Abstract
Parasitic diseases are of enormous public health significance in developing countries—a situation compounded by the toxicity of and resistance to many current chemotherapeutics. We investigated a focused library of 18 structurally diverse bis-acridine compounds for in vitro bioactivity against seven protozoan and one helminth parasite species and compared the bioactivities and the cytotoxicities of these compounds toward various mammalian cell lines. Structure-activity relationships demonstrated the influence of both the bis-acridine linker structure and the terminal acridine heterocycle on potency and cytotoxicity. The bioactivity of polyamine-linked acridines required a minimum linker length of approximately 10 Å. Increasing linker length resulted in bioactivity against most parasites but also cytotoxicity toward mammalian cells. N alkylation, but less so N acylation, of the polyamine linker ameliorated cytotoxicity while retaining bioactivity with 50% effective concentration (EC50) values similar to or better than those measured for standard drugs. Substitution of the polyamine for either an alkyl or a polyether linker maintained bioactivity and further alleviated cytotoxicity. Polyamine-linked compounds in which the terminal acridine heterocycle had been replaced with an aza-acridine also maintained acceptable therapeutic indices. The most potent compounds recorded low- to mid-nanomolar EC50 values against Plasmodium falciparum and Trypanosoma brucei; otherwise, low-micromolar potencies were measured. Importantly, the bioactivity of the library was independent of P. falciparum resistance to chloroquine. Compound bioactivity was a function of neither the potential to bis-intercalate DNA nor the inhibition of trypanothione reductase, an important drug target in trypanosomatid parasites. Our approach illustrates the usefulness of screening focused compound libraries against multiple parasite targets. Some of the bis-acridines identified here may represent useful starting points for further lead optimization.
Vector-borne protozoan and helminth diseases are of serious concern in many tropical and subtropical areas in terms of direct mortality and morbidity and the consequential detriment to economic productivity. Protozoan diseases such as malaria, leishmaniasis, the African and South American trypanosomiases, and the helminth infection schistosomiasis afflict hundreds of millions of people. Falciparum malaria kills one child every 30 s, and estimates of the number of those at risk from schistosomiasis in Africa have been revised upward from 600 to 700 million people (14). African trypanosomiases are not only lethal but also a major constraint to livestock farming in Africa, where more than a third of the land (8.7 million km2) is infested with the tsetse fly (24).
Set against this background is the increasing incidence of drug resistance to current therapies, many of which were introduced decades ago. Widespread parasite resistance to the 4-aminoquinoline compound chloroquine (CQ) has rendered this drug, the mainstay of malaria therapy for over 50 years, ineffective in many areas of endemicity (18). In addition, some current therapies for the treatment of parasitic diseases have narrow therapeutic indices, for example, the arsenical compound melarsoprol for the treatment of late-stage African trypanosomiasis. This compound elicits a fatal reactive encephalopathy in up to 10% of treated patients (7). Therefore, there is an urgent need for novel, safe, and cost-effective therapeutics for the treatment of these parasitic infections.
Since the development in the 1930s of the antimalarial acridine compound quinacrine (QA), acridine derivatives have been explored as potential antiparasitics. Acridines have shown efficacy against the protozoans Leishmania (9, 39), Trypanosoma brucei (15), and Trypanosoma cruzi (19) and against the flatworm bloodfluke Schistosoma mansoni (11). Bis-acridine compounds are dimeric acridine analogs, whereby two bioactive acridine heterocycles are tethered via a flexible linker. They were first developed as tumorostatic agents based on their enhanced DNA-binding properties compared to those of the respective monoacridines (1, 2, 37). Such dimeric ligands, including those derived from quinoline-based drugs, have been employed both to improve ligand potency by increasing the local concentration of the bioactive species and to avoid the cellular efflux mechanisms associated with multidrug resistance to the respective monomeric counterparts (17, 36). Bis-acridines have demonstrated bioactivity in mice infected with Plasmodium berghei (16) and in vitro against Plasmodium falciparum and trypanosomatid parasites (17).
Academic drug discovery has recognized the value of screening focused compound libraries rather than the exhaustive screening of chemical space often favored in commercial drug discovery settings. Extending this academic paradigm, we quantified the bioactivity and derived structure-activity relationship (SAR) data in vitro for a discrete library of 18 diverse bis-acridine compounds against seven protozoan and one helminth parasite species causing severe disease in humans. We reasoned that a suitable, privileged scaffold that demonstrates bioactivity against one parasite might provide a new lead against other targets. Many of the bis-acridines tested were effective at nanomolar concentrations, with bioactivities similar to or better than those of established drugs. Also, common structural motifs, necessary to distinguish specific parasiticidal activity from general cytotoxicity toward two mammalian cell lines, were identified.
MATERIALS AND METHODS
Compound synthesis.
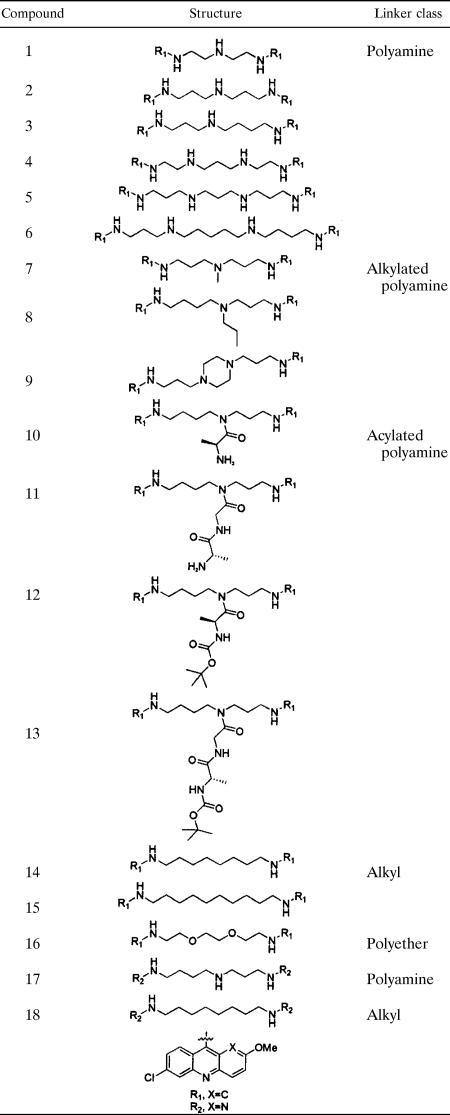
Bis-acridine (bis-6-dichloro-2-methoxyacridine) and bis-aza-acridine (bis-7-dichloro-2-methoxybenzo[b][1,5]naphthyridine) compounds (Table 1) were synthesized as previously described (26). The purities and identities of all compounds were confirmed by 1H nuclear magnetic resonance and liquid chromatography-mass spectrometry prior to use. CQ, QA, melarsoprol, amphotericin B, praziquantel (PZQ), metronidazole, and dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). The cysteine protease inhibitor K11777 was a gift from James Palmer (Rigel Pharmaceuticals, Inc., South San Francisco, CA). Compounds were prepared as DMSO stocks (10 mM) and stored at −20°C prior to use.
