Abstract
The occurrence of Plasmodium vivax resistance to chloroquine has been reported in several countries of Asia and South America. However, the resistance of P. vivax is insufficiently documented for three reasons: it has received far less attention than P. falciparum; in vivo investigations are handicapped by the existence of hypnozoites, which make it difficult to distinguish between recrudescences due to drug failure and relapses due to dormant forms in the liver; and in vitro studies are greatly limited by the poor growth of P. vivax. We report on the adaptation to P. vivax of a colorimetric double-site Plasmodium lactate dehydrogenase antigen capture enzyme-linked immunosorbent assay previously developed for P. falciparum. The assay proved remarkably sensitive, as under optimal conditions it could detect P. vivax parasitemia levels as low as 10−8. The technique, which relies on the detection of protein synthesis by the parasite, yielded steep drug-response curves, leading to the precise determination of the 50% inhibitory concentrations for a high proportion of isolates. Chloroquine-resistant parasites were identified in an area where this phenomenon had been documented by in vivo methods. Thus, the results indicate that the in vitro susceptibility of P. vivax can now be monitored easily and efficiently. The data suggest that the threshold of resistance is similar to that of P. falciparum, i.e., in the range of 100 nM for chloroquine and 15 nM for pyronaridine. However, further studies are required to precisely define the cutoff for resistance and the sensitivity to each drug.
The resistance of human malaria parasites to antimalarial compounds has become of considerable concern, particularly in view of the fast speed of emergence of resistant parasites, the fast spread of resistant parasites, and the shortage of novel classes of antimalarial drugs. The vast majority of studies have so far dealt with Plasmodium falciparum, the species responsible for most of the fatalities from malaria.
However, over the past decade, several reports have documented the emergence of resistance of P. vivax, at least to chloroquine. Although P. vivax seldom kills, it is nevertheless as prevalent as P. falciparum and carries a morbidity load as large as that of P. falciparum (16, 21).
The first case of P. vivax resistance to chloroquine was reported from Papua New Guinea in 1989 (22). Further cases were observed as well in Indonesia (1, 8, 25, 26, 29, 30), Myanmar (15, 17), India (12, 28), the Philippines (2), and Thailand (14), as well as in South America (11, 18, 23), thereby indicating that it may be a worldwide, yet underestimated, problem.
The prevalence of P. vivax resistance is indeed most likely widely underestimated for two reasons: in contrast to P. falciparum, there is no method for the cultivation of P. vivax as effective as that for the cultivation of P. falciparum that can be used for in vitro studies; conversely, under in vivo conditions, the reemergence of parasites following treatment is usually attributed in primary health care centers to the reemergence of parasites from the liver more than to treatment failure. The reemergence of the parasites is due to the existence of the dormant hypnozoite stages characteristic of P. vivax, which can resume growth and generate a new erythrocytic infection at regular intervals. Thus, when treatment failures occur, they are usually disregarded, due to the difficulty of distinguishing recrudescences from relapses (except for relapses caused by the Chesson and Panama strains, however, the interval of relapse is usually longer than that of the posttreatment follow-up). These two serious technical limitations have led to a scarcity of studies compared to the numbers conducted with P. falciparum, and the small number of investigations performed constitutes a third factor that has resulted in underestimation of the prevalence of P. vivax resistance to drugs.
We have recently described a colorimetric assay that can be used to measure P. falciparum drug resistance (10). The assay relies on the measurement of the synthesis of an enzyme, parasite lactate dehydrogenase (pLDH), by growing parasites. This assay differs from the classical [3H]hypoxanthine incorporation method, in that it assesses protein production by the parasite and not DNA replication.
P. vivax can grow to a certain extent under in vitro conditions, although with a very low reinvasion rate (i.e., it essentially matures), but the number of reinvaded red blood cells (RBCs) is quite low. We reasoned that the measurement of protein synthesis, which is active during the maturation stage, could provide a more sensitive and, hence, more reliable marker than nucleic acid synthesis. Therefore, monoclonal antibodies (MAbs) able to react with P. vivax LDH were used to measure protein synthesis during P. vivax maturation and thereafter to assess the ability of P. vivax to be inhibited by effective drugs under in vitro conditions in a double-site Plasmodium LDH antigen capture enzyme-linked immunosorbent assay (DELI).
