Abstract
To date, only a limited number of studies have reported finding an influence of ordinary nutrients on hepatitis C virus (HCV) RNA replication. However, the effects of other nutrients on HCV RNA replication remain largely unknown. We recently developed a reporter assay system for genome-length HCV RNA replication in hepatoma-derived HuH-7 cells (OR6). Here, using this OR6 assay system, we comprehensively examined 46 nutrients from four nutrient groups: vitamins, amino acids, fatty acids, and salts. We found that three nutrients—β-carotene, vitamin D2, and linoleic acid—inhibited HCV RNA replication and that their combination caused additive and/or synergistic effects on HCV RNA replication. In addition, combined treatment with each of the three nutrients and interferon alpha or beta or fluvastatin inhibited HCV RNA replication in an additive manner, while combined treatment with cyclosporine synergistically inhibited HCV RNA replication. In contrast, we found that vitamin E enhanced HCV RNA replication and negated the effects of the three anti-HCV nutrients and cyclosporine but not those of interferon or fluvastatin. These results will provide useful information for the treatment of chronic hepatitis C patients who also take anti-HCV nutrients as an adjunctive therapy in combination with interferon. In conclusion, among the ordinary nutrients tested, β-carotene, vitamin D2, and linoleic acid possessed anti-HCV activity in a cell culture system, and these nutrients are therefore considered to be potential candidates for enhancing the effects of interferon therapy.
Hepatitis C virus (HCV) is a major pathogen of chronic hepatitis (CH) and leads to fatal liver diseases, such as liver cirrhosis and hepatocellular carcinoma (1, 23, 38). Approximately 170 million people worldwide are infected with HCV (40). Therefore, HCV infection is a global health problem. The combination of pegylated interferon (IFN) with ribavirin is currently the most effective therapy for CH C (11, 28), and long-term treatment has been shown to improve the sustained virological response (SVR) rate (37). However, the SVR rate still remains at approximately 55% (11). Combination therapy occasionally also causes adverse effects, such as severe anemia (10) and cerebral vascular lesions (7), and reduction of the dosage leads to insufficient treatment. These adverse effects are more serious in older patients. Therefore, it remains necessary to identify alternative agents that have fewer side effects to couple with IFN. In developing countries, it is difficult to administer expensive IFN therapy. Hence, in such countries, inexpensive anti-HCV reagents are especially desirable (36).
The lack of a small-animal model and a cell culture system to support efficient HCV RNA replication hampered the development of anti-HCV reagents. Since an HCV subgenomic replicon system was developed in 1999 (25), the mechanism of HCV RNA replication has been gradually elucidated by a number of groups (2). However, this subgenomic replicon system may not necessarily reflect actual HCV RNA replication in hepatocyte cell lines, due to the absence of structural proteins. To overcome this problem, a cell culture system for genome-length HCV RNA replication was developed by several groups (4, 15, 33). We also developed a genome-length HCV RNA (strain O of genotype 1b) replication system (OR6) with luciferase as a reporter, which facilitated the prompt and precise monitoring of HCV RNA replication in hepatocyte cell lines (13, 30). In the OR6 assay system, the luciferase activity was well correlated with HCV RNA levels when cells were treated with IFN-α (13). Therefore, we quantified the luciferase activity instead of HCV RNA for the indirect evaluation of HCV RNA replication, although the OR6 assay system doesn't strictly quantify the HCV RNA replication. More recently, several groups have developed cell culture systems that produce infectious viral particles (genotype 2a), which can be used to reconstruct the life cycle of HCV infection in hepatocyte cell lines (24, 39, 45).
Using this OR6 assay system, we demonstrated that mizoribine (30), as an immunosuppressant, and fluvastatin (FLV) (14), as the reagent for hypercholesterolemia, inhibited HCV RNA replication in hepatocyte cell lines. Another immunosuppressant, cyclosporine (CsA), was also identified as an anti-HCV reagent in a subgenomic replicon system (41). These results suggested the expansion of the primary application of the existing therapeutic drugs to new anti-HCV therapy.
Other studies have also revealed that certain ordinary nutrients contained in common foods can influence HCV RNA replication (5, 8, 9, 17, 21, 26). However, in these studies, only a limited number of nutrients were tested. To date, there has been no comprehensive analysis of the effects of nutrients on HCV RNA replication. Thus, we inclusively investigated the effects of nutrients on genome-length HCV RNA replication using the OR6 assay system. Here, we report finding that three nutrients, namely, β-carotene (BC), vitamin D2 (VD2), and linoleic acid (LA), inhibited HCV RNA replication and that combination treatments including each of these nutrients and CsA synergistically inhibited HCV RNA replication. Furthermore, we found that vitamin E (VE) enhanced HCV RNA replication, and that the anti-HCV activities of each of the three nutrients and CsA were abrogated by VE.
MATERIALS AND METHODS
Reagents.
Vitamin B12, vitamin K1 (VK1), vitamin K3, elaidic acid, and vaccenic acid were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). BC, vitamin A (VA), vitamin B1, vitamin B2, vitamin B3, vitamin B6, vitamin C (VC), VD2, vitamin D3 (VD3), VE, vitamin K2 (VK2), pantothenic acid, biotin, folic acid, inositol, leucine, isoleucine, valine, tryptophan, phenylalanine, tyrosine, lauric acid, palmitic acid, stearic acid, behenic acid, oleic acid, elaidic acid, LA, arachidonic acid (AA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), Fe(II)SO4, Na2SeO4, Fe(III)(NO3)3, ZnCl2, NaCl, KCl, CaCl2, PCl3, MgCl2, CuCl2, MnCl2, and IFN-α were purchased from Sigma (St. Louis, MO), and CsA and FLV were purchased from Calbiochem (Los Angeles, CA). IFN-β was kindly provided by Toray Industries, Inc. Fatty acids, except for LA-albumin and oleic acid-albumin compounds (Sigma), were compounded with fatty acid-free bovine serum albumin (Sigma) as described in a previous report (6). We treated the HCV RNA-harboring cells at various concentrations of salts [Fe(II)SO4 at 5, 25, and 50 μM, Fe(III)(NO3)3 at 10, 100, and 200 μM, ZnCl2 at 20, 50, and 100 μM, Na2SeO4 at 1, 2.5, and 5 μM, NaCl at 100, 150, and 300 μM, KCl at 5, 10, and 20 μM, CaCl2 at 2, 4, and 8 μM, PCl3 at 1, 2.5, and 5 μM, MgCl2 at 0.5, 2.5, and 5 μM, CuCl2 at 20, 50, and 100 μM, and MnCl2 at 30, 60, and 120 μM]. Dimethyl sulfoxide or ethanol was used for the dissolution of the liposoluble nutrients, and the final concentrations (0.1%) of each were equal for the cells with nutrients and those without.
Cell cultures.
