Abstract
Yellow fever virus (YFV) causes 30,000 deaths worldwide, despite the availability of a vaccine. There are no approved antiviral therapies for the treatment of YFV disease in humans, and, therefore, these studies were designed to investigate the anti-YFV properties of T-1106, a substituted pyrazine, in a hamster model of YFV disease. Intraperitoneal (i.p.) treatment with 100 mg/kg of body weight/day of T-1106 starting 4 h prior to virus inoculation and continuing twice daily through 7 days post-virus inoculation (dpi) resulted in significantly improved survival, alanine aminotransferase levels in the serum, weight gain, and mean day to death. Virus titer in the liver at 4 dpi was significantly reduced in treated animals, as determined by both quantitative real-time PCR and infectious cell culture assay. No toxicity (weight loss or mortality) was observed at a dose of 100 mg/kg/day in sham-infected control animals. The observed minimal effective dose of T-1106 was 32 mg/kg/day administered either by oral or i.p. treatment. Therapeutic treatment was effective in significantly improving survival when T-1106 was administered beginning as late as 4 days after virus challenge with twice-daily treatment for 8 days at a dose of 100 mg/kg/day. With favorable safety, bioavailability, and postviral challenge treatment efficacy, T-1106 was effective in the treatment of disease in hamsters infected with YFV and should be further studied for potential use as a therapy for human YFV disease.
Yellow fever virus (YFV) continues to be a serious human health concern, causing approximately 30,000 deaths each year, despite the availability of a vaccine (17). Approximately 15% of infected individuals will develop severe disease, with a fatality rate of 20 to 50%, making this one of the most lethal viral infections of humans (8). No approved therapies specific for treatment of YFV are available. Although the virus is endemic to Africa and South America, there is potential for outbreaks of YFV outside the natural range of the virus and such imported cases have been reported (2, 7, 10). Therefore, the development of therapies is important, not only for treatment of cases in areas of endemicity but also for cases of imported viral disease and fulminant disease as a result of vaccination. A desirable therapy for YFV disease would be one that is efficacious when given after the onset of clinical signs.
Nonhuman primates have been important models for antiviral and vaccine research with YFV, although viral pathogenesis in these models tends to be more fulminant, with higher mortality rates and greater liver pathology compared with YFV disease in humans (1, 9). A hamster model of YFV disease has been characterized and is similar to infection of humans in many ways, and YFV disease in this model tends to be less virulent than infection in nonhuman primates (13, 16, 18). This hamster model has shown utility in antiviral studies and has been used for the evaluation of ribavirin and interferon alfacon-1 (6, 12).
T-1106 (3,4-dihydro-3-oxo-4-β-d-ribofuranosyl-2-pyrazinecarboxamide) (Fig. 1) is a chemical analog of T-705, a compound that has been shown to be active against influenza virus in in vivo experiments (3, 4, 14). Regarding the mechanism of action for T-705, it has been reported that T-705 can be converted to the phosphoribosylated metabolites (T-705RMP and T-705RTP) by host cellular kinases and the T-705RTP inhibits the influenza virus polymerase (4). As in the case of T-705, T-1106 has been shown to inhibit bovine viral diarrhea virus (BVDV) in vitro, and the phosphorylation of this compound was observed in several mammalian cells and in the livers of animals treated with T-1106 (5). In addition, T-1106 triphosphate also inhibited the viral RNA polymerase of hepatitis C virus in an enzyme assay. The objective of this research was to determine the efficacy of T-1106 against YFV infection in the hamster model of YFV disease.
FIG. 1.
Chemical structures of T-1106 and T-705.
MATERIALS AND METHODS
Animals.
Female Syrian golden hamsters with an average weight of 100 g were used. After a 24-h quarantine period and 7-day acclimation period, animals were randomly assigned to cages and individually marked with ear tags.
Test articles.
T-1106 was provided by Toyama Chemical Company, Ltd., Tokyo, Japan. The compound was suspended in sterile saline or 0.4% carboxymethyl cellulose at various concentrations. Ribavirin was provided by Valeant Pharmaceuticals (Costa Mesa, CA) and was used as a positive control in these studies at a dose of 50 mg/kg of body weight/day prepared in sterile saline. Interferon alfacon-1 (Infergen), a consensus-type interferon, was provided by L. Blatt (InterMune, Inc., Brisbane, CA) as an aqueous solution and used as a positive control in cell culture assays.
Virus.
