Abstract
The susceptibilities of 77 dermatophytes to miltefosine (MI), 1,12-bis(4-pentylpyridinium)dodecane (PYR), 1,12-bis(tributylammonium)dodecane (AM), itraconazole (ITC), terbinafine (TRB), and butenafine (BTF) were compared. Geometric mean MICs of TRB, BTF, ITC, MI, PYR, and AM were 0.039, 0.059, 1.718, 0.671, 6.006, and 4.771 μg/ml, respectively. MI was more active than ITC (P < 0.001).
Dermatophytoses are an important public health problem (5, 9). Despite therapy, relapse is frequent and disease persists in up to 25% of cases (5, 26). Furthermore, resistance to and clinical failures with itraconazole (ITC) and terbinafine (TRB), the two oral agents commonly used to treat chronic and severe disease, have been reported previously (11, 16).
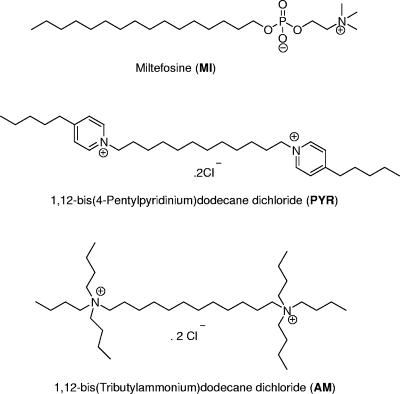
Our approach to the evaluation of new antidermatophyte compounds arose from the finding that extracellular phospholipase B produced by the fungus Cryptococcus neoformans is important for fungal survival and disease dissemination (2, 21, 23, 27). Moreover, we have demonstrated previously that novel inhibitors of cryptococcal phospholipase B exhibit broad-spectrum antifungal activity (8, 19, 31). One of these, the alkylphosphocholine miltefosine (MI), is of particular interest because it is already licensed (Impavido; Aeterna Zentaris, Frankfurt, Germany) for the treatment of visceral leishmaniasis in many countries (28, 29). Since dermatophytes also produce phospholipase(s) (3, 15), we compared the in vitro activities of MI and two biscationic salts (Fig. 1) against common dermatophytes with those of established antifungal agents by using the Clinical and Laboratory Standards Institute (CLSI) M38-A method (17) with recently determined optimal test conditions (7, 24, 25). MICs of test agents for Trichophyton rubrum as measured by using unfiltered (hyphae and microconidia) and filtered (microconidia) inocula were also compared.
FIG. 1.
Structures of three investigational antifungal compounds.
Seventy-seven isolates (nine species) were studied. These included 74 clinical strains (19 T. rubrum, 17 Trichophyton mentagrophytes, 15 Trichophyton tonsurans, 3 Trichophyton soudanense, 3 Trichophyton violaceum, 5 Epidermophyton floccosum, 5 Microsporum canis, 5 Microsporum gypseum, and 2 Microsporum cookei isolates) and 3 American Type Culture Collection (Rockville, MD) strains (T. rubrum ATCC 28188, T. mentagrophytes ATCC 28185, and T. tonsurans ATCC 28942). Isolates were stored as suspensions in sterile water at 25°C until required. Prior to testing, each isolate was subcultured onto potato dextrose agar to ensure purity and growth. Quality control strains Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included with each test run as described previously (18).
MI was obtained from Cayman Chemical Company (Ann Arbor, MI). Details of the synthesis of 1,12-bis(tributylammonium)dodecane dichloride (AM) have been published previously (19), and those of the synthesis of 1,12-bis(4-pentylpyridinium)dodecane dichloride (PYR) are presently unpublished (details are available upon request). All three compounds were prepared as 128-μg/ml stock solutions in RPMI 1640 medium (Sigma-Aldrich, St Louis, MO), providing a final concentration range of 64 to 0.125 μg/ml (17). ITC (Janssen Research Foundation, Beerse, Belgium) was dissolved in 100% dimethyl sulfoxide, and TRB (Novartis, North Ryde, Australia) and butenafine (BTF; Sequoia Research Products, Oxford, United Kingdom) were dissolved in 95% dimethyl sulfoxide-5% Tween 80 (vol/vol) (11). Stock solution concentrations of the three antifungal drugs (1,600 μg/ml) were diluted in accordance with CLSI M38-A methodology (17) to yield final concentration ranges of 16 to 0.031 μg/ml for ITC and 2 to 0.0039 μg/ml for TRB and BTF.