OR6 cells, polyclonal genome-length HCV RNA (ORN/C-5B/KE)-replicating cells, and subgenomic replicon (ORN/3-5B/KE) cells, which were derived from hepatoma cell line, HuH-7, were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, streptomycin (designated as the control medium in this study), and G418 (300 μg/ml) (Geneticin; Invitrogen, Carlsbad, CA), and the cells were passaged twice a week at a 5:1 split ratio. ORN/C-5B/KE and ORN/3-5B/KE are derived from HCV-O (strain O of genotype 1b). OR6c cells are cured OR6 cells from which genome-length HCV RNA was eliminated by IFN-α treatment (500 IU/ml for 2 weeks) without G418, as previously described (13).
Luciferase reporter assay.
For the Renilla luciferase (RL) assay, approximately 1.0 × 104 to 1.5 × 104 OR6 cells (72-hour treatment) or 0.5 × 104 OR6 cells (120-hour treatment) were plated onto 24-well plates in triplicate and cultured for 24 h. The cells were treated with each nutrient or compound for 72 or 120 h. Then, the cells were harvested with Renilla lysis reagent (Promega, Madison, WI) and subjected to the RL assay according to the manufacturer's protocol.
Western blot analysis.
For Western blot analysis, 4 × 104 to 4.5 × 104 OR6c cells harboring HCV-O/KE/EG (strain O of genotype 1b) (K. Abe, M. Ikeda, and N. Kato, unpublished data) were plated onto six-well plates and cultured for 24 h and then were treated with each nutrient or compound for 72 h. Preparation of the cell lysates, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunoblotting were then performed as previously described (18). The antibodies used in this study were those specific to HCV core antigen (CP11; Institute of Immunology, Tokyo) and β-actin (Sigma). The epitope of CP11 was located within amino acid positions 21 to 40 of the core antigen. Immunocomplexes on the membranes were detected by enhanced chemiluminescence assay (Renaissance; Perkin Elmer Life Science, Wellesley, MA).
Cell viability.
To examine the suppressive effects of nutrients on OR6 cell viability, approximately 4.5 × 104 to 5 × 104 OR6 cells (72-hour viability assay) or approximately 1 × 104 to 1.5 × 104 cells (120-hour viability assay) were plated onto six-well plates in triplicate and were cultured for 24 h. The cells were treated without nutrients or with each nutrient for 72 or 120 h, and then the number of viable cells was counted after trypan blue dye treatment as previously described (30).
Statistical analysis and synergistic statistics.
Differences between the anti-HCV activities of the nutrients at each concentration and controls were tested using Student's t test. P values of less than 0.05 were considered statistically significant. Then, an isobologram method was used to evaluate the effects of a combination of nutrients or compounds on HCV RNA replication (21). OR6 cells were treated with each combination of nutrients or compounds at various concentrations for 72 h. The 50% effective concentration (EC50) against HCV RNA replication in each combination treatment was analyzed by sigmoid regression, and isoboles of EC50 were plotted using the resulting data.
RESULTS
Effects of ordinary nutrients on HCV RNA replication.
To date, information about the anti-HCV effects of ordinary nutrients has been limited to only a few studies, and in those studies, a plasmid (26), a subgenomic replicon (21), and recombinant HCV proteins (5, 8, 9) were used in the assays. We recently developed OR6 assay system by the selection after introducing genome-length ORN/C-5B/KE RNA (Fig. 1A) into HuH-7 cells. Our OR6 assay system renders it possible to carry out the prompt and precise evaluation of genome-length HCV RNA replication (13, 30). Therefore, we comprehensively analyzed 46 ordinary nutrients to determine their effects on HCV RNA replication using our novel OR6 assay system (Table 1). The effects of the preexistent nutrients in the medium on HCV RNA replication were under a significant level, because the concentrations of the nutrients already contained in the medium were less than a one-thousandth part of the minimum concentration in the treatment.
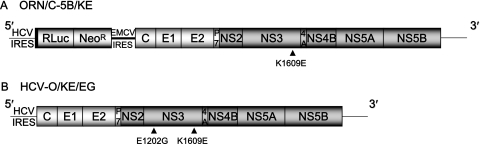
FIG. 1.
Schematic gene organization of genome-length HCV RNA. (A) ORN/C-5B/KE RNA replicated in OR6 cells. RL (RLuc) is expressed as a fusion protein with neomycin phosphotransferase (NeoR). The position of an adaptive mutation, K1609E, is indicated by a black triangle. (B) Authentic HCV RNA, HCV-O/KE/EG, was introduced into the OR6c cells by electroporation as previously described (13). The positions of two adaptive mutations, E1202G and K1609E, are indicated by black triangles. IRES, internal ribosome entry site; EMCV, encephalomyocarditis virus.
TABLE 1.
Summary of anti-HCV activities of various nutrients
| Nutrient type | Nutrient(s) with the indicated characteristic for HCVa
|
||
|---|---|---|---|
| Inhibitory | Enhancing | Ineffective | |
| Vitamins | |||
| Liposoluble | BC, VD2 | VA (retinol), VE, VK1, VK2, VK3 | VD3 |
| Water soluble | VC | VB1, VB2, VB3 (niacin), VB6, VB12, pantothenic acid, biotin, folic acid, inositol | |
| Amino acids | |||
| Branched-chain | Leucine, isoleucine, valine | ||
| Aromatic | Tryptophan | Phenylalanine, tyrosine | |
| Fatty acids | |||
| Saturated | Lauric acid (C12), palmitic acid (C16), stearic acid (C18), behenic acid (C22) | ||
| Mono-unsaturated | Oleic acid (C18; 9-unsaturated), elaidic acid (C18; trans-form of oleic acid), vaccenic acid (C18; 11-unsaturated) | ||
| Polyunsaturated | LA (C18:2, n-6), AA (C20:4, n-6), EPA (C20:5, n-3), DHA (C22:6, n-3) | ||
| Salts | Fe(II)SO4, Fe(III)(NO3)3, ZnCl2 | Na2SeO4 | NaCl, KCl, CaCl2, PCl3, MgCl2, CuCl2, MnCl2 |
Nutrients already contained in the medium are indicated in italics. VB1, vitamin B1; VB2, vitamin B2; VB3, vitamin B3; VB6, vitamin B6; VB12, vitamin B12; VK3, vitamin K3.