The Jimenez strain of hamster-adapted YFV was obtained as a generous gift from R. B. Tesh (University of Texas Medical Branch, Galveston, TX). The virus was inoculated intraperitoneally (i.p.) into five adult female hamsters. The livers of the infected hamsters were removed 3 days post-virus injection (dpi) and homogenized in a 2× volume of sterile phosphate-buffered saline. This liver homogenate had a titer of 106.0 50% cell culture infectious doses/ml (CCID50) when titrated on Vero cells.
In vitro evaluation of T-1106.
The antiviral activities of T-1106 were evaluated in vitro by cytopathic effect (CPE) inhibition assays and were determined by visual (microscopic) examination of the cells, increase of neutral red (NR) dye uptake into cells, and virus yield reduction. This method was previously developed by Sidwell and Huffman (15). Seven concentrations of T-1106 were evaluated against the Jimenez strain of YFV in 96-well flat-bottom microplates plated with Vero or CV-1 cells. T-1106 was added 5 to 10 min prior to the addition of virus. Virus was added at 10 CCID50 per well. Tests were read after incubation at 37°C for 6 days. For NR uptake, dye (0.34% in medium) was added to plates after visual examination for 2 h, after which the dye was eluted from the cells and absorbed dye was quantified. Antiviral activity was expressed as the 50% effective concentration (EC50), and a selective index (SI) value was obtained by dividing the 50% cytotoxicity concentration (CC50), obtained from uninfected cells treated with T-1106, by the EC50.
QRT-PCR.
Primer pairs (forward, AGTTGATTCCATCTTGGGCTTC; reverse, ACCTCTTCCTCTCCATCCCATC) and TaqMan probe (5′ 6-carboxyfluorescein-CCTATGGTGGCTCATGGAAGTTGGAAGG-6-carboxy-N,N,N′,N′-tetramethyl- rhodamine-3′) specific for nucleotides 4767 to 4860 of the Asibi YFV vaccine strain (AY640589.1) were used (QIAGEN, Valencia, CA). The one-step Brilliant quantitative real-time PCR (QRT-PCR) master mix one-step kit (Stratagene, La Jolla, CA) was used for reverse transcription and amplification of YFV RNA with primers and probe at 0.2 μM. One microliter of total cellular RNA (from a total of 100 μl) extracted from infected or control tissues was used. Samples were run on a DNA Engine Opticon 2 (MJ Research, Inc., Waltham, MA). Reverse transcription of cellular RNA was performed for 30 min at 50°C, followed by PCR, which consisted of 40 cycles of 15 s at 95°C and 60 s at 61°C. Results are given in terms of relative equivalents, reflecting the amount of YFV present in the sample as extrapolated from a standard curve obtained from amplification of a dilution of total RNA obtained 2 dpi from Vero cells infected with Jimenez YFV.
Serum ALT assay.
Serum was collected antemortem by ocular sinus collection from all of the animals in each group. Alanine aminotransferase (ALT) (SGPT) reagent (Teco Diagnostics, Anaheim, CA) was used, and the protocol was altered for use in 96-well flat-bottomed microplates. A volume of 50 μl of ALT substrate was placed in each well of a 96-well plate, and 10 μl of sample was added at timed intervals. The samples were incubated for 30 min at 37°C, after which 50 μl of ALT color reagent was added to each sample and incubated for 10 min as above. A volume of 200 μl of ALT Color Developer was then added to each well and incubated for 5 min. The plate was then read at 505 nm on a spectrophotometer, and the ALT concentration was determined per the manufacturer's instructions.
Infectious cell culture assay.
Vero cells were obtained from the American Type Culture Collection, Rockville, MD, and were cultured in 96-well flat-bottom microplates 1 day before use. Tissue samples, obtained at necropsy from five infected hamsters from each group 4 dpi, were homogenized and then serially diluted at log concentrations in sterile culture medium from 10−1 to 10−8. The dilutions of tissue homogenate were added to four columns of a microplate with semiconfluent Vero cells. Plates were placed in a CO2 incubator for 6 days, after which the cells were observed microscopically for virus CPE. The virus titer of the samples was extrapolated based on tissue weight and observed titer in dilutions of tissue samples.
Experimental design for animal studies.
