Abstract
Piperaquine is being developed as a long-acting component in artemisinin combination therapies. It was highly active in vitro and drug interaction studies showed that dihydroartemisinin combinations with piperaquine, chloroquine, and amodiaquine were indifferent tending toward antagonism. Competitive uptake of radiolabeled chloroquine and dihydroartemisinin in combination with other antimalarials was observed.
Bis-4-aminoquinoline piperaquine (PPQ) and its analogues have been shown to be potent against chloroquine (CQ)-sensitive (CQS) and CQ-resistant (CQR) parasites in vitro (1, 5) and in the field (18). Artemisinin derivatives are being evaluated as combination regimens (ACTs) to treat malaria and in particular to combat multidrug-resistant Plasmodium falciparum. It is hoped that combination chemotherapy will delay or at best prevent the onset of resistance to new agents and avoid cross-resistance to existing ones (20). PPQ has been used successfully for mass prophylaxis and treatment in China (18) and is increasingly being developed as a long-acting component in ACTs (7). One recent study assessed the in vitro interaction between PPQ and dihydroartemisinin (DHA), finding antagonism for K1 (CQR) and no interaction for 3D7 (CQS) strains of P. falciparum (7). An antagonistic drug combination may compromise efficacy and possibly increase the chances of resistance developing and spreading (10), and there may be situations, such as when treatment is incomplete, in which an antagonistic interaction could become significant (7). Our aim was to assess the in vitro effects of DHA in combination with PPQ against a range of P. falciparum strains with various degrees of drug resistance and to compare these results with DHA combined with the common 4-aminoquinolines CQ and amodiaquine (AQ). In addition, we examined the effect of a range of antimalarial drugs on the in vitro uptake of radiolabeled DHA and CQ.
Dose-response assays to obtain 50% inhibitory concentration (IC50) values of individual drugs and fixed ratio combination assays were carried out as previously published (10) at 1% parasitemia and 1% hematocrit. Uptake of [3H]DHA (Moravek Biochemicals) at 1.4 Ci/mmol and [3H]CQ (DuPont NEN) at 50.4 Ci/mmol was done at 37°C for 90 min. The experiment was initiated with addition of trophozoite parasites (5% parasitemia, 1.5% hematocrit) to microtubes containing both the unlabeled antimalarial and the radiolabeled drug (12). Samples were then centrifuged through silicon oil (AnalaR; BDH) and processed (12), and the radioactivity was determined on a Beckman liquid scintillation spectrometer. Uptake was represented as a percentage of control parasitized erythrocytes minus the uptake of drug in uninfected erythrocytes (± the relative standard deviation). All experiments were repeated at least twice in triplicate.
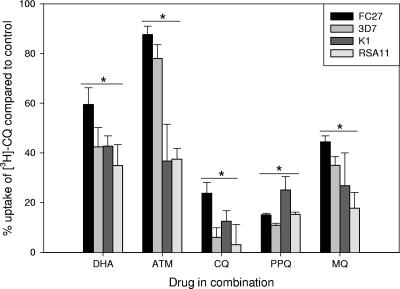
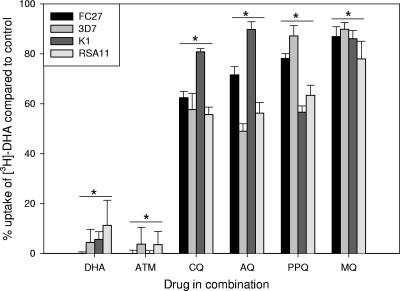