TABLE 1.
Focused library of bis-acridine compounds
Parasite cultures and cytotoxicity assays for P. falciparum.
Cultures of the CQ-sensitive P. falciparum strain 3D7 and CQ-resistant P. falciparum strain W2 were maintained in purified human red blood cells cultured in RPMI 1640 medium supplemented with 0.25% Albumax II (Gibco Life Technologies, San Diego, CA), 24 mM sodium bicarbonate, 0.1 mM hypoxanthine, 25 mM HEPES (pH 7.4), and 50 μg/liter gentamicin at 37°C, 5% O2, and 6% CO2. Growth inhibition of P. falciparum cultures was quantified using a fluorescence-activated cell sorting assay, as previously described (38). CQ and QA were used as drug controls to monitor the quality of the assay (Z′ = 0.6 to 0.8, where Z′ is a measure of the discrimination between the positive and negative controls on a screen plate). Dose-response curves were determined from three independent experiments. Compound bioactivity was expressed as EC50, the effective concentration of compound causing 50% parasite death.
T. brucei brucei and HL-60 cells.
Bloodstream forms of the monomorphic T. b. brucei clone 427-221a (20) and human promyelocytic leukemia HL-60 cells (DSMZ, Braunschweig, Germany) were cultured in Baltz medium (4) and RPMI 1640 medium, respectively. Both media were supplemented with 16.7% heat-inactivated fetal bovine serum (FBS). All cultures were incubated in a humidified atmosphere at 37°C in 5% CO2. Compound bioactivity was determined as previously described (34). Briefly, trypanosomes and HL-60 cells were seeded onto 24-well tissue culture plates in 1 ml medium at initial densities of approximately 104 and 105 cells/ml, respectively. The test compound was added to the medium to give a range of final concentrations up to 10 μM. DMSO- and melarsoprol-treated cultures served as controls. In all experiments, the final DMSO concentration was 1%, which had no effect on survival (28). After 48 h, cell numbers were quantified using a Neubauer hemocytometer. Live and dead cells were distinguished either by movement (trypanosomes) or by the exclusion of the dye, trypan blue (HL-60 cells). From three independent experiments, compound bioactivity was determined by a regression method based on the S-shaped concentration-inhibition relationship as previously described (21) and expressed as an EC50 value, defined as the effective concentration of compound for reducing the culture density to 50% of that of a DMSO-treated culture. The MIC, defined as the lowest concentration of compound that was 100% lethal to the culture, was determined microscopically.
T. cruzi amastigotes.
The bioactivities of the test compounds were determined as previously described (12). Briefly, J774 macrophages and bovine embryo skeletal muscle (BESM) cells were cultured in RPMI 1640 medium supplemented with 5% heat-inactivated FBS at 37°C in 5% CO2. The Y strain of T. cruzi was maintained by serial passage in BESM cells. Trypomastigotes were collected from culture supernatants and used to infect previously irradiated (6,000 rads) J774 macrophages. Macrophages (106 cells/well) were plated onto 12-well tissue culture plates 24 h before infection with 105 trypomastigotes/well. Excess parasites were removed from the culture 2 h postinfection. Compounds, tested in triplicate, were added to the medium at a final concentration of 5 or 10 μM, such that the final DMSO concentration was <0.1%. Cultures treated with DMSO or the peptidomimetic cysteine protease inhibitor K11777 served as controls. The medium and test compound were replaced every 48 h over 27 days. Cultures were subsequently maintained without the test compound for a total of between 40 and 46 days and monitored daily by phase-contrast microscopy. In untreated control cells, T. cruzi completes its intracellular development in 5 days, at which time the infected macrophages rupture to release a new generation of invasive trypomastigotes. Bioactivity was measured as the increase in survival time of J774 macrophages in the presence of the compound over that in a DMSO-treated culture.
Leishmania donovani promastigotes.
Promastigotes (1.5 × 105 cells/ml) of L. donovani MHOM/SD/00/1S-2D were seeded into 25-cm2 polystyrene cell culture flasks containing 5 ml of medium 199 (Sigma-Aldrich) and supplemented with 10% heat-inactivated FBS (Omega Scientific, Tarzana, CA), as previously described (23). Compounds, tested in triplicate, were then added to a final concentration of 10 μM and the flasks sealed and incubated at 27°C up to a maximum of 7 days. Cultures exposed to K11777 or amphotericin B, at 10 μM, served as controls. Aliquots of the cultures (500 μl) were removed daily, and parasite viability was quantified using the CellTiter-Glo luminescent cell viability assay (Promega, WI) according to the manufacturer's instructions. Bioactivity was expressed as the percent reduction in parasite viability relative to that in DMSO-treated cultures.
Leishmania major amastigotes.
The cloned virulent L. major isolate MHOM/IL/81/FE/BNI was maintained by passage in BALB/c mice (Charles River Breeding Laboratories, Sulzfeld, Germany). Promastigotes were grown in blood agar cultures at 26°C in 5% CO2 and 95% humidity. Stationary-phase promastigotes were collected, washed twice with phosphate-buffered saline, and diluted to 108 cells/ml in complete medium (RPMI 1640 medium [Invitrogen, Karlsruhe, Germany] supplemented with 10% fetal calf serum [PAA Laboratories, Linz, Austria], 2 mM l-glutamine [Biochrom, Berlin, Germany], 10 mM HEPES buffer [pH 7.2; Invitrogen], 100 μg/ml penicillin, 160 μg/ml gentamicin, 7.5% NaHCO3, and 50 mM 2-mercaptoethanol [Sigma-Aldrich Chemikalien, Taufkirchen, Germany]). Resident peritoneal exudate cells (PECs) obtained from BALB/c mice (6 to 8 weeks old) were the source of peritoneal macrophages. Macrophages constituted the major population of PECs, with 80% of the cells expressing the macrophage surface marker F4/80. PECs were obtained by peritoneal washing with 5 ml of ice-cold complete medium. The cells were subsequently incubated for 10 min with ice-cold TAC buffer (10 mM NH4Cl in phosphate-buffered saline, pH 7.2) to eliminate erythrocytes, centrifuged, and suspended at 2 × 106 cells/ml in complete medium. Freshly isolated macrophages (4 × 105 cells/ml) were plated onto eight-well glass chamber slides (Nunc, Rochester, NY) and allowed to adhere for 2 h at 37°C and 5% CO2. Nonadherent cells were removed by extensive washing with complete medium. Subsequently, adherent cells were infected for 24 h with stationary-phase L. major promastigotes, at a parasite-to-macrophage ratio of 10 to 1, in a final volume of 500 μl of complete medium. After removal of extracellular parasites by thorough rinsing with fresh complete medium, cells were incubated for a further 48 h in the presence of the test compound over a concentration range of 0.0001 to 10 μM. DMSO-treated and untreated cultures served as controls. Intracellular parasites were quantified by staining with acridine orange (Sigma-Aldrich) and ethidium bromide (Sigma-Aldrich) and analyzed by fluorescence microscopy (emission/excitation wavelengths, 495/450 nm), as described previously (29). All experiments were repeated three times. Bioactivity was measured as the percentage of amastigotes cleared from macrophages in the presence of the compound relative to the number of amastigotes in DMSO-treated cultures.