Stock inoculum suspensions were prepared from 7- to 10-day-old cultures grown on potato dextrose agar slants at 28°C. The suspensions were adjusted to an optical density ranging from 0.9 to 1.1 McFarland (70 to 72% transmittance) and then diluted 1:50 in RPMI 1640 medium to obtain a final inoculum concentration of 0.3 × 104 to 6.4 × 104 CFU/ml (17). For T. rubrum isolates, a suspension consisting only of microconidia (final cell number, 2.5 × 104 to 5 × 104 CFU/ml as verified with a hemacytometer) was additionally prepared (25). Broth microdilution tests were prepared in accordance with the CLSI M38-A protocol (17). Plates were incubated at 28°C and read visually after 4 and 7 days of incubation. Each isolate was tested in duplicate in two independent experiments. For ITC, the MIC was defined as the lowest concentration showing 80% inhibition. For the other agents, the MIC was the lowest concentration showing 100% growth inhibition (concentrations producing 80 and 100% growth inhibition were identical). Geometric mean (GM) MICs for the different genera were compared by using the Kruskal-Wallis test or the Mann-Whitney U test with SPPS version 14.0 software (SPPS Inc., Chicago, IL). P values of <0.05 were statistically significant.
MICs were determined at 7 days since the growth of some Trichophyton strains was insufficient at 4 days; all isolates produced good growth after 6 to 7 days. ITC MICs for quality control Candida strains were within previously published limits (18). Table 1 summarizes the susceptibility data for the 77 isolates. MI demonstrated good antidermatophyte activity, with a significantly lower GM MIC than ITC (0.671 versus 1.718 μg/ml; P < 0.001); MICs of MI at which 50% and 90% of the isolates were inhibited were fourfold lower than those of ITC (Table 1). Also notable was the narrow range of MI MICs (0.25 to 2.0 μg/ml). ITC MICs for 15 strains (19.5%) were ≥4 μg/ml. PYR and AM were 100- to 200-fold less potent than the most active agents, TRB and BTF.
TABLE 1.
In vitro activity of three investigational compounds in comparison with three established antifungal agents against nine dermatophyte species
| Species (no. of isolates tested) | Compound | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| Range | GM | 50%a | 90%b | ||
| T. rubrum (20) | ITC | 0.25-4 | 1.955 | 2 | 4 |
| TRB | 0.008-0.063 | 0.035 | 0.031 | 0.063 | |
| BTF | 0.008-0.125 | 0.050 | 0.063 | 0.125 | |
| MI | 0.25-2 | 0.547 | 0.5 | 1 | |
| PYR | 4-16 | 10.41 | 8 | 16 | |
| AM | 2-8 | 5.876 | 4 | 8 | |
| T. mentagrophytes (18) | ITC | 0.125-4 | 1.682 | 2 | 4 |
| TRB | 0.008-0.5 | 0.030 | 0.031 | 0.063 | |
| BTF | 0.008-1 | 0.063 | 0.063 | 0.125 | |
| MI | 0.25-2 | 1.133 | 1 | 2 | |
| PYR | 2-32 | 9.007 | 8 | 16 | |
| AM | 2-8 | 4.609 | 4 | 8 | |
| T. tonsurans (16) | ITC | 0.25-4 | 1.645 | 2 | 4 |
| TRB | 0.008-0.063 | 0.030 | 0.031 | 0.063 | |
| BTF | 0.008-0.063 | 0.040 | 0.031 | 0.063 | |