The sensitivities of eight laboratory parasite lines were assessed to a range of antimalarial drugs (Table 1). Overall, three of the parasite lines were CQS, four were CQR, and line 106/1 was moderately CQR. CQ IC50 values correlated well with CQ uptake values since all CQR parasite lines accumulated ∼4-fold less [3H]CQ (P < 0.001; data not shown) in support of previous observations (9, 19). Parasite line 106/1 had a significantly higher CQ IC50 than did other CQS lines (P ≤ 0.024), and a significant decrease in CQ susceptibility was seen with 34-1/E. The CQS 106/1 clone contains all of the “ancillary” PfCRT mutations associated with CQ resistance but lacks the crucial lys76thr mutation seen in all resistant isolates or the lys76ile PfCRT mutation in 34-1/E (8). The marked increase in CQ IC50 with the single mutation in pfcrt confirms previous observations (8, 16). This IC50 difference could be explained, in part, by the decrease in [3H]CQ uptake demonstrated in 34-1/E (data not shown). PPQ was active in all parasite lines, although some cross-resistance with CQ in CQR lines K1, RSA11, and 7G8-mdr7G8 was observed. [3H]CQ uptake (Fig. 1) was significantly reduced in all four lines by DHA (P < 0.001) and artemether (ATM; P ≤ 0.008). However, in CQS FC27 and 3D7, ATM had a significantly weaker ability to reduce [3H]CQ uptake compared to DHA (P < 0.001). In all lines, both unlabeled CQ and PPQ strongly reduced [3H]CQ uptake (P < 0.001), but mefloquine (MQ) had a lesser effect (P ≤ 0.003). The study of [3H]DHA uptake in the four lines showed no significant difference in the rate of uptake or the final amount of drug accumulated (data not shown). In all lines, artemisinins DHA and ATM blocked majority of [3H]DHA uptake (Fig. 2), while the quinolines CQ, AQ, PPQ, and MQ all significantly (P ≤ 0.001) reduced uptake. The fractional inhibitory concentration (FIC) values in Table 2 indicate that the interactions between DHA and PPQ, CQ, or AQ in the six lines tested were indifferent, tending toward antagonism. Previous findings of antagonism between artemisinin derivatives and CQ support these results (4, 11, 17).
TABLE 1.
In vitro sensitivities of eight parasite lines to the antimalarial drugs CQ, AQ, and PPQa
| Parasite | Mean in vitro sensitivity (IC50 [nmol/liter]) ± SEM
|
||
|---|---|---|---|
| CQ | AQ | PPQ | |
| CQS | |||
| FC27 | 16.48 ± 0.74 | 6.68 ± 0.38 | 29.61 ± 3.75 |
| T996 | 23.37 ± 0.21 | 14.78 ± 0.21 | 19.93 ± 2.01 |
| 3D7 | 22.76 ± 0.51 | 18.36 ± 0.52 | 36.90 ± 2.16 |
| 106/1 | 48.52 ± 5.12 | 17.48 ± 0.55 | 22.22 ± 1.78 |
| CQR | |||
| K1 | 266.58 ± 15.06 | 22.38 ± 0.73 | 49.03 ± 1.79 |
| RSA11 | 220.36 ± 6.63 | 26.66 ± 1.89 | 51.38 ± 1.68 |
| 7G8-mdr7G8 | 290.05 ± 12.09 | 32.71 ± 4.03 | 49.71 ± 1.33 |
| 34-1/E | 299.45 ± 1.60 | 17.24 ± 0.51 | 16.38 ± 1.20 |
CQR lines are regarded as an IC50 of >100 nM.
FIG. 1.
Effects of antimalarials on the uptake of 2.5 nM [3H]CQ after 90 min in erythrocytes infected with P. falciparum. Each bar represents the average of at least two experiments. An asterisk signifies a statistically significant decrease (P < 0.05) in all four parasite lines compared to drug uptake alone.
FIG. 2.
Effects of antimalarials on the uptake of 3 nM [3H]DHA after 90 min in erythrocytes infected with P. falciparum. Each bar represents the average of at least two experiments. An asterisk signifies a statistically significant decrease (P < 0.05) in all four parasite lines compared to drug uptake alone.
TABLE 2.