Schistosoma mansoni.
A Puerto Rican isolate of S. mansoni was maintained in the snail Biomphalaria glabrata and in BALB/c mice as intermediate and definitive hosts, respectively (30). Twenty-day-old S. mansoni worms were obtained by perfusion (33) after infection with 200 cercariae and washed three times in RPMI 1640 medium (Invitrogen) supplemented with 10% FBS and 1% antibiotic-antimycotic solution (Sigma-Aldrich). After extensive washing in RPMI 1640 medium, approximately 20 worms were added to each well of a 24-well culture plate containing 800 μl Basch 169 medium (5) (custom prepared; UCSF Cell Culture Facility) supplemented with 10% bovine calf serum and 1% antibiotic-antimycotic solution. Worms were incubated for 24 h at 37°C and 5% CO2 prior to use. Test compounds were added to give final concentrations of 1, 3, and 6 μM, and DMSO- and PZQ-treated parasites served as controls. Compound bioactivity was assessed by observing worm vitality after 4 and 18 h in two separate experiments. Parameters for worm viability included feeding and flexing behaviors and gut peristalsis. Worm death was associated with a lack of motility and a shrunken, dark appearance.
Entamoeba histolytica.
Trophozoites of E. histolytica strain HM1:IMSS were axenically maintained in TYI-S-33 medium supplemented with 10% bovine adult serum (8) and plated into 96-well microtiter plates at approximately 104 cells/well. Test compound was added to 48-h-old cultures (in logarithmic growth phase) over a concentration range up to 10 μM. Cultures were then incubated at 37°C for 60 h in a GasPak EZ anaerobe gas-generating pouch system (BD Biosciences, San Jose, CA). DMSO- and metronidazole-treated cultures were used as controls. Trophozoite viability was determined using the CellTiter-Glo luminescent cell viability assay (Promega) according to the manufacturer's instructions. Compound bioactivity was determined in quadruplicate from two independent experiments and expressed as EC50.
Trichomonas vaginalis.
The G3 strain of T. vaginalis was cultured in standard Diamond's medium (8) at 37°C. Cultures were maintained by daily passage, whereby 1 ml of dense culture (approximately 106 cells/ml) was diluted to 10 ml with standard Diamond's medium. Cultures were established for compound screening by diluting 0.5 ml of dense culture into 5.0 ml of complete Diamond's medium. The test compound was added to give a final concentration of 10 μM and the culture incubated for 24 h at 37°C. Untreated and DMSO- and metronidazole-treated cultures served as controls. Cell density was determined by counting motile cells using a hemocytometer as previously described (27). Bioactivity was determined in five separate experiments and expressed as the percentage of dead parasites relative to the number in DMSO-treated cultures.
RESULTS
Compound bioactivity at low-nanomolar concentrations against P. falciparum is independent of resistance to CQ.
The 18 bis-acridine compounds in our library (Table 1) were similarly effective against both the CQ-sensitive (3D7) and the CQ-resistant (W2) strains of P. falciparum (Table 2). Of the polyamine-linked series 1 to 6, compounds 2 to 6 had comparable bioactivities, whereas compound 1 demonstrated approximately six- to sevenfold-lower activity. This suggests a structure-activity dependence on the distance separating the terminal acridine heterocycles, a feature noted previously with respect to the bis-intercalation of DNA (25) and the antimalarial activity (17) of the bis-acridine class. Alkylation of the linker amine of compound 2, to yield the N-methyl analog, compound 7, decreased the bioactivity against both P. falciparum strains 8- to 12-fold. Additionally alkylated compounds (compounds 8 and 9) retained bioactivity, the latter compound having previously been shown to display antimalarial activity (17). Functionalizing compound 3 via acylation of the polyamine linker to yield compounds 10 to 13 reduced the bioactivity relative to that of the parent compound. Alkyl-linked bis-acridine compounds 14 and 15 demonstrated bioactivities comparable to those of the equivalent polyamine-linked compounds 3 and 4. The polyether-linked compound 16 demonstrated improved potency relative to the equivalent polyamine (compound 3)- or alkyl (compound 14)-linked compounds. Modification of the acridine heterocycle to an aza-acridine heterocycle with an additional ring nitrogen (Table 1) (compounds 17 and 18) markedly improved the bioactivity relative to that of the equivalent bis-acridines, compounds 3 and 14. The potent antimalarial activities of compounds 16, 17, and 18 have not previously been described, and the EC50 values are only two- to sevenfold greater than those of QA for both malaria strains and of CQ for the 3D7 strain. The EC50 values of the same compounds for the W2 strain were four to eight times lower than that of CQ.
TABLE 2.
Bioactivities of bis-acridine compounds against chloroquine-sensitive (3D7) and -resistant (W2) strains of P. falciparum
| Compound | EC50 (nM)a for strain:
|
||
|---|---|---|---|
| 3D7 | W2 | FcB1Rb | |
| 1 | 618 | 791 | 63 ± 10 |
| 2 | 127 | 180 | 55 ± 9 |
| 3 | 62 | 135 | 61 ± 3 |
| 4 | 105 | 136 | |
| 5 | 121 | 147 | |
| 6 | 125 | 129 | |
| 7 | 790 | 1085 | |
| 8 | 120 | 319 | |
| 9 | 64 | 112 | 17 ± 10c |
| 10 | 310 | 303 | |
| 11 | 716 | 823 | |
| 12 | 598 | 629 | 330 ± 59 |
| 13 | 314 | 341 | |
| 14 | 115 | 124 | 19 ± 10 |
| 15 | 95 | 116 | 57 ± 30 |
| 16 | 61 | 74 | |
| 17 | 29 | 34 | |
| 18 | 62 | 69 | |
| CQ | 10 | 293 | |
| QA | 11 | 33 | |
Compounds were incubated with synchronous ring stage parasites for 72 h, and survival was quantified relative to DMSO-treated cultures. Compound bioactivity was expressed as EC50, i.e., the effective concentration lethal to 50% of the parasite culture. Standard deviations did not exceed 20% of the means of three independent experiments.
Data are from reference 17, and values expressed are the means ± standard deviations of the results of at least three experiments.
Compound 9 was also tested against P. falciparum strains 3D7, F32a, GP1, FCR3, FCM29, W2, and K1; see reference 17.
T. b. brucei and HL-60 cells.
The trypanocidal activities of the bis-acridine compounds were compared with their cytotoxicities against the human promyelocytic leukemia HL-60 cell line (Table 3). All but three of the bis-acridine compounds (compounds 1, 15, and 18) demonstrated EC50 values against T. b. brucei in the low-nanomolar-concentration range (Table 3). Compound 1 was approximately 200-fold less potent than the other polyamine-linked compounds (compounds 2 to 6), suggestive of a constraint on the bis-acridine linker length for bioactivity. Alkylation of the polyamine linker was tolerated (compounds 7 to 9), but acylation generally increased the EC50 (compounds 10 to 13). Compounds with alkyl (compounds 14 and 15) or polyether (compound 16) linkers were less active than the polyamine-linked equivalents (compounds 3 and 4). Replacement of the acridine heterocycle with an aza-acridine had differing effects depending on the linker; the polyamine (compound 17) retained bioactivity, whereas the alkyl (compound 18) was 10-fold less potent.