| MI | 0.25-1 | 0.398 | 0.25 | 1 | |
| PYR | 1-8 | 2.802 | 2 | 8 | |
| AM | 2-8 | 3.366 | 4 | 4 | |
| T. soudanense (3) | ITC | 1-4 | 3.420 | ||
| TRB | 0.031-0.063 | 0.039 | |||
| BTF | 0.031-0.125 | 0.059 | |||
| MI | 0.25-1 | 0.52 | |||
| PYR | 8-16 | 14.54 | |||
| AM | 4-8 | 6.604 | |||
| T. violaceum (3) | ITC | 2-8 | 3.302 | ||
| TRB | 0.063 | 0.063 | |||
| BTF | 0.063 | 0.063 | |||
| MI | 0.25-0.5 | 0.328 | |||
| PYR | 1-32 | 8.963 | |||
| AM | 2-4 | 2.885 | |||
| All Trichophyton spp. (60) | ITC | 0.125-8 | 1.993 | 2 | 4 |
| TRB | 0.008-0.5 | 0.044 | 0.031 | 0.063 | |
| BTF | 0.008-1 | 0.074 | 0.063 | 0.125 | |
| MI | 0.25-2 | 0.652 | 0.5 | 1 | |
| PYR | 1-32 | 6.981 | 8 | 16 | |
| AM | 2-8 | 4.467 | 4 | 8 | |
| E. floccosum (5) | ITC | 0.063-0.5 | 0.226 | ||
| TRB | 0.031-0.125 | 0.059 | |||
| BTF | 0.063-0.125 | 0.081 | |||
| MI | 0.25-1 | 0.548 | |||
| PYR | 2-16 | 7.635 | |||
| AM | 4-8 | 6.0 | |||
| M. canis (5) | ITC | 0.25-8 | 1.230 | ||
| TRB | 0.031-0.125 | 0.083 | |||
| BTF | 0.063-0.25 | 0.101 | |||
| MI | 0.25-2 | 0.555 | |||
| PYR | 1-4 | 2.954 | |||
| AM | 4 | 4 | |||
| M. gypseum (5) | ITC | 1-8 | 3.680 | ||
| TRB | 0.031-0.125 | 0.065 | |||
| BTF | 0.063-0.25 | 0.084 | |||
| MI | 0.5-1 | 0.842 | |||
| PYR | 1-4 | 2.408 | |||
| AM | 4-8 | 6 | |||
| M. cookei (2) | ITC | 4-8 | 6 | ||
| TRB | 0.063-0.125 | 0.077 | |||
| BTF | 0.125-0.25 | 0.153 | |||
| MI | 0.5-1 | 0.866 | |||
| PYR | 0.5-2 | 1.061 | |||
| AM | 4-16 | 8.485 | |||
| All Microsporum spp. (12) | ITC | 0.25-8 | 2.633 | 4 | 8 |
| TRB | 0.031-0.125 | 0.075 | 0.063 | 0.125 | |
| BTF | 0.063-0.25 | 0.106 | 0.125 | 0.125 | |
| MI | 0.25-2 | 0.750 | 1 | 1 | |
| PYR | 0.5-4 | 2.244 | 2 | 4 | |
| AM | 4-16 | 5.246 | 4 | 8 | |
| All isolates (77) | ITC | 0.063-8 | 1.718 | 2 | 4 |
| TRB | 0.008-0.5 | 0.039 | 0.031 | 0.063 | |
| BTF | 0.008-1 | 0.059 | 0.063 | 0.125 | |
| MI | 0.25-2 | 0.617 | 0.5 | 1 | |
| PYR | 0.5-32 | 6.006 | 8 | 16 | |
| AM | 2-16 | 4.771 | 4 | 8 | |
MIC at which 50% of the isolates were inhibited.
MIC at which 90% of the isolates were inhibited.
GM MICs for species of different genera varied significantly except for those of MI and AM (P < 0.001; data not shown). This variation was most evident with ITC, which was most active against E. floccosum (GM MIC for E. floccosum, 0.226 μg/ml, versus 1.993 μg/ml for Trichophyton spp. [P = 0.002] and 2.633 μg/ml for Microsporum spp. [P = 0.002]) (Table 1). Unlike those of ITC, MI MICs demonstrated little inter- or intraspecies variation (Table 1). Drug MICs for T. rubrum were within one dilution when this organism was tested by using filtered and unfiltered inocula; inoculum sizes were similar (data not shown).
Although TRB and BTF remain the most active drugs (this study; 7, 13, 24), cost is often a factor in dictating prescription choice. Furthermore, BTF is available only as a topical formulation and is unsuitable for use in treating onychomycosis.