Mean FICs of the interactions between DHA and either PPQ, CQ, or AQa
| Strain | Mean FIC ± SEM
|
||
|---|---|---|---|
| PPQ + DHA | CQ + DHA | AQ + DHA | |
| CQS | |||
| FC27 | 1.66 ± 0.08 | 1.37 ± 0.09 | 1.34 ± 0.10 |
| 3D7 | 1.52 ± 0.16 | 1.55 ± 0.08 | 1.38 ± 0.09 |
| T996 | 1.37 ± 0.11 | 1.60 ± 0.10 | 1.35 ± 0.07 |
| CQR | |||
| K1 | 1.37 ± 0.08 | 1.36 ± 0.06 | 1.48 ± 0.09 |
| RSA11 | 1.61 ± 0.15 | 1.48 ± 0.07 | 1.56 ± 0.07 |
| 7G8-mdr7G8 | 1.47 ± 0.13 | 1.49 ± 0.09 | 1.39 ± 0.06 |
The mean FIC was determined using IC50 values. CQR lines regarded as an IC50 of >100 nM.
Competition for uptake of CQ and DHA at the same site (12) or competition for ferriprotoporphyrin (FPIX), a by-product of hemoglobin breakdown, may lead to the observed antagonism. Since it was shown that CQ, AQ, and PPQ reduce DHA accumulation, and vice versa for CQ, it may be possible that this could be a contributing factor toward the antagonism seen here. CQ binds to FPIX extremely avidly (6), and this saturable CQ uptake into the digestive vacuole has been proposed as the cause of intracellular accumulation of drug by the parasite (3). Artemisinin reacts with FPIX to form an adduct (13, 14), and molecular modeling studies have shown that a stable docked configuration of artemisinin and FPIX could exist (15). Bound DHA might sterically protect the CQ from interacting with FPIX, which could cause a decrease in CQ accumulation. Drugs that bind to FPIX have been shown to competitively inhibit CQ uptake (2). On the other hand, bound CQ would sterically protect FPIX from DHA interaction, which may protect FPIX from the free-radical producing reaction with artemisinins (21), leading to antagonism. PPQ interacts in a similar fashion with FPIX in vitro since it has been shown to prevent β-hematin formation (19a) and could therefore antagonize similarly.
It is important to understand the effect of drug combinations at the parasite level in vitro since there is concern that if drug combinations are antagonistic in vivo, the efficacy of such regimens might be compromised. It is difficult to predict in vivo drug interactions in humans based on in vitro findings, and the significance of an antagonistic interaction at typical therapeutic doses may be less apparent. However, further studies on the biochemical mechanisms behind antagonism or synergy are necessary to enhance our understanding.
Acknowledgments
We thank Piero Olliaro (World Health Organization) for supplying the PPQ, David Fidock (Albert Einstein College of Medicine, New York, NY) for 106/1 and 34-1/E, Pete Smith (Department Pharmacology, University of Cape Town, Cape Town, South Africa) for RSA11, and Pat Bray and Steve Ward (Liverpool School of Tropical Medicine, Liverpool, United Kingdom) for purifying and supplying the [3H]DHA and for 7G8-mdr7G8, originally from Alan Cowman (Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia).
Q.L.F. was supported by the Association of Commonwealth Universities, and I.S.A. was supported by Romark Research laboratory. D.C.W. thanks UK PHLS for financial support.