TABLE 3.
Bioactivities of bis-acridine compounds against T. b. brucei
| Compound |
T. b. brucei
|
HL-60 cells
|
Selectivity
|
Bioactivity against T. brucei at compound concn of 1.56 μM (%)e | Toxicity for MRC-5 cells at compound concn of 3.13 μM (%)f | |||
|---|---|---|---|---|---|---|---|---|
| EC50 (nM)a | MIC (nM)b | EC50 (nM) | MIC (nM) | EC50 ratioc | MIC ratiod | |||
| 1 | 218.0 | 10,000 | 9,470 | >10,000 | 43 | >1 | 100 | 100 |
| 2 | 1.1 | 1,000 | 244 | 10,000 | 214 | 10 | 100 | 100 |
| 3 | 0.7 | 1,000 | 144 | 10,000 | 209 | 10 | 100 | 100 |
| 4 | 1.3 | 1,000 | 120 | 1,000 | 91 | 1 | ||
| 5 | 1.4 | 1,000 | 142 | 10,000 | 98 | 10 | ||
| 6 | 1.9 | 1,000 | 270 | 10,000 | 140 | 10 | ||
| 7 | 0.9 | 100 | 1,180 | 10,000 | 1,326 | 100 | ||
| 8 | 1.4 | 1,000 | 491 | 10,000 | 348 | 10 | ||
| 9 | 1.8 | 100 | 1,480 | 10,000 | 836 | 100 | 60 | 0 |
| 10 | 0.9 | 1,000 | 225 | 10,000 | 242 | 10 | ||
| 11 | 6.4 | 10,000 | 491 | >10,000 | 77 | >1 | ||
| 12 | 3.5 | 1,000 | 1,650 | 10,000 | 469 | 10 | 100 | 0 |
| 13 | 2.4 | 1,000 | 270 | 10,000 | 111 | 10 | ||
| 14 | 8.6 | 1,000 | 1,650 | 10,000 | 191 | 10 | 100 | 20 |
| 15 | 25.0 | 1,000 | 3,140 | 10,000 | 125 | 10 | 100 | 0 |
| 16 | 5.8 | 1,000 | 3,040 | 10,000 | 524 | 10 | ||
| 17 | 1.4 | 100 | 119 | 7,000 | 86 | 70 | ||
| 18 | 31.0 | 1,000 | 26,300 | >10,000 | 859 | >10 | ||
| Melarsoprol | 16.0 | 100 | 4,270 | 100,000 | 267 | >1,000 | ||
Compound bioactivity was measured after a 48-h incubation and expressed as EC50, i.e., the effective concentration of compound for reducing the culture density to 50% of that of DMSO-treated cultures.
Compound bioactivity was also expressed as the MIC, i.e., the compound concentration that was 100% lethal.
Defined as EC50(HL-60 cells)/EC50(T. b. brucei).
Defined as MIC(HL-60 cells)/MIC(T. b. brucei). Each compound was tested in three separate experiments, and the standard deviations never exceeded 20% of the means.
Data are from reference 17.
Data are from reference 17. MRC-5 is a human diploid embryonic lung cell line.
In general, individual cytotoxicities measured for the library members against the HL-60 cell line mirrored those bioactivities recorded with T. b. brucei. Thus, compound 1 was approximately 70-fold less cytotoxic than the other polyamine-linked compounds 2 to 6, again suggesting that cytotoxicity required a polyamine linker of minimal length. Compounds 2 to 6 were generally the most cytotoxic library components, with mid-nanomolar EC50 values. However, cytotoxicity could be alleviated by certain alkyl (compounds 7 and 9) and acyl (compound 12) modifications to the polyamine linker. Likewise, the alkyl (compounds 14 and 15)- and polyether (compound 16)-linked compounds were less cytotoxic than the polyamine-linked equivalents (compounds 3 and 4). Cytotoxicity of the polyamine-linked compounds could not be decreased by replacing the acridine heterocycle with an aza-acridine (compound 17). However, when the polyamine linker was replaced with an alkyl, a relatively nontoxic compound resulted (compound 18).
With respect to the MIC, most bis-acridines had values of 100 or 1,000 nM against T. b. brucei, and by this measure were 10- to 100-fold more cytotoxic to the parasite than to HL-60 cells (Table 3). Considering both the EC50 and the MIC ratios, compounds 7 and 9 demonstrated the most favorable therapeutic indices. Compared to melarsoprol, the standard therapy for late-stage African trypanosomiasis involving the central nervous system, six bis-acridines had better EC50 ratios. However, the MIC ratio for melarsoprol was the highest of any compound screened, being at least 10 times greater than that of the best of the bis-acridines (compounds 7 and 9).
T. cruzi amastigotes.
The bis-acridine library was screened against the intracellular amastigote stage of T. cruzi and bioactivity measured as the increase in survival time of J774 host macrophages in the presence of compound relative to that in cultures treated with DMSO. All bis-acridines were ineffective at 5 μM, and compound 3 was directly cytotoxic to macrophages. At 10 μM, most compounds were cytotoxic; only compound 15 prevented lysis of macrophages 5 days after infection. However, the effect was trypanostatic rather than trypanocidal, with amastigotes persisting for 22 days postinfection, at which time macrophage lysis occurred. In contrast, 10 μM of the cysteine protease inhibitor K11777 cleared the macrophages of amastigotes.
L. donovani promastigotes.
In the presence of 10 μM of each compound, differential bioactivity was evident against L. donovani promastigotes. Table 4 compares the bioactivities of the bis-acridines at the 72-h time point, at which time the maximum differential bioactivities were recorded. As found for P. falciparum and T. b. brucei, compound 1 was relatively ineffective, as were the compounds with an acylated linker (compounds 11 to 13). In contrast, whereas the piperazinyl compound 9 was potent against P. falciparum and T. b. brucei, it displayed only 46% lethality against L. donovani promastigotes. Also of note was the alkyl-linked aza-acridine compound 18, which was less potent than the parent alkyl-linked compound 14. The peptidomimetic cysteine protease inhibitor K11777 was ineffective at 10 μM. At the same concentration, the antibiotic and a current therapy for leishmaniasis, amphotericin B, were 100% lethal.
TABLE 4.
Bioactivity of bis-acridine compounds against L. donovani promastigotes
| Compound | % Parasite deatha |
|---|---|
| 1 | 47 |
| 2 | 95 |
| 3 | 96 |
| 4 | 99 |
| 5 | 73 |
| 6 | 98 |
| 7 | 99 |
| 8 | 99 |
| 9 | 46 |
| 10 | 93 |
| 11 | 16 |
| 12 | 49 |
| 13 | 37 |
| 14 | 99 |
| 15 | 99 |
| 16 | 96 |
| 17 | 99 |
| 18 | 42 |
| K11777b | 6 |
| Amphotericin B | 100 |
Parasites were incubated with 10 μM compound for 72 h, and parasite survival was quantified relative to DMSO-treated control cultures.