The results obtained in the laboratory indicate that even at a very low density P. vivax parasites can be reliably detected by the technique. The results obtained in the field with wild isolates from an area where P. vivax isolates are chloroquine resistant indicate that the in vitro susceptibility of P. vivax can now be easily and efficiently monitored by this new colorimetric DELI based on protein synthesis detection. In addition, the remarkable sensitivity of the technique is well suited to the usually low parasite densities characteristic of P. vivax infection as well as to the modest proportion of parasites that actually grow under in vitro conditions.
MATERIALS AND METHODS
Study area.
The field study was conducted in the province of Dawae, Myanmar, in a region which had been the object of extensive entomological, parasitological, and clinical surveys by the Department of Medical Research in collaboration with the authors and 12 physicians working in dispensaries or small regional hospitals. The area is mesoendemic for P. falciparum and P. vivax, with spleen infection rates varying from 2 to 38% and parasite prevalence ranging from 6 to 52%, depending on the village. P. falciparum was more prevalent in villages in the hills near the Thai border, whereas P. vivax was dominant in the villages near the coast.
Patients.
Twenty-two P. vivax isolates were obtained from patients (age range, 2 to 36 years; mean age, 17.6 years) attending the clinic in Kambauk for fever because of a pure P. vivax infection, detected by the presence of asexual blood stages. Thorough examination of thin and thick smears did not reveal the presence of other malaria parasite species, and the submicroscopic presence of P. falciparum was excluded by using P. falciparum-specific MAbs in a DELI, performed as described previously (10). The mean level of parasitemia was 0.36% (range, 0.01 to 1%), with a large majority of trophozoites. None of the patients had a history of intake of chloroquine or other antimalarials during the week before sampling. Thirty-eight P. falciparum isolates from the same area, where chloroquine resistance is highly prevalent, were tested in parallel.
In vitro microtest.
Blood collected on heparin was washed twice in RPMI 1640 medium (Gibco BRL, Paisley, United Kingdom) and once with complete culture medium. Complete culture medium was a 3:1 mix (vol/vol) of RPMI 1640 medium containing HEPES (25 mM), NaHCO3 (25 mM), and Waymouth medium (Flow Laboratories, Irvine, United Kingdom) supplemented with 12% (vol/vol) human type AB Rhesus negative serum and hypoxanthine (10 μg/liter). Chloroquine sulfate was obtained from Rhone Poulenc (Vitry sur Seine, France), and pyronaridine tetraphosphate was kindly provided by WHO (Geneva, Switzerland). Stock solutions of chloroquine sulfate (600 ng/ml) and pyronaridine tetraphosphate (600 ng/ml) were prepared in sterile distilled water and were used in twofold dilutions with the culture medium in 96-well culture microplates (Nunc, Denmark) to obtain nine final dilutions (600 to 2.34 ng/ml for chloroquine) and (150 to 0.58 ng/ml for pyronaridine). Parasitized RBCs were added to each well and to three control wells without drug to a final volume of 250 μl/well at a 2% hematocrit. Each isolate was tested in duplicate. The plates were incubated in a candle jar at 37°C for 48 h. At the end of the assay, the plates were frozen and thawed three times to hemolyze the RBCs.
The DELI was performed as described previously by Druilhe et al. (10) for the assay of P. falciparum, with some modifications. Briefly, 100 μl of lysate from each well was transferred into 96-well plates (Maxisorb; Nunc, Denmark) coated with capture MAb 6C9, which reacts with the pLDH of P. vivax, and the plates were incubated for 1 h at 37°C. After five washings with phosphate-buffered saline (PBS)-1% bovine serum albumin (BSA; Boehringer Mannheim, Mannheim, Germany) secondary biotinylated anti-pLDH MAb 19G7 (19) was added and the plates were incubated for 1 h at 37°C. After five washings with PBS-1% BSA, the plates were incubated with streptavidin-peroxidase (Boehringer Mannheim) for 30 min at room temperature. After nine washings with PBS-1% BSA, the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry, Gaithersburg, MD) was added. After 5 min at room temperature the reaction was stopped by addition of 1 M phosphoric acid, and the yellow color that developed was quantified with a spectrophotometer (Titertek Multiscan MCC/340) at 450 nm. The drug sensitivity was expressed as the concentration of drug that inhibited parasite growth by 50% (IC50), determined from the maximal optical density (OD) values for the drug-free control wells compared to the OD values for the wells containing the dilutions of drug. Additional laboratory studies were performed with MAb 11d, which reacts only with P. vivax, as the capture MAb in place of MAb 6C9, which is panspecific; and MAb 6C9 was used for both the laboratory and the field studies.