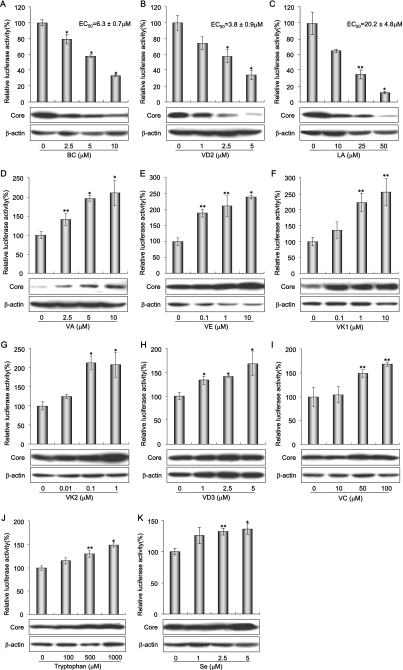
We first examined 8 liposoluble vitamins and 10 water-soluble vitamins to investigate their effects on HCV RNA replication. Among the liposoluble vitamins, VA (Fig. 2D), VE (Fig. 2E), and VK (Fig. 2F and G) significantly enhanced HCV RNA replication. However, BC and VD2 significantly inhibited HCV RNA replication in a dose-dependent manner (the mean EC50s ± standard deviations [SDs] were 6.3 ± 0.7 μM and 3.8 ± 0.9 μM, respectively) (Fig. 2A and B). In contrast with VD2, VD3 apparently enhanced relative luciferase activity, but this promotive effect was thought to result from cell proliferation, since the amount of β-actin increased in a manner similar to that of HCV core antigen (Fig. 2H). Most of the water-soluble vitamins exerted no effect on HCV RNA replication (data not shown), while only VC moderately enhanced HCV RNA replication (Fig. 2I).
FIG. 2.
Effects of ordinary nutrients on HCV RNA-replicating cells. (A through K) Reporter assay and Western blot analysis of nutrient sensitivity of HCV RNA replication. OR6 cells were treated with each nutrient at a four-grade-modulated concentration in the medium. After 72 h of treatment, the RL assay was performed as described in Materials and Methods. Shown here are the percent relative luciferase activities calculated when the RL activity of untreated cells was assigned the value of 100%. The data indicate means ± SDs of triplicate samples from at least three independent experiments. Subsequently, OR6c cells, into which authentic HCV RNA was introduced, were treated with nutrients exhibiting either inhibitory effects, i.e., BC (A), VD2 (B), and LA (C), or promotive effects, i.e., VA (D), VE (E), VK1 (F), VK2 (G), VD3 (H), VC (I), tryptophan (J), and Se (K) at the same concentrations as those used in the OR6 assay (bar graphs). After 72 h of treatment, the production of HCV core antigen was analyzed by immunoblotting using antibody specific to HCV core antigen (upper lanes). β-Actin was used as a control for the amount of protein loaded per lane (lower lanes). *, P < 0.01; **, P < 0.05.
We next examined three branched-chain amino acids and three aromatic amino acids for their effects on HCV RNA replication. We tested the six amino acids at concentrations of 0, 100, 500, and 1,000 μM, and only tryptophan exerted moderate promotive effects on HCV RNA replication (Fig. 2J).
We further examined four saturated fatty acids, three monounsaturated fatty acids, and four polyunsaturated fatty acids (PUFAs) to assess their effects on HCV RNA replication. As has been noted in previous reports (17, 21), all of the PUFAs, i.e., LA, AA, EPA, and DHA, inhibited HCV RNA replication in OR6 cells in a dose-dependent manner (the mean EC50s ± SDs were 20.2 ± 4.8 μM, 22.1 ± 1.7 μM, 36.2 ± 2.5 μM, and 37.0 ± 3.6 μM, respectively). However, we found that with the exception of LA, treatment with 50 μM of PUFA resulted in the suppression of cell growth due to cytotoxicity (data not shown). These data indicate that among PUFAs, only LA exhibited a significant inhibitory effect on HCV RNA replication without concomitant cytotoxicity (Fig. 2C and 3).
FIG. 3.
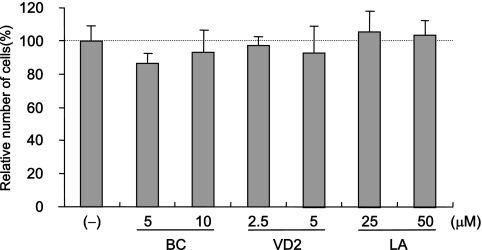
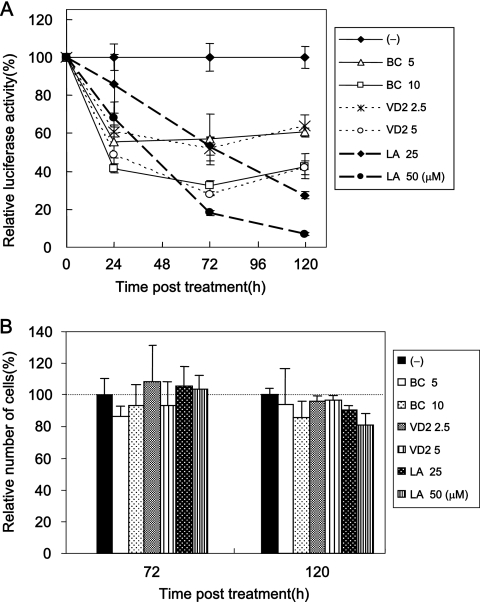
The anti-HCV activities of three nutrients were not due to the suppression of cell growth. Cell viabilities after treatment with BC, VD2, and LA are shown. OR6 cells were cultured in control medium [(−)] and in the presence of BC (5 and 10 μM), VD2 (2.5 and 5 μM), and LA (25 and 50 μM) for 72 h, and then the number of viable cells was counted after trypan blue dye treatment. Shown here are the percent relative cell numbers calculated when the relative cell number of untreated cells was assigned as 100%. The data indicate means ± SDs of triplicates from at least two independent experiments.
Finally, we examined 11 salts in order to assess their effects on HCV RNA replication. Iron [Fe(II) in the form of FeSO4 and Fe(III) in the form of Fe(NO3)3] and zinc (in the form of ZnCl2) exhibited anti-HCV effects without cytotoxicity at concentrations of up to 50% inhibition, but beyond 50% inhibition, cell growth was dose-dependently affected by the cytotoxicity of these minerals. Selenium (in the form of Na2SeO4), a typical antioxidant, slightly enhanced HCV RNA replication (Fig. 2K). We also confirmed these results using authentic HCV RNA-replicating cells (Fig. 1B and 2A through K).
These results suggest that the ordinary nutrients tested here have different profiles in terms of their effects on HCV RNA replication. The results are summarized in Table 1. Most of the nutrients were found to have no effect on HCV RNA replication. Eight nutrients enhanced HCV RNA replication, and the antioxidant nutrients VA, VC, VE, and Se were included in this group. Among the 46 nutrients tested with the OR6 assay system, we found that BC, VD2, and LA exerted anti-HCV effects without cytotoxicity. To the best of our knowledge, this is the first study to demonstrate the anti-HCV effects of BC and VD2. Therefore, we focused on the anti-HCV effects of BC, VD2, and LA in the following study.
The effects of BC, VD2, LA, and VE on polyclonal genome-length and subgenomic HCV RNA replication.