K11777, a peptidomimetic inhibitor of cysteine proteases (12). Each compound was tested three times, and the standard deviations never exceeded 20% of the means.
L. major amastigotes.
Peritoneal macrophages infected with L. major amastigotes were incubated for 48 h at 37°C with 10-fold dilutions of bis-acridines ranging in concentration from 0.0001 to 10 μM. Interpretation of SARs was complicated due to toxicity to the macrophages. For example, all compounds, except compounds 5 and 7, were cytotoxic at 10 μM. Compounds 2, 4, 10, and 16 were cytotoxic at 1 μM. EC50 values (means ± standard deviations) were obtained for five compounds as follows: compound 3, 0.80 ± 0.18 μM; compound 5, 3.00 ± 0.30 μM; compound 6, 0.60 ± 0.06 μM; compound 7, 6.00 ± 2.21 μM; and compound 11, 0.70 ± 0.14 μM. All other compounds were ineffective at or below 1 μM.
S. mansoni.
Bis-acridine compounds were incubated with 20-day-old schistosomes at concentrations of 1, 3, and 6 μM, and bioactivities were scored based on worm vitality (Table 5). Dead worms were shrunken, nonmotile, and darker in appearance than those in DMSO-treated cultures. After 18 h at 1 μM, no compound was bioactive. After 4 h at 3 and 6 μM, the polyamine-linked analogs, compounds 2 to 4 and 6, the N-methyl compound 7, and the aza-acridine compound 17 were effective. Polyamine compound 5 was effective only at 6 μM over the same time course. All other compounds were ineffective after 18 h at 6 μM. PZQ, the standard drug to treat schistosomiasis, was lethal to worms after 4 h at concentrations of 3 and 6 μM.
TABLE 5.
Bioactivities of bis-acridine compounds against 20-day-old S. mansoni parasites
| Compound | Score at concn (μM)a of:
|
||
|---|---|---|---|
| 1 | 3 | 6 | |
| 1 | − | − | − |
| 2 | − | ++ | ++ |
| 3 | − | ++ | ++ |
| 4 | − | ++ | ++ |
| 5 | − | − | ++ |
| 6 | − | + | ++ |
| 7 | − | ++ | ++ |
| 8 | − | − | − |
| 9 | − | − | − |
| 10 | − | − | − |
| 11 | − | − | − |
| 12 | − | − | − |
| 13 | − | − | − |
| 14 | − | − | − |
| 15 | − | − | − |
| 16 | − | − | − |
| 17 | − | + | ++ |
| 18 | − | − | − |
| PZQ | − | + | ++ |
Compounds were incubated with parasites at the indicated concentrations. After 4 h, parasite survival was scored visually as follows: −, no effect; +, dying; ++, dead. A second independent experiment yielded identical results.
E. histolytica.
After a 48-h incubation, the SAR of the bis-acridine library against E. histolytica trophozoites was atypical relative to that against the other parasites. Compounds 7, 14, 15, and 17 gave EC50 values between 0.5 and 1.0 μM. All other compounds were less potent, with EC50 values between 5.0 and 10.0 μM, including the polyamine-linked analogs, compounds 2 to 6, that demonstrated bioactivity against the other cell systems tested. The EC50 value of metronidazole, a drug used to treat amebiasis, was 2 μM.
T. vaginalis.
After 24 h with 10 μM compound, trichomonad densities were quantified in a hemocytometer. Similar to the low bioactivities recorded for the other parasites and HL-60 cells, the polyamine-linked compound 1 was inactive (Table 6). In contrast to the other cell systems, however, the polyamine compounds 2 to 6 were either ineffective (compound 5) or only partially active against T. vaginalis. Bioactivity could be restored upon N alkylation of the polyamine linker (compounds 7 and 8), with the notable exception of compound 9, which was potent against most other parasite targets. N acylation of the polyamine linker (compounds 10 to 13) yielded compounds of various potencies. The alkyl (compounds 14 and 15)- and polyether (compound 16)-linked compounds were either as bioactive as or more bioactive than the polyamine-linked equivalents (compounds 3 and 4). Bioactivity due to the polyamine linker could be increased by replacing the acridine heterocycle with an aza-acridine (compound 17 versus compound 3). However, when the polyamine linker was replaced with an alkyl, considerable bioactivity was lost (compound 18). Metronidazole, a current therapy for trichomoniasis, was 100% lethal at 1 μM.
TABLE 6.
Bioactivities of bis-acridine compounds against T. vaginalis
| Compound | % of parasites that dieda |
|---|---|
| 1 | 0 |
| 2 | 23 |
| 3 | 53 |
| 4 | 75 |
| 5 | 0 |
| 6 | 66 |
| 7 | 92 |
| 8 | 90 |
| 9 | 10 |
| 10 | 80 |
| 11 | 0 |
| 12 | 95 |
| 13 | 53 |
| 14 | 100 |
| 15 | 100 |
| 16 | 75 |
| 17 | 85 |
| 18 | 59 |
| Metronidazole | 100 |
Parasites were incubated for 24 h with compound at 10 μM or metronidazole at 1 μM, and parasite survival was quantified relative to DMSO-treated control cultures. Each compound was tested five times, and the standard deviations never exceeded 15% of the means.
DISCUSSION
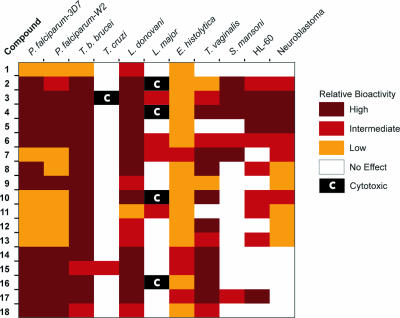
We have screened a focused library of bis-acridine compounds for bioactivity against various parasite targets and derived an SAR for the class, as summarized in Fig. 1. In developing chemotherapeutics for the treatment of parasitic infections that affect large populations, it is important to separate compounds that display general cytotoxicity from those with greater or selective bioactivity toward the given parasite target. Such bioactivity might be the result of a number of mutually nonexclusive factors, including selective uptake and/or concentration of the compound by the parasite, lower concentrations of the molecular target, a lack of functional redundancy of the target, or higher rates of mitosis relative to mammalian cells, thereby accelerating the appearance of bioactivity. Therefore, we complemented our parasite screens with cytotoxicity assays using mammalian HL-60 cells (Table 3; Fig. 1). Serving as host cells in the L. major and T. cruzi amastigote assays, macrophages also provided a useful indication of compound cytotoxicity. Finally, we incorporated into Fig. 1 previous data describing the cytotoxicity of the library in mouse neuroblastoma N2a cells (26), a cell line of relevance when considering therapies targeting late-stage T. brucei infection of the central nervous system. The accumulated screening data identified two important structural determinants to differentiate bis-acridine bioactivity from cytotoxicity, namely, the interacridine linker and the terminal acridine heterocycle.