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Basco, L. K., and P. Ringwald. 2003. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 47:1391-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. A. Ward. 1999. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, P. G., M. Mungthin, R. G. Ridley, and S. A. Ward. 1998. Access to hematin: the basis of chloroquine resistance. Mol. Pharmacol. 54:170-179. [DOI] [PubMed] [Google Scholar]
- 4.Chawira, A. N., and D. C. Warhurst. 1987. The effect of artemisinin combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. J. Trop. Med. Hyg. 90:1-8. [PubMed] [Google Scholar]
- 5.Chen, L. 1991. Recent studies on antimalarial efficacy of piperaquine and hydroxypiperaquine. Chin. Med. J. 104:161-163. [PubMed] [Google Scholar]
- 6.Chou, A. C., R. Chevli, and C. D. Fitch. 1980. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry 19:1543-1549. [DOI] [PubMed] [Google Scholar]
- 7.Davis, T. M. E., J. Hamzah, K. F. Ilett, H. A. Karunajeewa, J. C. Reeder, K. T. Batty, S. Hackett, and P. H. Barrett. 2006. In vitro interactions between piperaquine, dihydroartemisinin, and other conventional and novel antimalarial drugs. Antimicrob. Agents Chemother. 50:2883-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fidock, D. A., T. Nomura, A. K. Talley, R. A. Cooper, S. M. Dzekunov, T. M. Ferdig, L. M. Ursos, A. B. Sidhu, B. Naude, K. W. Deitsch, X. Z. Su, J. C. Wootton, P. D. Roepe, and T. E. Wellems. 2000. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitch, C. D. 1970. Plasmodium falciparum in owl monkeys: drug resistance and chloroquine binding capacity. Science 169:289-290. [DOI] [PubMed] [Google Scholar]
- 10.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fivelman, Q. L., J. C. Walden, P. J. Smith, P. I. Folb, and K. I. Barnes. 1999. The effect of artesunate combined with standard antimalarials against chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum in vitro. Trans. R. Soc. Trop. Med. Hyg. 93:429-432. [DOI] [PubMed] [Google Scholar]
- 12.Gu, H. M., D. C. Warhurst, and W. Peters. 1984. Uptake of [3H]dihydroartemisinine by erythrocytes infected with Plasmodium falciparum in vitro. Trans R. Soc. Trop. Med. Hyg. 78:265-270. [DOI] [PubMed] [Google Scholar]
- 13.Hong, Y. L., Y. Z. Yang, and S. R. Meshnick. 1994. The interaction of artemisinin with malarial hemozoin. Mol. Biochem. Parasitol. 63:121-128. [DOI] [PubMed] [Google Scholar]
- 14.Meshnick, S. R., A. Thomas, A. Ranz, C. M. Xu, and H. Z. Pan. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181-189. [DOI] [PubMed] [Google Scholar]
- 15.Shukla, K. L., T. M. Gund, and S. R. Meshnick. 1995. Molecular modeling studies of the artemisinin (qinghaosu)-hemin interaction: docking between the antimalarial agent and its putative receptor. J. Mol. Graph. 13:215-222. [DOI] [PubMed] [Google Scholar]
- 16.Sidhu, A. B. S., D. Verdier-Pinard, and D. A. Fidock. 2002. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298:210-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stahel, E., P. Druilhe, and M. Gentilini. 1988. Antagonism of chloroquine with other antimalarials. Trans. R. Soc. Trop. Med. Hyg. 82:221. [DOI] [PubMed] [Google Scholar]
- 18.Tran, T. H., C. Dolecek, P. M. Pham, T. D. Nguyen, T. T. Nguyen, H. T. Le, T. H. Dong, T. T. Tran, K. Stepniewska, N. J. White, and J. Farrar. 2004. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomised clinical trial. Lancet 363:18-22. [DOI] [PubMed] [Google Scholar]
- 19.Verdier, F., J. Le Bras, F. Clavier, I. Hatin, and M. C. Blayo. 1985. Chloroquine uptake by Plasmodium falciparum-infected human erythrocytes during in vitro culture and its relationship to chloroquine resistance. Antimicrob. Agents Chemother. 27:561-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Warhurst, D. C., J. C. Craig, I. S. Adagu, R. K. Guy, P. B. Madrid, and Q. L. Fivelman. Activity of piperaquine and other 4-aminoquinoline antiplasmodial drugs against chloroquine-sensitive and -resistant blood-stages of Plasmodium falciparum. Role of beta-haematin inhibition and drug concentration in vacuolar water- and lipid-phases. Biochem. Pharmacol., in press. [DOI] [PubMed]
- 20.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 21.Wright, C. W., and D. C. Warhurst. 2002. The mode of action of artemisinin and its derivatives, p. 249-288. In C. W. Wright (ed.), Artemisia. Taylor & Francis, London, England.