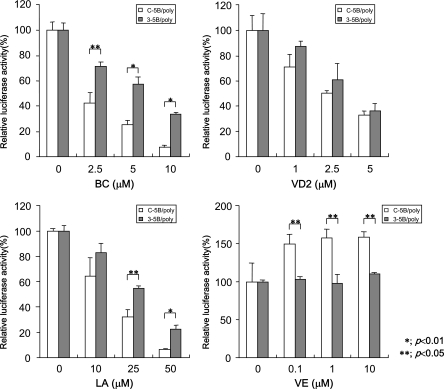
OR6 cells are among the cloned cell lines selected by G418. Therefore, we examined polyclonal cells harboring genome-length HCV RNA (ORN/C-5B/KE/poly) to exclude the possibility that the anti-HCV effects of BC, VD2, and LA were an OR6 clone-specific phenomenon. Furthermore, polyclonal cells harboring subgenomic HCV RNA (ORN/3-5B/KE/poly) were also used to examine the effects of the anti-HCV nutrients on RNA replication in the absence of the structural HCV proteins. The results revealed that all of these three nutrients exhibited a dose-dependent suppression of HCV RNA replication in both cell systems, although the three nutrients had stronger anti-HCV effects in the polyclonal genome-length HCV RNA-replicating cells than they did in the subgenomic HCV RNA-replicating cells (Fig. 4). These results indicated that the anti-HCV activities of these nutrients were not due to cell clonality, and the sensitivities of the reagents were found to differ between subgenomic and genome-length HCV RNA-replicating cells. One possible explanation of this difference is that the different genome sizes of subgenomic (9-kb) and genome-length (12-kb) HCV RNA might affect the replication efficiencies and lead to the difference in the sensitivities of antiviral reagents. These differences were significant, especially in BC- and LA-treated cells. A subgenomic replicon system may underestimate the effects of anti-HCV reagents and therefore might fail to identify potentially effective anti-HCV reagents. Therefore, our genome-length HCV RNA replication system (OR6) is advantageous for evaluating anti-HCV candidates.
FIG. 4.
The effects of BC, VD2, LA, and VE on polyclonal genome-length and subgenomic HCV RNA replication. Both polyclonal genome-length HCV RNA-replicating cells (ORN/C-5B/KE/poly) and subgenomic replicon cells (ORN/3-5B/KE/poly) were treated with BC, VD2, LA, or VE according to the same protocol as that used for the OR6 assay. The RL assay was performed at 72 h postapplication, and then RL activity was calculated as described in the legend to Fig. 2.
We also tested VE's effect on subgenomic and genome-length HCV RNA-replicating cells. VE enhanced the replication of genome-length HCV RNA. However, interestingly, VE did not affect subgenomic HCV RNA replication. These results suggest that the subgenomic HCV RNA replication system may not be able to evaluate the reagent-enhancing HCV RNA replication.
Anti-HCV activities of three nutrients were not due to inhibition of cell growth.
Since it has been reported that HCV RNA replication is dependent on cell growth (34), we examined whether the anti-HCV activities of three nutrients were due to their respective cytotoxicities. OR6 cells were treated with each nutrient (BC, 5 and 10 μM; VD2, 2.5 and 5 μM; LA, 25 and 50 μM) for 72 h. These results suggest that the anti-HCV effects of BC, VD2, and LA are not due to their cytotoxicities.
Time course assay of inhibitory effects of three nutrients on HCV RNA replication.
A kinetics analysis of the anti-HCV effects of reagents provides information about inhibitory mechanisms and optimized drug administration. Therefore, we conducted a time course assay (24, 72, and 120 h after treatment) of the anti-HCV effects of three nutrients, BC, VD2, and LA, using our OR6 assay system. The results revealed that BC and VD2 exhibited stronger inhibition of HCV RNA replication than did LA at 24 h after treatment. However, the anti-HCV activities of BC and VD2 only slightly increased at 72 or 120 h after treatment (Fig. 5A). On the other hand, LA inhibited HCV RNA replication in dose- and time-dependent manners. It is noteworthy that about 90% inhibition of RL activity was observed at 120 h after LA (50 μM) treatment of OR6 cells (Fig. 5A).
FIG. 5.
Time course assay of the anti-HCV activities of three nutrients. (A) Time course of the inhibitory effects of three nutrients on HCV RNA replication. OR6 cells were treated with control medium [(−)], BC (5 and 10 μM), VD2 (2.5 and 5 μM), or LA (25 and 50 μM), and the RL assay was performed at 24, 72, and 120 h postapplication. Relative RL activity was calculated as described in the legend to Fig. 2. (B) Time course of cell viability after the application of three nutrients. OR6 cells were cultured in the control medium and in the presence of BC (5 and 10 μM), VD2 (2.5 and 5 μM), or LA (25 and 50 μM), and at 72 and 120 h postapplication, the number of viable cells was counted after trypan blue dye treatment. Shown here are the percent relative cell numbers calculated as described in the legend to Fig. 3.
We examined whether these reductions in relative RL activity induced by all three nutrients at 120 h were due to the suppression of cell growth. Compared to the number of untreated cells, at 120 h after treatment with each nutrient (BC, 5 and 10 μM; VD2, 2.5 and 5 μM; LA, 25 and 50 μM), no significant reduction in the number of treated cells was observed (Fig. 5B). These results indicate that the anti-HCV effects of these three nutrients were not due to their respective cytotoxicities.
HCV RNA replication was additively inhibited by each combination of three nutrients and was synergistically inhibited by all three.
As described above, we found that BC, VD2, and LA possessed anti-HCV activities. However, these nutrients appeared to be insufficient for eliminating HCV by mono-treatment. Therefore, we examined the anti-HCV effects of two or three nutrients in combination.
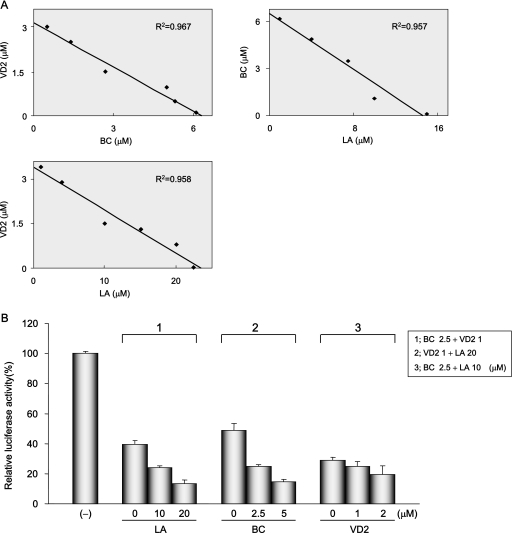
To evaluate the effects of each combination treatment, OR6 cells were cotreated with two nutrients at the listed concentrations for 72 h (BC, approximately 0 to 5 μM; VD2, approximately 0 to 3 μM; LA, approximately 0 to 20 μM). Isoboles of 50% inhibition of HCV RNA replication were obtained for each data point. An analysis of the 50% isoboles of each combination treatment graphed nearly a straight line in each case. These results indicate that the inhibitory effects of all combinations on HCV RNA replication were additive (Fig. 6A).