The 38 P. falciparum isolates were tested with chloroquine and pyronaridine, as described previously (10), and served as controls.
RESULTS
Development of a reliable DELI with high sensitivity for detection of very low level P. vivax parasite densities.
We relied on two parasite-specific MAbs that had previously been selected and developed for use for the detection of P. vivax and other species by a rapid immunochromatographic (dipstick) method. We also investigated a series of other MAbs, developed by Flow Inc. (Portland, OR); however, the combination of MAbs 6C9 and 19G7, which are used in the dipstick assay, or MAbs 11d and 19G7 ultimately provided the best sensitivity (data not shown).
To identify the optimal conditions of sensitivity and reproducibility, blood obtained from a patient with 1% P. vivax parasitemia was used to prepare a 10-fold series of dilutions in RBCs from a healthy individual, resulting in levels of parasitemia ranging from 10−3 to 10−9. The various parameters which could influence the results of the capture enzyme-linked immunosorbent assay (ELISA) were thereafter assessed in a systematic manner, as previously described for the detection of P. falciparum (10), by varying, e.g., the concentrations of the capture MAb or the detection MAb, the type of buffer used to coat the ELISA wells, the buffer used for dilution of the secondary biotin-labeled antibody, or the temperature used either during the coating of the plates or during the assay run. The conditions found to be optimal are those described in the Materials and Methods section. The main modification compared to the previous DELI (10) is the higher concentration of the capture MAb of 5 μg/ml instead of 1 μg/ml for P. falciparum.
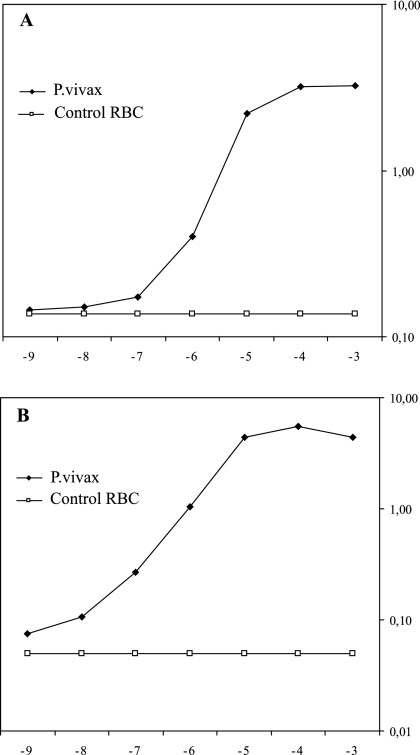
The results obtained are summarized in Fig. 1, which represents the results of one of the experiments performed to assess reproducibility. A direct relationship between the OD values and the parasite concentrations was obtained (Fig. 1). This result incidentally indicates that the assay can be used for the fast and semiautomated assessment of P. vivax parasite densities in patient blood, an important objective in view of the usually low parasite densities found in P. vivax-infected patients. The results were very similar when either panspecific MAb 6C9 (Fig. 1A) or P. vivax-specific MAb 11d (Fig. 1B) was used as the capture MAb.
FIG. 1.
Correlation between ODs and P. vivax parasite densities in the DELI. The results shown are those of a typical experiment performed with either MAb 6C9 (A) or MAb 11d (B), in which P. vivax-infected erythrocytes were serially diluted 10-fold with noninfected RBCs to obtain parasite densities ranging from 10−2 to 10−9 and the pLDH contents in the P. vivax lysates were determined by the DELI.