FIG. 1.
Relative antiparasitic bioactivities and mammalian cell cytotoxicities of bis-acridine compounds. Compounds were assayed for bioactivity against the parasite indicated and scored as follows. For P. falciparum 3D7 and W2, EC50 values were categorized as high (<150 nM), intermediate (150 to 300 nM), or low (>300 nM). For T. b. brucei bloodstream forms, EC50 values were categorized as high (<10 nM), intermediate (10 to 100 nM), or low (>100 nM). For T. cruzi amastigotes, the compound at 5 μM was categorized as either having no effect or being cytotoxic to macrophages. At 10 μM, only compound 15 was trypanostatic and is indicated as “intermediate.” For L. donovani promastigotes, percentages of parasite deaths at a compound concentration of 10 μM were categorized as high (>66%), intermediate (33 to 66%), or low (<33%). For L. major amastigotes, all compounds (except 5 and 7) were toxic to macrophages at 10 μM; otherwise, EC50 values were categorized as high (<1.0 μM), intermediate (1 to 10 μM), or no effect (>10 μM). Compounds cytotoxic to macrophages at 1 μM are indicated. For E. histolytica, EC50 values were categorized as intermediate (0.5 to 1.0 μM) or low (5.0 to 10 μM). For T. vaginalis; percentages of parasite deaths at a compound concentration of 10 μM were categorized as high (>66%), intermediate (33 to 66%), low (<33%), or no effect (0). For S. mansoni 20-day-old worms, the effects of a compound concentration of 3 μM were categorized as high (dead), intermediate (dying), or no effect (viable). For HL-60 cells, EC50 values were categorized as high (100 to 200 nM), intermediate (200 to 500 nM), low (500 to 1,000 nM), or no effect (>1,000 nM). For neuroblastoma N2a cells, percent cytotoxicity of each compound at 200 nM relative to untreated control cells (26) was categorized as high (>66%), intermediate (33 to 66%), or low (<33%). The figure was prepared using the software JColorGrid (22).
Bioactivity and/or cytotoxicity of the polyamine-linked compounds 1 to 6 required a minimum length of the interacridine linker (approximately 10 Å) such that compound 1 was generally inactive toward parasites and noncytotoxic (Fig. 1). Increasing the length of the polyamine linker to yield compound 2 resulted in bioactivity against a number of parasites; however, elongation of the linker also resulted in penalties with respect to cytotoxicity against mammalian cells. Thus, the polyamine-linked compounds 2 to 6 were cytotoxic to HL-60 cells, N2a cells, and macrophages.
The bioactivity and cytotoxicity of the polyamine-linked bis-acridine compounds were independent of a compound's potential to bis-intercalate with DNA. Le Pecq et al. (25) demonstrated that polyamine-linked bis-(9-amino-6-chloro-2-methoxyacridine) compounds 3 and 5 have a suitable separation of the acridine heterocycles to bis-intercalate between adjacent nucleic acid bases of a DNA strand. However, when the interacridine linker is below a length threshold, as is the case in compound 1, bis-intercalation is inhibited and monointercalation predominates. It is presumed that the available secondary amines of the polyamine linker also contribute to DNA binding through hydrogen bonding to the phosphate backbone and/or bases. In the present study, compounds with polyamine linkers of sufficient length to potentially bis-intercalate manifested both bioactivity and cytotoxicity (e.g., compounds 3 and 6). Likewise, compounds with polyamine linkers too short to bis-intercalate were nonetheless potent (e.g., compound 2), perhaps due to monointercalation by the acridine heterocycle. Overall, given the general cytotoxicity of the polyamine-linked class, it seems unlikely that these compounds would make suitable lead compounds for further drug development.
Various structural modifications to the interacridine linker and/or the terminal acridine heterocycle were shown to alleviate cytotoxicity but, on the whole, to retain bioactivity (Fig. 1). The addition of steric bulk and conformational constraint to the polyamine linker through alkylation of the linker (compounds 7 to 9) generally reduced cytotoxicity, while acylation (compounds 10 to 13) generated compounds with diminished bioactivity (e.g., toward P. falciparum) (Table 2). In addition, alkyl (compounds 14 to 15)- and polyether (compound 16)-linked analogs were less cytotoxic than the corresponding polyamines. The bis-aza-acridine compound 17 demonstrated less cytotoxicity (except against HL-60 cells) than the equivalently linked bis-acridine compound 3 (Fig. 1), suggesting that the cytotoxicity of the polyamine linker could be mitigated by an appropriately substituted terminal heterocycle. The alkyl-linked bis-aza-acridine compound 18 was also less toxic than the polyamine class but had slightly less bioactivity than the equivalently alkyl-linked compound 14.
The bis-acridine compounds demonstrated excellent bioactivity against P. falciparum, with mid- to low-nanomolar EC50 values. Also, activity was independent of resistance to CQ. The most effective bis-acridines gave bioactivities comparable to those of the antimalarial drugs CQ and QA. These drugs are thought to act on P. falciparum by disrupting the storage of toxic heme as polymeric hematin (3, 10, 35). Given that the acridine heterocycle is a common structural feature of both QA and the bis-acridine compounds, it is conceivable that the present compounds operate via a similar mechanism. The potent compounds 9, 15, 16, and 18 were shown to be nontoxic to both HL-60 and N2a cells at the concentrations used, suggesting that further structural modification may yield more-potent and -selective antimalarial bis-acridines.
In the case of T. b. brucei, all but three of the bis-acridines were extremely effective, with EC50 values equivalent to or better than those of the current antitrypanosomal drug, melarsoprol. Three compounds (compounds 7, 9, and 17) also had the same MICs as melarsoprol, of which two, compounds 7 and 9, scored well with respect to their EC50 and MIC selectivity indices relative to HL-60 cells. With respect to N2a cells, compounds 9 and 17 were slightly toxic (10% cell death) and nontoxic at 200 nM, respectively, whereas for compound 7, 40% cell death was reported (26); this concentration is twice the MIC measured for T. b. brucei, indicating that further cytotoxicity tests are advisable. The aza-acridine compound 18 requires special notice. Despite its relatively poor EC50 value against T. b. brucei, its EC50 selectivity index with respect to HL-60 cells was the second highest recorded. The result, combined with the compound's lack of cytotoxicity toward N2a cells, suggests that further modification of this compound may improve its bioactivity against T. b. brucei.
For both P. falciparum and T. b. brucei, the piperazinyl compound 9 demonstrated encouraging therapeutic indices, thus identifying this compound as a promising starting point for further optimization. Our results support those of Girault and colleagues (17), who noted that compound 9 was bioactive against the same parasites and lacked cytotoxicity to MRC-5 embryonic lung cells (see also Table 3). In addition, the present report highlights the novel and potent antimalarial activities of compounds 16, 17, and 18, with EC50 values comparable to those recorded for CQ and QA against either P. falciparum strain.