FIG. 6.
Effects of treatment with each combination or all of the three nutrients on HCV RNA replication. (A) Isobole plots of 50% inhibition of HCV RNA replication. OR6 cells were treated with each combination of three nutrients, BC (0, 0.5, 1, 2, 3, 4, and 5 μM), VD2 (0, 0.1, 0.5, 1, 1.5, 2, and 3 μM), and LA (0, 1, 5, 10, 15, and 20 μM), for 72 h, and RL assay was performed as described in the legend to Fig. 2 to obtain 50% isoboles. The R2 value indicates the coefficient of determination. (B) The effect of treatment with all three nutrients on HCV RNA replication was synergistic. OR6 cells were treated with LA (0, 10, and 20 μM) in addition to 2.5 μM BC plus 1 μM VD2, BC (0, 2.5, and 5 μM) in addition to 1 μM VD2 plus 20 μM LA, or VD2 (0, 1, and 2 μM) in addition to 2.5 μM BC plus 10 μM LA. After 72 h of treatment, the RL assay was performed, and then relative RL activity was calculated as described in the legend to Fig. 2.
Treatment with all three nutrients at various concentrations resulted in stronger suppression of HCV RNA replication in OR6 cells than we had predicted as an additive effect (Fig. 6B). For instance, in the sample cotreated with 2.5 μM BC (≈EC20) in addition to 1 μM VD2 (≈EC30) plus 20 μM LA (≈EC50) (Fig. 2A through C), the actual effect on HCV RNA replication was 90% inhibition, which was 20% greater than we had originally estimated (i.e., approximately 70%; 1 − 0.8 × 0.7 × 0.5 = 0.72) (Fig. 6B). In addition, no suppression of cell growth was observed during these cotreatments (data not shown). These results suggest that treatment with a mixture of these three nutrients may exert synergistic inhibitory effects on HCV RNA replication.
Treatment with each of three nutrients in combination with IFN or FLV additively inhibited HCV RNA replication, and CsA synergistically inhibited HCV RNA replication.
Recently, CsA was proposed as a novel candidate to be paired with IFN in similar studies using a cell culture system (41). We have also reported findings obtained with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, statins, exerted diverse anti-HCV effects, and FLV was found to exert the strongest inhibitory effect on HCV RNA among the statins tested (14).
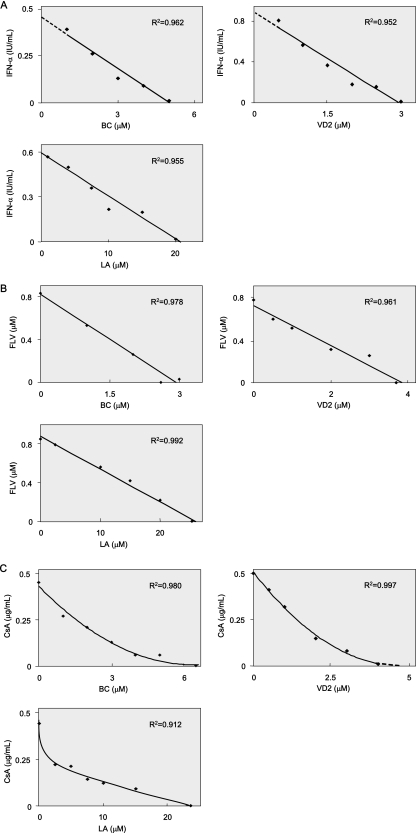
Therefore, we examined the anti-HCV effects of each of three nutrients in combination with IFN, FLV, or CsA by using OR6 cells. OR6 cells were treated for 72 h with IFN-α (0, 0.2, 0.5, and 1 IU/ml) in combination with each of the nutrients at various concentrations (BC, approximately 0 to 5 μM; VD2, approximately 0 to 4 μM; LA, approximately 0 to 20 μM) (Fig. 7A). FLV (approximately 0 to 2 μM) or CsA (approximately 0 to 1 μg/ml) was also used for treatment in combination with BC, VD2, or LA at the concentration mentioned above (Fig. 7B and C). Isoboles of 50% inhibition of HCV RNA replication were generated from each sample. An analysis of 50% isoboles in combinations using each nutrient and IFN-α or FLV graphed nearly straight lines in each case, indicating that the suppressive effects of these cotreatments on HCV RNA replication were additive (Fig. 7A and B). Similar additive effects were obtained in combination with IFN-β (data not shown). It was noteworthy that all cotreatments with each nutrient and CsA resulted in curved, concave plots of 50% isoboles, thus suggesting that these combinations with CsA exerted synergistic inhibitory effects on HCV RNA replication (Fig. 7C). These results indicate that these three nutrients, administered as a supportive nutritional therapy, could potentially improve the SVR rate associated with IFN therapy alone.
FIG. 7.
Additive inhibitory effects of each of three nutrients in combination with IFN-α or FLV on HCV RNA replication, and synergistic effects observed with Cs. (A to C) Isobole plots of 50% inhibition of HCV RNA replication. OR6 cells were treated with BC (0, 1, 2, 3, 4, and 5 μM), VD2 (0, 0.5, 1, 2, 3, and 4 μM), and LA (0, 2.5, 5, 10, 15, and 20 μM) in combination with IFN-α (0, 0.2, 0.5, and 1 IU/ml) (A), FLV (0, 0.5, 1, and 2 μM) (B), or CsA (0, 0.2, 0.5, and 1 μg/ml) (C) for 72 h, and the RL assay was performed as described in the legend to Fig. 2 to obtain 50% isoboles. The R2 value indicates the coefficient of determination.
The anti-HCV activities of BC, VD2, and LA, as well as that of CsA but not those of IFN and FLV, were completely canceled by VE.