Moreover, as was the case for P. falciparum, the sensitivity of the antigen capture assay was impressive. Indeed, parasite densities as low as 1 infected RBC in 108 RBCs could be reproducibly detected under optimal laboratory conditions, i.e., at parasite densities that are 100-fold lower than the lowest limit of microscopic detection performed by very well trained technicians (ca. 1 infected RBC per 106 RBCs).
Assessment of drug responses in P. vivax isolates from Myanmar.
Since P. vivax cannot be cultured in the laboratory, the ability of the technique to determine the P. vivax response to antimalarial drugs was studied in the field near the Thai- Myanmar border, one of the few areas in the world where P. vivax resistance to chloroquine is documented.
P. vivax LDH protein synthesis by in vitro maturing parasites was clearly detectable by the DELI. The ODs increased, on average, 4.2-fold over the 48-h in vitro growth period (range, 3.1- to 6-fold).
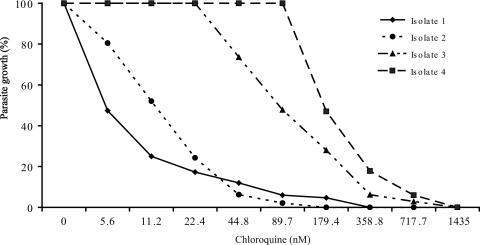
Therefore, by the DELI with parasites cultured over a range of chloroquine or pyronaridine concentrations, dose-response curves essentially similar to those obtained by the DELI with P. falciparum were obtained (Fig. 2). In contrast to [3H]hypoxanthine incorporation, the differences in OD values between wells in which growth was fully inhibited by high drug concentrations and control wells without drug were marked (e.g., the ODs increased from 0.25 to 1.18 and 0.29 to 1.11 over 48 h for isolates 1 and 4 in Fig. 2, respectively). The curves obtained indicated a clear drug concentration-dependent effect, leading to precise determinations of the IC50s. Figure 2 provides two examples of P. vivax parasites which are likely susceptible to chloroquine (isolates 1and 2; IC50s, 5.5 and 11.9 nM, respectively) and two examples of P. vivax parasites which are likely resistant to the effect of chloroquine (e.g., isolates 3 and 4; IC50s, 89.9 and 180 nM, respectively), as indicated by the measurement of P. vivax LDH synthesis. The profile of the dose-response curve obtained also permits calculation of the IC90. Although determination of the IC90 is less precise than that of the IC50, as the IC90 is in the asymptotic part of the curve, it can also be of value for epidemiological purposes, i.e., for comparison of isolates from two areas where P. vivax resistance is prevalent or to indicate a trend toward resistance when IC90s are high, even though the IC50 remains below the threshold value of resistance.
FIG. 2.
Dose-response to chloroquine of four P. vivax isolates from Myanmar. The inhibition of P. vivax growth by various concentrations of chloroquine in two isolates sensitive to chloroquine and two P. vivax isolates likely resistant to chloroquine is shown. The parasites were cultured for 48 h in the presence of each drug concentration, the contents of each duplicate well were lysed, and the P. vivax LDH antigen concentration was determined by the DELI with MAbs 6C9 and 19G7. Since the starting levels of parasitemia differed among the four isolates, the maximal OD values recorded without drug were adjusted to 100% and results are expressed as the percentage of decrease in the OD values obtained with the various drug concentrations.
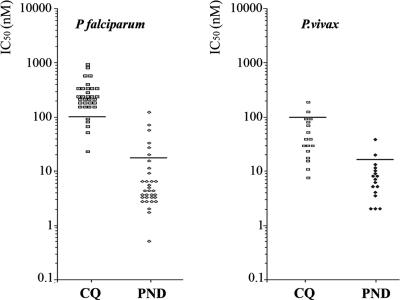
Of 22 P. vivax isolates cultured from samples from patients attending the clinic at Kambauk, the measurement of P. vivax LDH synthesis showed satisfactory dose-response curves for 17 of them. The distribution of the corresponding IC50s is shown in Fig. 3B, together with results obtained for 33 of the 38 P. falciparum isolates from the same area (Fig. 3A). The results show a range of various levels of susceptibility of P. vivax to chloroquine, with the IC50s for two isolates being above the cutoff value of 100 nM and those for two isolates reaching this value; the last two isolates were thus borderline susceptible and all four isolates had reduced susceptibilities to chloroquine. The responses obtained with pyronaridine indicated susceptibility to low concentrations of this drug, as is the case for P. falciparum. The response by one isolate was above the threshold determined for the latter species (20); i.e., it may have been resistant. Altogether the results indicate that P. vivax growth can be monitored precisely by determination of pLDH synthesis and suggest that P. vivax response to drugs can be evaluated by this new technique, yielding results essentially similar to those obtained for P. falciparum by the same technique.