In order to gain insight into the mechanism of action of bis-acridines against T. b. brucei, we attempted to visualize compounds 2, 7, and 9 and the monoacridine QA-mustard in live parasites by virtue of the inherent fluorescence of the acridine scaffold (results not shown). Compounds 2 and 7 were localized in the nucleus and kinetoplast of the parasite, suggestive, therefore, of binding to DNA. QA-mustard was also localized in the same structures, which is of interest given that it is an active-site-directed, irreversible inhibitor of trypanothione reductase (TryR) (31)—a cytosolic enzyme (32). Compound 9 was undetectable but had been previously localized in the nucleus of P. falciparum and in the kinetoplast of insect-stage T. cruzi (17). By contrast, in murine muscle L-6 cells, compound 9 was localized in the lysosomes (17), thus suggesting that the cell type under study is an important factor in determining the cellular localization and any possible mode of action of the specific bis-acridine.
We also tested the possibility that the present bis-acridines may target TryR (results not shown), a flavoenzyme central to the trypanothione-based redox metabolism of parasitic trypanosomatids and considered to be an important drug target (6, 13). Two π-stacked QA-mustard heterocycles bind within the active site of TryR (31). Therefore, we hypothesized that dimeric bis-acridine molecules may have the capacity to simultaneously bind adjacent acridine-binding sites in TryR, leading to improved bioactivity over that of monoacridines. However, for compounds 1, 7, 9, and 18, the 50% inhibitory concentration values in biochemical assays with TryR (performed according to reference 31) were approximately 3 to 5 orders of magnitude greater than the respective EC50 values determined with the parasite (results not shown). Also, there was no correlation between the 50% inhibitory concentration and EC50 values. Therefore, it seems unlikely that the bioactivity of the bis-acridines against T. b. brucei is as a result of binding to TryR, unless the compounds are greatly concentrated by the parasite in the appropriate cellular compartment.
The bioactivity of the bis-acridine library was tested against parasites and life cycle stages other than P. falciparum and T. b. brucei (summarized in Fig. 1). Most compounds were potent against insect-stage L. donovani promastigotes; however, against the intracellular amastigote stages of L. major and T. cruzi, the acridine compounds either were cytotoxic to the macrophage host cells or had no effect on the parasite. The indications are, therefore, that this class of compounds is not suitable for further development as antileishmanial or antichagasic compounds. For E. histolytica, four compounds (compounds 7, 14, 15, and 17) had micromolar EC50 values similar to those of the current treatment, metronidazole, with compounds 14 and 15 being noncytotoxic to both HL-60 and N2a cells. Interestingly, compounds 14 and 15 were also active (100% lethal at 10 μM) against T. vaginalis, raising the possibility that one or both of these compounds might be a useful lead to treat both amebiasis and trichomoniasis, as is the case with metronidazole. Against the bloodfluke S. mansoni, no outstanding compound was identified. Compound 17 was schistosomicidal but demonstrated differential cytotoxicity against HL-60 and N2a cells, suggesting that additional cytotoxicity screening is warranted.
In conclusion, we have taken a novel approach to identifying potentially useful antiparasitic compounds whereby a small, focused compound library is screened for bioactivity against a large number of parasite targets. Using this strategy, we have identified a number of bis-acridine compounds that demonstrate bioactivity against more than one parasite yet have relatively low cytotoxicity toward mammalian cells. These compounds may be useful starting points for drug discovery.
Acknowledgments
Research at the SCBRPD was supported by the Sandler Family Supporting Foundation and NIAID grant TDRU AI35707. The support of the Wellcome Trust (A.H.F.) and the James Irvine Foundation (K.M.L.) is acknowledged, as is that of the Department of Biological Sciences, Office of the Provost, and Office of Research and Sponsored Programs at the University of the Pacific, Stockton, CA (K.M.L.).
We thank Leslie W. Deady and coworkers of the Department of Chemistry, La Trobe University, Victoria 3086, Australia, for their assistance in the preparation of bis-acridine compounds. T.S., H.M., and A.P.-S. are grateful to Martina Schultheis for technical assistance.
Footnotes
Published ahead of print on 19 March 2007.
REFERENCES
- 1.Antonini, I., P. Polucci, A. Magnano, B. Gatto, M. Palumbo, E. Menta, N. Pescalli, and S. Martelli. 2003. Design, synthesis, and biological properties of new bis(acridine-4-carboxamides) as anticancer agents. J. Med. Chem. 46:3109-3115. [DOI] [PubMed] [Google Scholar]
- 2.Antonini, I., P. Polucci, A. Magnano, S. Sparapani, and S. Martelli. 2004. Rational design, synthesis, and biological evaluation of bis(pyrimido[5,6,1-de]acridines) and bis(pyrazolo[3,4,5-kl]acridine-5-carboxamides) as new anticancer agents. J. Med. Chem. 47:5244-5250. [DOI] [PubMed] [Google Scholar]
- 3.Auparakkitanon, S., W. Noonpakdee, R. K. Ralph, W. A. Denny, and P. Wilairat. 2003. Antimalarial 9-anilinoacridine compounds directed at hematin. Antimicrob. Agents Chemother. 47:3708-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baltz, T., D. Baltz, C. Giroud, and J. Crockett. 1985. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 4:1273-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basch, P. F. 1981. Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J. Parasitol. 67:179-185. [PubMed] [Google Scholar]
- 6.Borges, A., M. L. Cunningham, J. Tovar, and A. H. Fairlamb. 1995. Site-directed mutagenesis of the redox-active cysteines of Trypanosoma cruzi trypanothione reductase. Eur. J. Biochem. 228:745-752. [DOI] [PubMed] [Google Scholar]
- 7.Bouteille, B., O. Oukem, S. Bisser, and M. Dumas. 2003. Treatment perspectives for human African trypanosomiasis. Fundam. Clin. Pharmacol. 17:171-181. [DOI] [PubMed] [Google Scholar]
- 8.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 9.Di Giorgio, C., F. Delmas, N. Filloux, M. Robin, L. Seferian, N. Azas, M. Gasquet, M. Costa, P. Timon-David, and J. P. Galy. 2003. In vitro activities of 7-substituted 9-chloro and 9-amino-2-methoxyacridines and their bis- and tetra-acridine complexes against Leishmania infantum. Antimicrob. Agents Chemother. 47:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, T. J., R. Hunter, C. H. Kaschula, H. M. Marques, A. Misplon, and J. Walden. 2000. Structure-function relationships in aminoquinolines: effect of amino and chloro groups on quinoline-hematin complex formation, inhibition of beta-hematin formation, and antiplasmodial activity. J. Med. Chem. 43:283-291. [DOI] [PubMed] [Google Scholar]