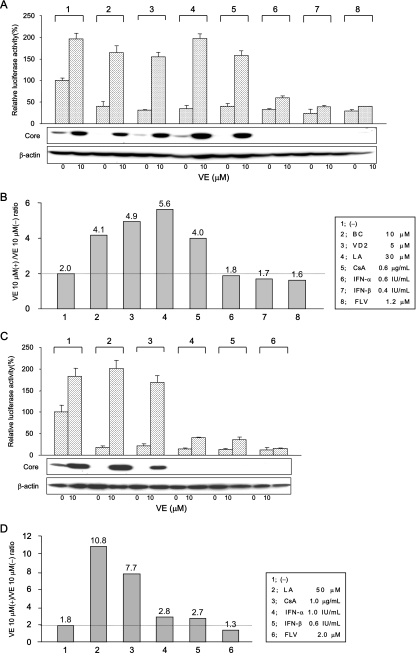
Among the 46 nutrients tested, BC and VD2 exhibited inhibitory effects on HCV RNA replication up to 70%, and LA exhibited inhibitory effects up to 90%, without exhibiting any cytotoxicity (Fig. 5A). In contrast, most of the liposoluble vitamins enhanced HCV RNA replication in OR6 cells. We used VE in the following studies because VE is one of the most common vitamins in the daily diet and it exerts a strong enhancing effect on HCV RNA replication. To clarify the mechanism of these opposing effects, we investigated whether the anti-HCV effects of BC, VD2, and LA were canceled by the addition of VE. We also tested representative anti-HCV compounds (i.e., CsA, IFN-α, IFN-β, and FLV) in combination with VE. We first examined the influence of 10 μM VE on the nutrients and compounds at the 70% inhibitory concentration level (Fig. 8A and B). The inhibitory effects of IFN-α, IFN-β, and FLV were hardly influenced by cotreatment with VE, whereas the anti-HCV effects of BC, VD2, LA, and CsA were canceled to a significant level by VE in the OR6 cells (Fig. 8A, upper panel). These results were also confirmed using authentic HCV RNA-replicating cells (Fig. 8A, lower panel). To normalize these results, we divided the luciferase value observed in the presence of VE by that in the absence of VE, and we considered this value to represent the effects of VE. When this value was larger than the value obtained in the absence of anti-HCV reagent (2.0; column 1 in Fig. 8B), we interpreted it as indicative of a reagent whose anti-HCV effects were canceled by VE. According to this criterion, BC (4.1), VD2 (4.9), LA (5.6), and CsA (4.0) were evaluated to be reagents for which the anti-HCV effects were canceled by VE (columns 2, 3, 4, and 5 in Fig. 8B). The anti-HCV effects of IFN-α, IFN-β, and FLV were not affected by VE (columns 6, 7, and 8 in Fig. 8B). We next examined the influence of 10 μM VE on the anti-HCV nutrients and compounds at the 90% inhibitory concentration level (Fig. 8C and D). BC and VD2 were not assessed in this experiment, because the maximum inhibitory effect was 70% in the case of these nutrients (Fig. 5A). Similar results were obtained in this experiment. LA (10.8) and CsA (7.7) were evaluated to be reagents for which the anti-HCV effects were canceled by VE (compare columns 2 and 3 to column 1 in Fig. 8D), although IFN-α (2.8) and IFN-β (2.7) were slightly affected by VE at this concentration (Fig. 8D, compare columns 4 and 5 to column 1). Judging by these results, it appears that BC, VD2, LA, and CsA may share some mechanism by which VE negated their anti-HCV activities.
FIG. 8.
VE canceled the anti-HCV activities of BC, VD2, LA, and CsA. (A and B) Effects of VE on the nutrients and compounds at the 70% inhibitory concentration. Both OR6 cells and OR6c cells, into which authentic HCV RNA was introduced, were treated with control medium [(−)], 10 μM BC, 5 μM VD2, 30 μM LA, 0.6 μg/ml of CsA, 0.6 IU/ml of IFN-α, 0.4 IU/ml of IFN-β, or 1.2 μM FLV in either the absence or presence of 10 μM VE for 72 h. After treatment, an RL assay of harvested OR6 cell samples was performed, and then the relative RL activity was calculated as described in the legend to Fig. 2. Subsequently, the production of HCV core antigen in OR6c cells was analyzed by immunoblotting using antibody specific to HCV core antigen. β-Actin was used as a control for the amount of protein loaded per lane (A). Then, the ratio of RL activity in the presence of 10 μM VE (+) to the RL activity in the absence of VE (−) was calculated. The horizontal line indicates the promotive effect of 10 μM VE alone on HCV RNA replication as a baseline (B). (C and D) Effects of VE on the nutrients and compounds at the 90% inhibitory concentration. Both OR6 cells and OR6c cells were treated with control medium, 50 μM LA, 1 μg/ml of CsA, 1 IU/ml of IFN-α, 0.6 IU/ml of IFN-β, and 2 μM FLV in either the absence (−) or presence (+) of 10 μM VE for 72 h. After treatment, the RL assay and Western blot analysis were performed (C), and then the ratio of RL activity in the presence of 10 μM VE to the RL activity in the absence of VE was calculated in the same manner as that described above (D).
DISCUSSION
The differential effects of BC and VA, as well as those of VD2 and VD3, which belong to the same categories as VA and VD, respectively, are of interest. We observed that whereas BC and VD2 inhibited HCV RNA replication, VA enhanced it, and VD3 exhibited basically no effect. The mechanism governing how these vitamins from the same category exert different effects on HCV RNA remains to be elucidated. However, liposoluble vitamins have been reported to exhibit various physiological activities with each nuclear receptor, consequently acting as hormone-like substances (19, 20, 27, 35). Differences in the gene products induced by each of these vitamins may lead to differences in the effects on HCV RNA replication. Another explanation might be considered in the light of findings suggesting that VA is an antioxidant, and yet recently, BC has been reported to induce oxidative stress (32, 43). This diversity of activities among vitamins in the same category, VA, might result in a variety of influences on HCV RNA replication. Further studies are still needed to account for why these different consequences are generated.
Previous studies have demonstrated that PUFAs such as AA, EPA, and DHA inhibit HCV RNA replication in cell culture systems (17, 21). However, saturated and mono-unsaturated fatty acids have been shown to enhance HCV RNA replication (17). In the prior studies, the cells tolerated the presence of PUFAs at concentrations of up to 50 μM. In contrast, in our study, 50 μM PUFAs were toxic, with the exception of LA. Furthermore, saturated and mono-unsaturated fatty acids hardly exhibited any effects on HCV RNA replication in our OR6 cell culture system. These discrepancies might be due to differences in both the clonalities of the cells and the HCV strains used in each experiment.
Here, we demonstrated that three nutrients, BC, VD2, and LA, exhibited anti-HCV effects in polyclonal genome-length and subgenomic HCV RNA (strain O of genotype 1b)-replicating cells. These results indicated that the inhibitory activities of at least three anti-HCV nutrients are not limited to a specific cell clone (OR6).
Moreover, IFN or FLV exhibited additive anti-HCV effects when the cells were cotreated with each of the three anti-HCV nutrients. However, CsA showed synergistic anti-HCV effects in combination with each of these three nutrients. Interestingly, these results coincided with the experiment using VE, as VE canceled the anti-HCV effects of CsA but not those of IFN or FLV. It was recently demonstrated that the anti-HCV effects of CsA are related to the inhibition of cyclophilin (31, 42). CsA is also known as an oxidant that can cause renal or vascular dysfunction, and interestingly, antioxidants, including VE, attenuate these CsA-induced side effects (16, 22). Furthermore, we confirmed that another antioxidant, Se, also weakened the anti-HCV effects of BC, VD2, and LA (data not shown). Therefore, BC, VD2, and LA may possess an anti-HCV mechanism similar to that of CsA, and oxidative stress may be involved in these anti-HCV effects to some extent. Among the nutrients tested, VA, VC, VE, and Se enhanced HCV RNA replication, and these nutrients functioned as antioxidants. In contrast, four PUFAs inhibited HCV RNA replication, and they served as oxidants (29, 44). These results are further evidence of the involvement of oxidative stress in HCV RNA replication.