FIG. 3.
Distribution of IC50s among 17 P. vivax isolates (B) and 33 P. falciparum isolates (A). P. falciparum and P. vivax isolates from Dawae Province in Myanmar were cultured in the presence of various concentrations of chloroquine (CQ) or pyronaridine (PRN), and the IC50s were determined and plotted on the graph. The horizontal bars represent the likely thresholds between sensitivity and resistance.
DISCUSSION
There are now numerous indications that P. vivax resistance to antimalarial drugs is prevalent in various regions of Asia and South America, at least to chloroquine and at least in those locations where it has been investigated.
However, the assessment of resistance in P. vivax remains difficult. There is a severe shortage of reliable methods for monitoring of the phenomenon. The techniques that have been available to date have substantial drawbacks and therefore have not been widely used.
Under in vivo conditions, the follow-up of drug-treated P. vivax cases frequently shows the reemergence of parasites; however, recrudescences due to drug failure cannot be distinguished with certainty from relapses due to the presence of hypnozoites. However, as the relapse interval in this area of the world is usually ≥10 weeks, evidence for resistance had been based on either the persistence or the recurrence of an asexual parasitemia within 4 weeks after chloroquine treatment, together with an effective blood level of drug (3). The only reliable method actually requires detection of cases in individuals under prophylaxis with the drug to be tested. Baird (4) reported 29% and 61% rates of resistance of P. vivax measured in this manner in a cohorts of 94 individuals and in another cohort of 41 individuals, respectively, undergoing medically controlled chloroquine prophylaxis (5 mg/kg of body weight/week) for 18 to 52 weeks and monitored medically and by the use of thick smears. Comparative studies in the same area led to the determination that following radical cure, cases in whom parasites persisted for more than 4 days after treatment or recrudesced before day 35 posttreatment were likely to be infected with parasites resistant to chloroquine (4).
Under in vitro conditions the proportion of P. vivax parasites that successfully grow is usually very low. Among the parasites put into culture, only a few parasites actually mature; and when they do, few of the released merozoites reinvade new RBCs, usually leading to decreasing levels of parasitemia over time. This, in turn, implies that the effect of a given drug against only a small proportion of the parasites initially put into culture can be observed. The difficulty is increased by the lower level of parasitemia due to P. vivax than to P. falciparum, and so the proportion of isolates that can actually be studied is very small.
By microscopy, successful growth and successful determination of the IC50s were obtainable for only 5 to 15% of the isolates (6). The measurement of drug inhibition by microscopy is troublesome and requires the differential counting of each stage of schizogonic development, not just the total level of parasitemia. By the [3H]hypoxanthine incorporation method, similar values were obtained for P. vivax and P. ovale (5). Moreover, the difference between wells with growing and inhibited parasites was very low; i.e., the counts were usually too low for the reliable determination of an IC50. This ratio was in the range of 2 in our hands for not more than 15 to 25% of the isolates tested (unpublished material). A picogreen assay has recently been proposed, although it was not claimed to result in a major improvement over microscopy (13).
In contrast, in the P. vivax DELI, protein synthesis by the parasite under in vitro conditions was sufficient to yield dose-response curves with steep slopes. The clear difference in OD values for protein synthesis between wells with chloroquine-inhibited and noninhibited parasites led to dose-effect response curves from which the IC50s and the IC90s could be determined for P. vivax as precisely as they are for P. falciparum by the same technique (10).