- 11.Elslager, E. F., F. H. Tendick, L. M. Werbel, and D. F. Worth. 1969. Antimalarial and antischistosomal effects of proximal hydrazine and hydroxylamine analogs of chloroquine and quinacrine. J. Med. Chem. 12:970-974. [DOI] [PubMed] [Google Scholar]
- 12.Engel, J. C., P. S. Doyle, I. Hsieh, and J. H. McKerrow. 1998. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J. Exp. Med. 188:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairlamb, A. H., and A. Cerami. 1992. Metabolism and functions of trypanothione in the Kinetoplastida. Annu. Rev. Microbiol. 46:695-729. [DOI] [PubMed] [Google Scholar]
- 14.Fenwick, A. 2006. Waterborne infectious diseases-could they be consigned to history? Science 313:1077-1081. [DOI] [PubMed] [Google Scholar]
- 15.Gamage, S. A., D. P. Figgitt, S. J. Wojcik, R. K. Ralph, A. Ransijn, J. Mauel, V. Yardley, D. Snowdon, S. L. Croft, and W. A. Denny. 1997. Structure-activity relationships for the antileishmanial and antitrypanosomal activities of 1′-substituted 9-anilinoacridines. J. Med. Chem. 40:2634-2642. [DOI] [PubMed] [Google Scholar]
- 16.Girault, S., S. Delarue, P. Grellier, A. Berecibar, L. Maes, L. Quirijnen, P. Lemiere, M. A. Debreu-Fontaine, and C. Sergheraert. 2001. Antimalarial in-vivo activity of bis(9-amino-6-chloro-2-methoxyacridines). J. Pharm. Pharmacol. 53:935-938. [DOI] [PubMed] [Google Scholar]
- 17.Girault, S., P. Grellier, A. Berecibar, L. Maes, E. Mouray, P. Lemiere, M. A. Debreu, E. Davioud-Charvet, and C. Sergheraert. 2000. Antimalarial, antitrypanosomal, and antileishmanial activities and cytotoxicity of bis(9-amino-6-chloro-2-methoxyacridines): influence of the linker. J. Med. Chem. 43:2646-2654. [DOI] [PubMed] [Google Scholar]
- 18.Guinovart, C., M. M. Navia, M. Tanner, and P. L. Alonso. 2006. Malaria: burden of disease. Curr. Mol. Med. 6:137-140. [DOI] [PubMed] [Google Scholar]
- 19.Hammond, D. J., J. Hogg, and W. E. Gutteridge. 1985. Trypanosoma cruzi: possible control of parasite transmission by blood transfusion using amphiphilic cationic drugs. Exp. Parasitol. 60:32-42. [DOI] [PubMed] [Google Scholar]
- 20.Hirumi, H., K. Hirumi, J. J. Doyle, and G. A. Cross. 1980. In vitro cloning of animal-infective bloodstream forms of Trypanosoma brucei. Parasitology 80:371-382. [DOI] [PubMed] [Google Scholar]
- 21.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 22.Joachimiak, M. P., J. L. Weisman, and B. C. H. May. 2006. JColorGrid: software for the visualization of biological measurements. BMC Bioinformatics 7:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi, P. B., D. L. Sacks, G. Modi, and W. R. McMaster. 1998. Targeted gene deletion of Leishmania major genes encoding developmental stage-specific leishmanolysin (GP63). Mol. Microbiol. 27:519-530. [DOI] [PubMed] [Google Scholar]
- 24.Kristjanson, P. M., B. M. Swallow, G. J. Rowlands, R. L. Kruska, and P. N. de Leeuw. 1999. Measuring the costs of African animal trypanosomosis, the potential benefits of control and returns to research. Agric. Syst. 59:79-98. [Google Scholar]
- 25.Le Pecq, J. B., M. Le Bret, J. Barbet, and B. Roques. 1975. DNA polyintercalating drugs: DNA binding of diacridine derivatives. Proc. Natl. Acad. Sci. USA 72:2915-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.May, B. C., A. T. Fafarman, S. B. Hong, M. Rogers, L. W. Deady, S. B. Prusiner, and F. E. Cohen. 2003. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc. Natl. Acad. Sci. USA 100:3416-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meingassner, J. G., H. Mieth, R. Czok, D. G. Lindmark, and M. Müller. 1978. Assay conditions and the demonstration of nitroimidazole resistance in Tritrichomonas foetus. Antimicrob. Agents Chemother. 13:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merschjohann, K., F. Sporer, D. Steverding, and M. Wink. 2001. In vitro effect of alkaloids on bloodstream forms of Trypanosoma brucei and T. congolense. Planta Med. 67:623-627. [DOI] [PubMed] [Google Scholar]
- 29.Ponte-Sucre, A., Y. Campos, M. Fernandez, H. Moll, and A. Mendoza-Leon. 1998. Leishmania sp.: growth and survival are impaired by ion channel blockers. Exp. Parasitol. 88:11-19. [DOI] [PubMed] [Google Scholar]
- 30.Ruppel, A., Y. E. Shi, and N. A. Moloney. 1990. Schistosoma mansoni and S. japonicum: comparison of levels of ultraviolet irradiation for vaccination of mice with cercariae. Parasitology 101:23-26. [DOI] [PubMed] [Google Scholar]
- 31.Saravanamuthu, A., T. J. Vickers, C. S. Bond, M. R. Peterson, W. N. Hunter, and A. H. Fairlamb. 2004. Two interacting binding sites for quinacrine derivatives in the active site of trypanothione reductase: a template for drug design. J. Biol. Chem. 279:29493-29500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, K., F. R. Opperdoes, and A. H. Fairlamb. 1991. Subcellular distribution of trypanothione reductase in bloodstream and procyclic forms of Trypanosoma brucei. Mol. Biochem. Parasitol. 48:109-112. [DOI] [PubMed] [Google Scholar]
- 33.Smithers, S. R., and R. J. Terry. 1965. The infection of laboratory hosts with cercariae of Schistosoma mansoni and the recovery of the adult worms. Parasitology 55:695-700. [DOI] [PubMed] [Google Scholar]
- 34.Steverding, D., R. W. Spackman, H. J. Royle, and R. J. Glenn. 2005. Trypanocidal activities of trileucine methyl vinyl sulfone proteasome inhibitors. Parasitol. Res. 95:73-76. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan, D. J., Jr., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vennerstrom, J. L., W. Y. Ellis, A. L. Ager, Jr., S. L. Andersen, L. Gerena, and W. K. Milhous. 1992. Bisquinolines. 1. N,N-bis(7-chloroquinolin-4- yl)alkanediamines with potential against chloroquine-resistant malaria. J. Med. Chem. 35:2129-2134. [DOI] [PubMed] [Google Scholar]
- 37.Wakelin, L. P., T. S. Creasy, and M. J. Waring. 1979. Equilibrium constants for the binding of an homologous series of monofunctional and bifunctional intercalating diacridines to calf thymus DNA. FEBS Lett. 104:261-265. [DOI] [PubMed] [Google Scholar]
- 38.Weisman, J. L., A. P. Liou, A. A. Shelat, F. E. Cohen, R. K. Guy, and J. L. DeRisi. 2006. Searching for new antimalarial therapeutics amongst known drugs. Chem. Biol. Drug Des. 67:409-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werbovetz, K. A., E. K. Lehnert, T. L. Macdonald, and R. D. Pearson. 1992. Cytotoxicity of acridine compounds for Leishmania promastigotes in vitro. Antimicrob. Agents Chemother. 36:495-497. [DOI] [PMC free article] [PubMed] [Google Scholar]