CH C patients may take excessive doses of VE during the course of interferon therapy, because as an antioxidant, VE has been expected to prevent injury to hepatocytes caused by oxidative stress. However, our results suggest that the potentially negative effects of VE on therapy for CH C patients should be carefully considered. To date, the significance of the role played by ordinary nutrients in viral infections has not been well characterized and has even been underestimated. We believe that our findings will shed light on the field of viral infection from the perspective of the nutritional sciences.
It is difficult to determine the blood concentrations of the nutrients tested in this study because the administration conditions may affect the concentrations in the blood. Rühl et al. (35) reported that the concentrations of BC in human serum are between 0.34 to 0.54 μM and that the average concentration in the human liver is 4.4 μM. Hagenlocher et al. (12) reported that the concentration of LA in human serum is 0.8 to 11.9 μg/100 μl. Armas et al. (3) reported that the concentration of VD2 in human serum at 24 h after a 50,000-IU administration is about 50 nM. The concentration of the nutrient in this study is higher than that in those reports. Therefore, monotreatment of the nutrient may not eliminate HCV. However, these nutrients may boost the effect of IFN treatment in combination like ribavirin does.
It is worth trying to examine the effects of BC, VD2, and LA on the recently developed JFH1 infectious virus production system in a future study. Here, it remains unclear whether these three nutrients affect the production of the virus. Furthermore, the comparison of the effects of these three nutrients between HCV genotypes 1 and 2 will provide useful information for the HCV therapy, as HCV genotypes 1 and 2 respond differentially to IFN treatment.
The precise mechanism underlying the anti-HCV activities of the nutrients has not been clarified in this study. The nutrients may inhibit viral RNAs and proteins, including the internal ribosome entry site, NS3-4A serine protease, and NS5B polymerase. Further in vitro study will be needed to clarify the targets of the nutrients responsible for their anti-HCV activities. Another possibility is that the nutrients inhibit the cellular proteins required for HCV RNA replication. We are now planning a study to clarify the mechanism underlying the nutrients’ anti-HCV activities.
In conclusion, we found that three nutrients, BC, VD2, and LA, inhibited HCV RNA replication in a cell culture system and that Se, tryptophan, and various vitamins (A, C, E, and K) enhanced HCV RNA replication. The anti-HCV effects of BC, VD2, and LA were reversed by VE. These results are expected to provide useful information for the improvement of the SVR rates of patients receiving the currently standard IFN therapy. In addition, these findings may contribute to the development of a nutritional supplement specific to the treatment of people with CH C.
Acknowledgments
We thank Atsumi Morishita for her technical assistance and Yasuo Ariumi for his helpful discussions.
This work was supported by a Grant-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control and by a Grant-in-Aid for research on hepatitis, both from the Ministry of Health, Labor, and Welfare of Japan. K.A. was supported by a Research Fellowship from the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Alter, H. J., R. H. Purcell, J. W. Shih, J. C. Melpolder, M. Houghton, Q. L. Choo, and G. Kuo. 1989. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N. Engl. J. Med. 321:1494-1500. [DOI] [PubMed] [Google Scholar]
- 2.Appel, N., T. Schaller, F. Penin, and R. Bartenschlager. 2006. From structure to function: new insights into hepatitis C virus RNA replication. J. Biol. Chem. 281:9833-9836. [DOI] [PubMed] [Google Scholar]
- 3.Armas, L. A., B. W. Hollis, and R. P. Heaney. 2004. Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 89:5387-5391. [DOI] [PubMed] [Google Scholar]
- 4.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bougie, I., S. Charpentier, and M. Bisaillon. 2003. Characterization of the metal ion binding properties of the hepatitis C virus RNA polymerase. J. Biol. Chem. 278:3868-3875. [DOI] [PubMed] [Google Scholar]
- 6.Cousin, S. P., S. R. Hugl, C. E. Wrede, H. Kajio, M. G. Myers, Jr., and C. J. Rhodes. 2001. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology 142:229-240. [DOI] [PubMed] [Google Scholar]
- 7.Ferencz, S., and R. Batey. 2003. Intracerebral haemorrhage and hepatitis C treatment. J. Viral Hepat. 10:401-403. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari, E., J. Wright-Minogue, J. W. Fang, B. M. Baroudy, J. Y. Lau, and Z. Hong. 1999. Characterization of soluble hepatitis C virus RNA-dependent RNA polymerase expressed in Escherichia coli. J. Virol. 73:1649-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fillebeen, C., A. M. Rivas-Estilla, M. Bisaillon, P. Ponka, M. Muckenthaler, M. W. Hentze, A. E. Koromilas, and K. Pantopoulos. 2005. Iron inactivates the RNA polymerase NS5B and suppresses subgenomic replication of hepatitis C virus. J. Biol. Chem. 280:9049-9057. [DOI] [PubMed] [Google Scholar]
- 10.Fried, M. W. 2002. Side effects of therapy of hepatitis C and their management. Hepatology 36:S237-S44. [DOI] [PubMed] [Google Scholar]
- 11.Hadziyannis, S. J., H. Sette, Jr., T. R. Morgan, V. Balan, M. Diago, P. Marcellin, G. Ramadori, H. Bodenheimer, Jr., D. Bernstein, M. Rizzetto, S. Zeuzem, P. J. Pockros, A. Lin, and A. M. Ackrill. 2004. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 140:346-355. [DOI] [PubMed] [Google Scholar]
- 12.Hagenlocher, T., J. Nair, N. Becker, A. Korfmann, and H. Bartsch. 2001. Influence of dietary fatty acid, vegetable, and vitamin intake on etheno-DNA adducts in white blood cells of healthy female volunteers: a pilot study. Cancer Epidemiol. Biomarkers Prev. 10:1187-1191. [PubMed] [Google Scholar]
- 13.Ikeda, M., K. Abe, H. Dansako, T. Nakamura, K. Naka, and N. Kato. 2005. Efficient replication of a full-length hepatitis C virus genome, strain O, in cell culture, and development of a luciferase reporter system. Biochem. Biophys. Res. Commun. 329:1350-1359. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda, M., K. Abe, M. Yamada, H. Dansako, K. Naka, and N. Kato. 2006. Different anti-HCV profiles of statins and their potential for combination therapy with interferon. Hepatology 44:117-125. [DOI] [PubMed] [Google Scholar]