Another major advantage of the colorimetric DELI compared to previously used techniques is the proportion of isolates for which an IC50 can be determined. By assessment of pLDH protein synthesis, the proportion of isolates which showed a clear dose-response to chloroquine, 17 of 22 (77%), was remarkably high. This success rate is not markedly different from that obtained with 38 P. falciparum isolates tested in the same location, which reached 86% for chloroquine and pyronaridine.
The distribution of the IC50 for chloroquine assessed in this manner in Myanmar showed that 15 of the 17 isolates (88.2%) that were successfully cultured had IC50s below the 100 nM threshold of sensitivity (9), whereas 2 isolates had IC50s above that value and could be considered resistant to chloroquine and 2 isolates had borderline values; thus, 4 isolates (23%) showed reduced susceptibilities to chloroquine (Fig. 2). For pyronaridine, 13 of the 14 isolates (92.8%) successfully tested had IC50s below the threshold of 15 nM/liter (20), and only one could be considered resistant (Fig. 2).
The Thai-Myanmar border is one of the areas where P. falciparum parasites have the highest levels of multidrug resistance and is one of the few areas, together with Indonesia, where P. vivax resistance to chloroquine has already been documented (15, 17). We have confirmed these results in the area of Kanbauk, Myanmar, by using an in vivo protocol of prophylaxis: the emergence of P. vivax resistant parasites was observed in 3 of 60 individuals who received 100 mg of chloroquine prophylaxis daily for 3 months and in whom adequate blood chloroquine concentrations were determined (unpublished). We therefore chose to study the responses of P. vivax parasites from that area to assess the value of the new assay.
The percentage of P. vivax isolates resistant to chloroquine in the area of Kanbauk determined by the DELI is in agreement with the proportion determined by in vivo methods in the same area by Myint Oo and colleagues (15) and by our chloroquine prophylaxis studies. In contrast, P. vivax isolates studied by the DELI in Sri Lanka in 1999 were all found to be sensitive, i.e., with IC50s below 100 nM and in agreement with the fact that resistance of P. vivax to chloroquine has not been reported in Sri Lanka (S. Handunnetti et al., unpublished results). However, further studies are clearly required to more precisely determine the cutoff between resistance and sensitivity for data generated by the P. vivax DELI. The data presented here and those reported by others who used microscopic methods (7, 24, 27) would support the idea that the threshold is similar to that for P. falciparum, i.e., in the range of 80 to 100 nM for chloroquine and 15 nM for pyronaridine (20).
In summary, the results obtained in the laboratory as well as in the field indicate that the DELI has the potential to determine the in vitro susceptibility of P. vivax and is well suited to the low parasite densities characteristic of P. vivax. It could be of value for the high-throughput detection of low-grade P. vivax infections in human populations for epidemiological purposes. Finally, it also provide a means to assess the effects of novel compounds on the second most important malaria parasite species in the world, P. vivax, before such compounds are subjected to costly clinical development efforts.
Footnotes
Published ahead of print on 26 March 2007.