This study provides the first indication that MI has good activity against clinically important dermatophytes. Indeed, its MIC range (Table 1) was comparable to those of the azoles (7, 13). Not only was the overall GM MIC of MI significantly lower than that of ITC (P > 0.001), but the MICs of MI at which 90% of the Trichophyton and Microsporum spp. strains were inhibited were also lower. Given that mean ITC concentrations in plasma range from 0.8 to 1.5 μg/ml (4), it is possible that the relatively high ITC MICs (≥4 μg/ml) noted for nearly 20% of the isolates may be associated with clinical failures and/or relapses. Conversely, serum MI concentrations achieved in rat models of infection (44.8 μg/ml) (14, 30) and patients with leishmaniasis (median, >20 μg/ml) (1) significantly exceed the observed MIC of MI at which 90% of the isolates tested were inhibited (1 μg/ml). We also noted, in contrast to findings of previous studies (7, 13), that ITC MICs for species of different genera varied. The reasons for these differences are unknown. Further study is required to determine if these differences are sufficient to caution against prescribing ITC for the treatment of infections with certain Microsporum spp. (Table 1). AM and PYR showed less promise as antidermatophyte agents, having higher MICs than MI. However, as MICs of both compounds for Candida, Cryptococcus, and other molds range from 0.6 to 13.4 μg/ml (our unpublished results), we are continuing to evaluate these simple salts as potential topical antifungal agents.
The ideal incubation temperature and time for susceptibility testing of dermatophytes remain incompletely resolved. Although others have proposed higher incubation temperatures (35 or 37°C) (10, 20, 22), we conducted susceptibility testing at 28°C. Adequate growth with clear MIC end points was observed in all instances. Regarding incubation times, 4 days was insufficient for adequate growth for some Trichophyton strains, in contrast to previous reports (6, 10, 12); 7 days was necessary to obtain reproducible MICs. In one study, the separation of hyphae from conidial structures influenced MICs (25). Our results do not support this observation for T. rubrum.
In conclusion, MI is as active as ITC against common dermatophytes and has a broader spectrum. The results of our study suggest that MI and related alkylphosphocholines could be exploited for the development of novel antifungal drugs.
Acknowledgments
We thank Heather Gidding for expert assistance with statistical analysis and Clarissa Ng and Daniel Obando for the synthesis of AM and PYR, respectively.
This work was supported by the National Health and Medical Research Council of Australia grant number 211040 and a Stream 1 infrastructure grant from New South Wales Health.
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Berman, J. 2005. Miltefosine to treat leishmaniasis. Expert Opin. Pharmacother. 6:1381-1388. [DOI] [PubMed] [Google Scholar]
- 2.Chen, S. C., M. Muller, J. Z. Zhou, L. C. Wright, and T. C. Sorrell. 1997. Phospholipase activity in Cryptococcus neoformans: a new virulence factor? J. Infect. Dis. 175:414-420. [DOI] [PubMed] [Google Scholar]
- 3.Das, S. K., and A. B. Banerjee. 1977. Lipolytic enzymes of Trichophyton rubrum. Sabouraudia 15:313-323. [PubMed] [Google Scholar]
- 4.Debruyne, D., and A. Coquerel. 2001. Pharmacokinetics of antifungal agents in onychomycoses. Clin. Pharmacol. 40:441-472. [DOI] [PubMed] [Google Scholar]
- 5.Elewski, B. E., and M. A. Charif. 1997. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch. Dermatol. 133:1172-1173. [PubMed] [Google Scholar]
- 6.Favre, B., B. Hofbauer, K-S. Hildering, and N. S. Ryder. 2003. Comparison of in vitro activities of 17 antifungal drugs against a panel of 20 dermatophytes by using a microdilution assay. J. Clin. Microbiol. 41:4817-4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Torres, B., F. J. Cabanes, A. J. Carrillo-Munoz, A. Esteban, I. Inza, L. Abarca, and J. Guarro. 2002. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J. Clin. Microbiol. 40:3999-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganendren, R., F. Widmer, V. Singhal, C. Wilson, T. Sorrell, and L. Wright. 2004. In vitro antifungal activities of inhibitors of phospholipases from the fungal pathogen Cryptococcus neoformans. Antimicrob. Agents Chemother. 48:1561-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum, M., N. Isham, R. Hajjeh, M. Cano, F. Al-Hasawi, D. Yearick, J. Warner, L. Long, C. Jessup, and B. Elewski. 2003. Tinea capitis in Cleveland: survey of elementary school students. J. Am. Acad. Dermatol. 48:189-193. [DOI] [PubMed] [Google Scholar]
