2471-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1993. Update: barrier protection against HIV infection and other sexually transmitted diseases. Morb. Mortal. Wkly. Rep. 42:589-597. [PubMed] [Google Scholar]
- 7.Cummins, J. E., Jr., J. M. Villanueva, T. Evans-Strickfaden, S. M. Sesay, S. R. Abner, T. J. Bush, T. A. Green, J. L. Lennox, T. Wright, T. M. Folks, C. E. Hart, and C. S. Dezzutti. 2003. Detection of infectious human immunodeficiency virus type 1 in female genital secretions by a short-term culture method. J. Clin. Microbiol. 41:4081-4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dezzutti, C. S., P. C. Guenthner, J. E. Cummins, Jr., T. Cabrera, J. H. Marshall, A. Dillberger, and R. B. Lal. 2001. Cervical and prostate primary epithelial cells are not productively infected but sequester human immunodeficiency virus type 1. J. Infect. Dis. 183:1204-1213. [DOI] [PubMed] [Google Scholar]
- 9.Dezzutti, C. S., V. N. James, A. Ramos, S. T. Sullivan, A. Siddig, T. J. Bush, L. A. Grohskopf, L. Paxton, S. Subbarao, and C. E. Hart. 2004. In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission. Antimicrob. Agents Chemother. 48:3834-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias, C., and C. Coggins. 2001. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: where have we been? Where are we going? J. Womens Health Gend. Based Med. 10:163-173. [DOI] [PubMed] [Google Scholar]
- 11.Fogh, J., W. C. Wright, and J. D. Loveless. 1977. Absence of HeLa cell contamination in 169 cell lines derived from human tumors. J. Natl. Cancer Inst. 58:209-214. [DOI] [PubMed] [Google Scholar]
- 12.Giard, D. J., S. A. Aaronson, G. J. Todaro, P. Arnstein, J. H. Kersey, H. Dosik, and W. P. Parks. 1973. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 51:1417-1423. [DOI] [PubMed] [Google Scholar]
- 13.Holmes, K. K., R. Levine, and M. Weaver. 2004. Effectiveness of condoms in preventing sexually transmitted infections. Bull. World Health Organ. 82:454-461. [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang, S. 1997. HIV-1-co-receptors binding. Nat. Med. 3:367-368. [DOI] [PubMed] [Google Scholar]
- 15.Kalichman, S. C., E. A. Williams, C. Cherry, L. Belcher, and D. Nachimson. 1998. Sexual coercion, domestic violence, and negotiating condom use among low-income African American women. J. Womens Health. 7:371-378. [DOI] [PubMed] [Google Scholar]
- 16.Keller, M. J., B. Zerhouni-Layachi, N. Cheshenko, M. John, K. Hogarty, A. Kasowitz, C. L. Goldberg, S. Wallenstein, A. T. Profy, M. E. Klotman, and B. C. Herold. 2006. PRO 2000 gel inhibits HIV and herpes simplex virus infection following vaginal application: a double-blind placebo-controlled trial. J. Infect. Dis. 193:27-35. [DOI] [PubMed] [Google Scholar]
- 17.Luo, W., H. Yang, K. Rathbun, C. P. Pau, and C. Y. Ou. 2005. Detection of human immunodeficiency virus type 1 DNA in dried blood spots by a duplex real-time PCR assay. J. Clin. Microbiol. 43:1851-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis, L., and R. Shattock. 2006. Selective transmission of CCR5-utilizing HIV-1: the ‘gatekeeper’ problem resolved? Nat. Rev. Microbiol. 4:312-317. [DOI] [PubMed] [Google Scholar]
- 19.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neurath, A. R., N. Strick, S. Jiang, Y. Y. Li, and A. K. Debnath. 2002. Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of “dead-end” gp41 six-helix bundles. BMC Infect. Dis. 2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neurath, A. R., N. Strick, Y. Y. Li, and A. K. Debnath. 2001. Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120. BMC Infect. Dis. 1:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinn, T. C., and J. Overbaugh. 2005. HIV/AIDS in women: an expanding epidemic. Science 308:1582-1583. [DOI] [PubMed] [Google Scholar]
- 23.Rohan, L. C., D. Ratner, K. McCullough, S. L. Hiller, and P. Gupta. 2004. Measurement of anti-HIV activity of marketed vaginal products and excipients using a PBMC-based in vitro assay. Sex. Transm. Dis. 31:143-148. [DOI] [PubMed] [Google Scholar]
- 24.Rusconi, S., M. Moonis, D. P. Merrill, P. V. Pallai, E. A. Neidhardt, S. K. Singh, K. J. Willis, M. S. Osburne, A. T. Profy, J. C. Jenson, and M. S. Hirsch. 1996. Naphthalene sulfonate polymers with CD4-blocking and anti-human immunodeficiency virus type 1 activities. Antimicrob. Agents Chemother. 40:234-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scordi-Bello, I. A., A. Mosoian, C. He, Y. Chen, Y. Cheng, G. A. Jarvis, M. J. Keller, K. Hogarty, D. P. Waller, A. T. Profy, B. C. Herold, and M. E. Klotman. 2005. Candidate sulfonated and sulfated topical microbicides: comparison of anti-human immunodeficiency virus activities and mechanisms of action. Antimicrob. Agents Chemother. 49:3607-3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinding, S. W. 2005. Does ‘CNN′ (condoms, needles and negotiation) work better than ′ABC’ (abstinence, being faithful and condom use) in attacking the AIDS epidemic? Int. Fam. Plan. Perspect. 31:38-40. [DOI] [PubMed] [Google Scholar]
- 27.Tien, D., R. L. Schnaare, F. Kang, G. Cohl, T. J. McCormick, T. R. Moench, G. Doncel, K. Watson, R. W. Buckheit, M. G. Lewis, J. Schwartz, K. Douville, and J. W. Romano. 2005. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res. Hum. Retrovir. 21:845-853. [DOI] [PubMed] [Google Scholar]
- 28.van der Straten, A., R. King, O. Grinstead, A. Serufilira, and S. Allen. 1995. Couple communication, sexual coercion and HIV risk reduction in Kigali, Rwanda. AIDS 9:935-944. [DOI] [PubMed] [Google Scholar]
- 29.Weller, S., and K. Davis. 2001. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database Syst. Rev. 2001:CD003255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witvrouw, M., V. Fikkert, W. Pluymers, B. Matthews, K. Mardel, D. Schols, J. Raff, Z. Debyser, E. De Clercq, G. Holan, and C. Pannecouque. 2000. Polyanionic (i.e., polysulfonate) dendrimers can inhibit the replication of human immunodeficiency virus by interfering with both virus adsorption and later steps (reverse transcriptase/integrase) in the virus replicative cycle. Mol. Pharmacol. 58:1100-1108. [PubMed] [Google Scholar]
- 31.Wu, Z., Z. Chen, and D. M. Phillips. 2003. Human genital epithelial cells capture cell-free human immunodeficiency virus type 1 and transmit the virus to CD4+ Cells: implications for mechanisms of sexual transmission. J. Infect. Dis. 188:1473-1482. [DOI] [PubMed] [Google Scholar]