REFERENCES
- 1.Baird, J. K., H. Basri, Purnomo, M. J. Bang, B. Subianto, I. Patchen, and S. L. Hoffman. 1991. Resistance to chloroquine by Plasmodium vivax in Irian Jaya. Am. J. Trop. Med. Hyg. 44:547-552. [DOI] [PubMed] [Google Scholar]
- 2.Baird, J. K., H. Basri, B. Subianto, D. J. Fryauff, P. D. McElroy, B. Leksana, T. L. Richie, S. Masbar, F. S. Wignall, and S. L. Hoffman. 1995. Treatment of chloroquine-resistant Plasmodium vivax with chloroquine and primaquine or halofantrine. J. Infect. Dis. 171:1678-1682. [DOI] [PubMed] [Google Scholar]
- 3.Baird, J. K., B. Leksana, S. Masbar, D. J. Fryauff, M. A. Sutanihardja, Suradi, F. S. Wignall, and S. I. Hoffman. 1997. Diagnosis of resistance to chloroquine by Plasmodium vivax: timing of recurrence and whole blood chloroquine concentrations. Am. J. Trop. Med. Hyg. 56:621-626. [DOI] [PubMed] [Google Scholar]
- 4.Baird, J. K. 2004. Chloroquine resistance in Plasmodium vivax. Antimicrob. Agents Chemother. 48:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basco, L. K., and J. Le Bras. 1994. Short term in vitro culture of Plasmodium vivax and P. ovale for drug-susceptibility testing. Parasitol. Res. 80:262-264. [DOI] [PubMed] [Google Scholar]
- 6.Brockelman, C. R., A. P. Tan-Ariya, and D. Bunnag. 1989. Development of in vitro microtest for the assessment of Plasmodium vivax sensitivity to chloroquine. South East Asian J. Trop. Med. Public Health 20:41-47. [PubMed] [Google Scholar]
- 7.Chotivanich, K., R. Udomsangpetch, W. Chierakul, P. N. Newton, R. Ruangveerayuth, S. Pukrittayakamee, S. Looareesuwan, and N. J. White. 2004. In vitro efficacy of antimalarial drugs against Plasmodium vivax on the western border of Thailand. Am. J. Trop. Med. Hyg. 70:395-397. [PubMed] [Google Scholar]
- 8.Collignon, P. 1991. Chloroquine resistance in Plasmodium vivax. J. Infect. Dis. 164:222-223. [DOI] [PubMed] [Google Scholar]
- 9.Congpuong, K., K. Na-Bangchang, K. Thimasarn, U. Tasanor, and W. H. Wernsdorfer. 2002. Sensitivity of Plasmodium vivax to chloroquine in Sa Kaeo Province, Thailand. Acta Trop. 83:117-121. [DOI] [PubMed] [Google Scholar]
- 10.Druilhe, P., A. Moreno, C. Blanc, P. Brasseur, and P. Jacquier. 2001. Colorimetric in vitro drug sensitivity assay for P. falciparum based on a highly sensitive double-site pLDH antigen capture ELISA assay. Am. J. Trop. Med. Hyg. 64:233-241. [DOI] [PubMed] [Google Scholar]
- 11.Garavelli, P. L., and E. Corti. 1992. Chloroquine resistance in Plasmodium vivax: the first case in Brazil. Trans. R. Soc. Trop. Med. Hyg. 86:128. [DOI] [PubMed] [Google Scholar]
- 12.Garg, M., N. Gopinathan, P. Bodhe, and N. A. Kshirsagar. 1995. Vivax malaria resistant to chloroquine: cases reports from Bombay. Trans. R. Soc. Trop. Med. Hyg. 89:656-657. [DOI] [PubMed] [Google Scholar]
- 13.Kosaisavee, V., R. Suwanarusk, F. Nosten, D. E. Kyle, M. Barrends, J. Jones, R. Price, B. Russel, and U. Lek-Uthai. 2006. Plasmodium vivax: isotopic, PicoGreen, and microscopic assays for measuring chloroquine sensitivity in fresh and cryopreserved isoplates. Exp. Parasitol. 114:34-39. [DOI] [PubMed] [Google Scholar]
- 14.Looareesuwan, S., P. Wilairatana, S. Krudsood, S. Treeprasertsuk, P. Singhasivanon, V. Bussaratid, W. Chokjindachai, P. Viriyavejakul, K. Chalermrut, D. S. Walsh, and J. White. 1999. Chloroquine sensitivity of Plasmodium vivax in Thailand. Ann. Trop. Med. Parasitol. 93:225-230. [PubMed] [Google Scholar]
- 15.Marlar-Than, Myat-Phone-Kyaw, Aye-Yu-Soe, Khaing-Khaing-Gyi, Ma-Sabai, and Myint-Oo. 1995. Development of resistance to chloroquine by Plasmodium vivax in Myanmar. Trans. R. Soc. Trop. Med. Hyg. 89:307-308. [DOI] [PubMed] [Google Scholar]