- 10.Ghannoum, M. A., V. Chaturverdi, A. Espinel-Ingroff, M. A. Pfaller, M. G. Rinaldi, W. Lee-Yang, and D. W. Warnock. 2004. Intra- and interlaboratory study of a method for testing antifungal susceptibilities of dermatophytes. J. Clin. Microbiol. 42:2977-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, A. K., and K. Yatika. 2003. Evaluation of in vitro resistance in patients with onychomycosis who fail antifungal therapy. Dermatology 207:375-380. [DOI] [PubMed] [Google Scholar]
- 12.Jessup, C. J., J. Warner, N. Isham, I. Hasan, and M. A. Ghannoum. 2000. Antifungal susceptibility testing of dermatophytes: establishing a medium for inducing conidial growth and evaluation of susceptibility of clinical isolates. J. Clin. Microbiol. 38:341-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karaca, N., and A. Nedret Koc. 2004. In vitro susceptibility testing of dermatophytes: comparison of disk diffusion and reference broth dilution methods. Diagn. Microbiol. Infect. Dis. 48:259-264. [DOI] [PubMed] [Google Scholar]
- 14.Marschner, N., J. Kotting, H. Eibl, and C. Unger. 1992. Distribution of hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine in rat tissues during steady-state treatment. Cancer Chemother. Pharmacol. 31:18-22. [DOI] [PubMed] [Google Scholar]
- 15.Muhsin, T. M., A. H. Aubaid, and A. H. Al-Duboon. 1997. Extracellular enzyme activities of dermatophytes and yeast isolates on solid media. Mycoses 40:465-469. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee, P. K., S. D. Leidich, N. Isham, L. Leitner, N. S. Ryder, and M. A. Ghannoum. 2003. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob. Agents Chemother. 47:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 18.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts, 2nd ed. Approved standard M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 19.Ng, C. K., D. Obando, F. Widmer, L. C. Wright, T. C. Sorrell, and K. A. Jolliffe. 2006. Correlation of antifungal activity with fungal phospholipase inhibition using a series of bisquaternary ammonium salts. J. Med. Chem. 49:811-816. [DOI] [PubMed] [Google Scholar]
- 20.Norris, H. A., B. E. Elewski, and M. A. Ghannoum. 1999. Optimal growth conditions for the determination of the antifungal susceptibility of three species of dermatophytes with the use of a microdilution method. J. Am. Acad. Dermatol. 40:S9-S13. [DOI] [PubMed] [Google Scholar]
- 21.Noverr, M. C., G. M. Cox, J. R. Perfect, and G. B. Huffnagle. 2003. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71:1538-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perea, S. A., W. Fothergill, D. A. Sutton, and M. G. Rinaldi. 2001. Comparison of in vitro activities of voriconazole and five established antifungal agents against different species of dermatophytes using a broth macrodilution method. J. Clin. Microbiol. 39:385-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santangelo, R. T., H. Zoellner, T. C. Sorrell, C. Wilson, C. Donald, J. Djordjevic, Y. Shounan, and L. Wright. 2004. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72:2229-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos, D. A., and J. S. Hamdam. 2005. Evaluation of broth microdilution antifungal susceptibility testing conditions for Trichophyton rubrum. J. Clin. Microbiol. 43:1917-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santos, D. A., M. E. S. Barros, and J. S. Hamdan. 2006. Establishing a method of inoculum preparation for susceptibility testing of Trichophyton rubrum and Trichophyton mentagrophytes. J. Clin. Microbiol. 44:98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scher, R. K., and R. Baran. 2003. Onychomycosis in clinical practice: factors contributing to recurrence. Br. J. Dermatol. 149(Suppl. 65):5-9. [DOI] [PubMed] [Google Scholar]
- 27.Siafakas, A. R., L. C. Wright, T. C. Sorrell, and J. T. Djordjevic. 2006. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot. Cell 5:488-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sindermann, H., S. L. Croft, K. R. Engel, W. Bommer, H. J. Eibl, C. Unger, and J. Engel. 2004. Miltefosine (Impavido): the first oral treatment against leishmaniasis. Med. Microbiol. Immunol. 193:173-180. [DOI] [PubMed] [Google Scholar]
- 29.Soto, J., and J. Berman. 2006. Treatment of New World cutaneous leishmaniasis with miltefosine. Trans. R. Soc. Trop. Med. Hyg. 100(Suppl. 1):S34-S40. [DOI] [PubMed] [Google Scholar]
- 30.Unger, C., E. Fleer, W. Damenz, P. Hilgard, G. Nagel, and H. Eibl. 1991. Hexadecylphosphocholine: determination of serum concentrations in rats. J. Lipid Mediat. 3:71-78. [PubMed] [Google Scholar]
- 31.Widmer, F., L. C. Wright, D. Obando, R. Handke, R. Ganendren, D. H. Ellis, and T. C. Sorrell. 2006. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]