- 15.Ikeda, M., M. Yi, K. Li, and S. M. Lemon. 2002. Selectable subgenomic and genome-length dicistronic RNAs derived from an infectious molecular clone of the HCV-N strain of hepatitis C virus replicate efficiently in cultured Huh7 cells. J. Virol. 76:2997-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins, J. K., H. Huang, K. Ndebele, and A. K. Salahudeen. 2001. Vitamin E inhibits renal mRNA expression of COX II, HO I, TGFbeta, and osteopontin in the rat model of cyclosporine nephrotoxicity. Transplantation 71:331-334. [DOI] [PubMed] [Google Scholar]
- 17.Kapadia, S. B., and F. V. Chisari. 2005. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc. Natl. Acad. Sci. USA 102:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, N., K. Sugiyama, K. Namba, H. Dansako, T. Nakamura, M. Takami, K. Naka, A. Nozaki, and K. Shimotohno. 2003. Establishment of a hepatitis C virus subgenomic replicon derived from human hepatocytes infected in vitro. Biochem. Biophys. Res. Commun. 306:756-766. [DOI] [PubMed] [Google Scholar]
- 19.Kliewer, S. A., J. M. Lehmann, and T. M. Willson. 1999. Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757-760. [DOI] [PubMed] [Google Scholar]
- 20.Landes, N., P. Pfluger, D. Kluth, M. Birringer, R. Ruhl, G. F. Bol, H. Glatt, and R. Brigelius-Flohe. 2003. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 65:269-273. [DOI] [PubMed] [Google Scholar]
- 21.Leu, G. Z., T. Y. Lin, and J. T. Hsu. 2004. Anti-HCV activities of selective polyunsaturated fatty acids. Biochem. Biophys. Res. Commun. 318:275-280. [DOI] [PubMed] [Google Scholar]
- 22.Lexis, L. A., A. Fenning, L. Brown, R. G. Fassett, and J. S. Coombes. 2006. Antioxidant supplementation enhances erythrocyte antioxidant status and attenuates cyclosporine-induced vascular dysfunction. Am. J. Transplant. 6:41-49. [DOI] [PubMed] [Google Scholar]
- 23.Liang, T. J., L. J. Jeffers, K. R. Reddy, M. De Medina, I. T. Parker, H. Cheinquer, V. Idrovo, A. Rabassa, and E. R. Schiff. 1993. Viral pathogenesis of hepatocellular carcinoma in the United States. Hepatology 18:1326-1333. [PubMed] [Google Scholar]
- 24.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 25.Lohmann, V., F. Korner, J. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 26.Lott, W. B., S. S. Takyar, J. Tuppen, D. H. Crawford, M. Harrison, T. P. Sloots, and E. J. Gowans. 2001. Vitamin B12 and hepatitis C: molecular biology and human pathology. Proc. Natl. Acad. Sci. USA 98:4916-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McDonnell, D. P., D. J. Mangelsdorf, J. W. Pike, M. R. Haussler, and B. W. O'Malley. 1987. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science 235:1214-1217. [DOI] [PubMed] [Google Scholar]
- 28.McHutchison, J. G., and M. W. Fried. 2003. Current therapy for hepatitis C: pegylated interferon and ribavirin. Clin. Liver Dis. 7:149-161. [DOI] [PubMed] [Google Scholar]
- 29.Miyamoto, S., G. R. Martinez, D. Rettori, O. Augusto, M. H. Medeiros, and P. Di Mascio. 2006. Linoleic acid hydroperoxide reacts with hypochlorous acid, generating peroxyl radical intermediates and singlet molecular oxygen. Proc. Natl. Acad. Sci. USA 103:293-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naka, K., M. Ikeda, K. Abe, H. Dansako, and N. Kato. 2005. Mizoribine inhibits hepatitis C virus RNA replication: effect of combination with interferon-alpha. Biochem. Biophys. Res. Commun. 330:871-879. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa, M., N. Sakamoto, Y. Tanabe, T. Koyama, Y. Itsui, Y. Takeda, C. H. Chen, S. Kakinuma, S. Oooka, S. Maekawa, N. Enomoto, and M. Watanabe. 2005. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology 129:1031-1041. [DOI] [PubMed] [Google Scholar]
- 32.Paolini, M., A. Antelli, L. Pozzetti, D. Spetlova, P. Perocco, L. Valgimigli, G. F. Pedulli, and G. Cantelli-Forti. 2001. Induction of cytochrome P450 enzymes and over-generation of oxygen radicals in beta-carotene supplemented rats. Carcinogenesis 22:1483-1495. [DOI] [PubMed] [Google Scholar]
- 33.Pietschmann, T., V. Lohmann, A. Kaul, N. Krieger, G. Rinck, G. Rutter, D. Strand, and R. Bartenschlager. 2002. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 76:4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietschmann, T., V. Lohmann, G. Rutter, K. Kurpanek, and R. Bartenschlager. 2001. Characterization of cell lines carrying self-replicating hepatitis C virus RNAs. J. Virol. 75:1252-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rühl, R., R. Sczech, N. Landes, P. Pfluger, D. Kluth, and F. J. Schweigert. 2004. Carotenoids and their metabolites are naturally occurring activators of gene expression via the pregnane X receptor. Eur. J. Nutr. 43:336-343. [DOI] [PubMed] [Google Scholar]
- 36.Sagoe-Moses, C., R. D. Pearson, J. Perry, and J. Jagger. 2001. Risks to health care workers in developing countries. N. Engl. J. Med. 345:538-541. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-Tapias, J. M., M. Diago, P. Escartin, J. Enriquez, M. Romero-Gomez, R. Barcena, J. Crespo, R. Andrade, E. Martinez-Bauer, R. Perez, M. Testillano, R. Planas, R. Sola, M. Garcia-Bengoechea, J. Garcia-Samaniego, M. Munoz-Sanchez, and R. Moreno-Otero. 2006. Peginterferon-alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 131:451-460. [DOI] [PubMed] [Google Scholar]
- 38.Tong, M. J., N. S. el-Farra, A. R. Reikes, and R. L. Co. 1995. Clinical outcomes after transfusion-associated hepatitis C. N. Engl. J. Med. 332:1463-1466. [DOI] [PubMed] [Google Scholar]
- 39.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 20:1-16. [DOI] [PubMed] [Google Scholar]
- 41.Watashi, K., M. Hijikata, M. Hosaka, M. Yamaji, and K. Shimotohno. 2003. Cyclosporin A suppresses replication of hepatitis C virus genome in cultured hepatocytes. Hepatology 38:1282-1288. [DOI] [PubMed] [Google Scholar]
- 42.Watashi, K., N. Ishii, M. Hijikata, D. Inoue, T. Murata, Y. Miyanari, and K. Shimotohno. 2005. Cyclophilin B is a functional regulator of hepatitis C virus RNA polymerase. Mol. Cell 19:111-122. [DOI] [PubMed] [Google Scholar]
- 43.Yeh, S. L., W. Y. Wang, C. S. Huang, and M. L. Hu. 2006. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem. Biol. Interact. 160:175-182. [DOI] [PubMed] [Google Scholar]
- 44.Yin, H., E. S. Musiek, L. Gao, N. A. Porter, and J. D. Morrow. 2005. Regiochemistry of neuroprostanes generated from the peroxidation of docosahexaenoic acid in vitro and in vivo. J. Biol. Chem. 280:26600-26611. [DOI] [PubMed] [Google Scholar]
- 45.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]