- 16.Mendis, K., B. J. Sima, B. Marchesinin, and R. Carter. 2001. The neglected burden of Plasmodium vivax malaria. Am. J. Trop. Med. Hyg. 64:97-106. [DOI] [PubMed] [Google Scholar]
- 17.Myat-Phone-Kyaw, Mynt-Oo, Mynt-Lwin, Thaw-zin, Kian-Hla-Aye, and New-New-Yin. 1993. Emergence of chloroquine-resitant Plasmodium vivax in Myanmar (Burma). Trans. R. Soc. Trop. Med. Hyg. 87:687. [DOI] [PubMed] [Google Scholar]
- 18.Phillips, E. J., J. S. Keystone, and K. C. Kain. 1996. Failure of combined chloroquine and high-dose primaquine therapy for Plasmodium vivax malaria acquired in Guyana, South America. Clin. Infect. Dis. 23:1171-1173. [DOI] [PubMed] [Google Scholar]
- 19.Piper, R., J. Le Bras, L. Wentworth, A. Hunt-Cooke, S. Houze, P. Chiodini, and M. Makler. 1999. Immunocapture diagnostic assays for malaria using Plasmodium lactate dehydrogenase (pLDH). Am. J. Trop. Med. Hyg. 60:109-118. [DOI] [PubMed] [Google Scholar]
- 20.Pradines, B., A. Tall, D. Parzy, A. Spiegel, T. Fusai, P. Hienne, J. F. Trape, and J. C. Doury. 1998. In vitro activity of pyronaridine and amodiaquine against African isolates (Senegal) of Plasmodium falciparum in comparison with standard antimalarial agents. J. Antimicrob. Chemother. 42:333-339. [DOI] [PubMed] [Google Scholar]
- 21.Ridley, R. G. 2002. Chemotherapeutic hope on the horizon for Plasmodium vivax malaria? Proc. Natl. Acad. Sci. USA 99:13362-13364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rieckmann, K. H., D. R. Davis, and D. C. Hutton. 1989. Plasmodium vivax resistance to chloroquine? Lancet ii:1183-1184. [DOI] [PubMed] [Google Scholar]
- 23.Ruebush, T. K., J. Zegarra II, J. Cairo, E. M. Andersen, M. Green, D. R. Pillai, W. Marquino, M. Huilca, E. Arevalo, C. Garcia, L. Solary, and K. C. Kain. 2003. Chloroquine-resistant Plasmodium vivax malaria in Peru. Am. J. Trop. Med. Hyg. 69:548-552. [PubMed] [Google Scholar]
- 24.Russell, B. M., R. Udomsangpetch, K. H. Rieckmann, B. M. Kotecka, R. E. Coleman, and J. Satabongkot. 2003. Simple in vitro assay for determining the sensitivity of Plasmodium vivax isolates from fresh human blood to antimalarials in areas where P. vivax is endemic. Antimicrob. Agents Chemother. 47:170-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuurkamp, G., P. E. Spicer, R. K. Kereu, P. K. Bulungol, and K. H. Rieckmann. 1992. Chloroquine resistant P. vivax in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 86:121-122. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz, I. K., E. M. Lackritz, and L. C. Patchen. 1991. Chloroquine resistant Plasmodium vivax from Indonesia. N. Engl. J. Med. 1991:927. [DOI] [PubMed] [Google Scholar]
- 27.Tasanor, O., H. Noedl, K. Na-Bangchang, K. Congpuong, J. Sirichaisinthop, and W. H. Wernsdorfer. 2002. An in vitro system for assessing the sensitivity of Plasmodium vivax chloroquine. Acta Trop. 83:49-61. [DOI] [PubMed] [Google Scholar]
- 28.Van Den Abbeele, K., E. Van Den Enden, and J. Van Den Ende. 1995. Combined chloroquine and primaquine resistant Plasmodium vivax malaria in a patient returning from India. Ann. Soc. Belge Med. Trop. 75:73-74. [PubMed] [Google Scholar]
- 29.Vinetz, J. M. 2006. Emerging chloroquine-resistant Plasmodium vivax (benign tertian) malaria: the need for alternative drug treatment. Clin. Infect. Dis. 42:1073-1074. [DOI] [PubMed] [Google Scholar]
- 30.Whitby, M., G. Wood, J. R. Veenendaal, and K. Rieckmann. 1989. Chloroquine-resistant Plasmodium vivax. Lancet ii:1395. [DOI] [PubMed] [Google Scholar]