Hamsters were randomly assigned to groups, and 8 to 15 animals were included in each. Toxicity controls, consisting of three animals per group, were included to determine if there was any apparent toxicity associated with treatment. Normal (healthy) control animals were also included. A 10−4 dilution (102.0 CCID50) of stock virus was prepared in minimal essential medium. Hamsters were injected i.p. with 0.1 ml of the diluted virus. A solution of T-1106 at 100 mg/kg/day was prepared and kept at 4°C until use. Animals were treated i.p., twice daily (b.i.d.) for 8 days, with treatments 12 h apart. Mortality was observed daily, and weight was recorded at 0, 3, and 6 dpi. Weight change was determined by the amount of weight lost or gained between 3 and 6 dpi. Serum was taken 6 dpi to determine the level of serum ALT. To determine liver virus titer, liver samples were taken on 4 dpi from 5 hamsters (from groups of 15 animals) and the remaining 10 animals were left for mortality, weight change, and serum ALT evaluation. Ribavirin, prepared in saline at a dose of 50 mg/kg/day, was used as a positive control compound, and saline was used as a placebo control.
In the first experiment, a simple efficacy study was conducted to determine the effect of T-1106 on hamsters infected with YFV. Follow-up studies were conducted to determine minimal effective dose (T-1106 tested at 10 and 32 mg/kg/day), route of treatment (i.p. versus oral gavage [p.o.] treatment), and how long after virus challenge that drug could be effectively administered (1 through 5 dpi). The above parameters were used to determine efficacy of T-1106 in treatment of YFV disease in hamsters, and T-1106 was given as outlined in the first antiviral experiment.
Statistical analysis.
Survival data were analyzed using the Wilcoxon log-rank survival analysis (JMP Software, The Statistical Discovery Software; SAS Institute, Inc). All other statistical analysis was done using one-way Students t test.
RESULTS
T-1106 was not active in CPE reduction assays in Vero or CV-1 cells and yielded an EC50 of >100 μg/ml (>369 μM) and an SI of 1. Interferon alfacon-1 was used as a positive control and had an EC50 of <0.003 μg/ml and an SI of >800 in CV-1 cells. No overt toxicity was observed as determined by CPE of uninfected cells treated with concentrations of T-1106 as high as 100 μg/ml (data not shown).
Intraperitoneal treatment with 100 mg/kg/day of T-1106, beginning 4 h prior to virus challenge and continuing b.i.d. through 7 dpi, significantly improved survival in hamsters challenged with YFV (Table 1). Animals treated with T-1106 did not appear to be sick, in contrast to placebo-treated infected controls, which displayed ruffled fur, hunching, and lethargy. T-1106 was also effective in significantly improving serum ALT levels and weight change compared with placebo treatment. T-1106 appeared to be well tolerated in the toxicity controls in all studies, with all animals surviving and exhibiting host weight gain (Table 1).
TABLE 1.
Effect of i.p. and p.o. T-1106 treatmenta on Syrian golden hamsters challenged with yellow fever virusb
| Treatment | Result forc:
|
|||||
|---|---|---|---|---|---|---|
| Toxicity controls
|
Infected and treated
|
|||||
| No. alive/total | Mean wt changed (g) ± SDe | No. alive/total | MDDf ± SD | Mean serum ALT concn (IU/liter) ± SD | Mean wt changed (g) ± SD | |
| Expt 1 | ||||||
| T-1106 (100 mg/kg/day i.p.) | 3/3 | 5.3 ± 3.2 | 12/12** | >21 ± 0.0*** | 56 ± 5** | 7.1 ± 1.8** |
| Saline | 3/3 | 6.0 ± 2.0 | 5/12 | 9.0 ± 2.7 | 127 ± 99 | −0.9 ± 7.1 |
| Expt 2 | ||||||
| T-1106 | ||||||
| 32 mg/kg/day i.p. | 3/3 | 8.3 ± 4.0 | 10/10** | >21 ± 0.0*** | 65 ± 14*** | 5.2 ± 1.8** |
| 10 mg/kg/day i.p. | —g | — | 8/10 | 8.0 ± 1.4 | 174 ± 97 | −1.8 ± 7.7 |
| Saline | 3/3 | 5.3 ± 2.9 | 5/10 | 6.6 ± 0.5 | 230 ± 88 | −5.7 ± 9.0 |
| Normal control | 3/3 | 4.3 ± 2.1 | — | — | — | — |
| Expt 3 | ||||||
| T-1106 | ||||||
| 100 mg/kg/day p.o. | — | — | 10/10** | >21 ± 0.0*** | 56 ± 3.3*** | 6.3 ± 3.0*** |
| 32 mg/kg/day p.o. | — | — | 10/10** | >21 ± 0.0*** | 61 ± 4.8** | 4.4 ± 4.0** |
| Ribavirin (50 mg/kg/day i.p.) | — | — | 7/8** | 6.0 ± 0.0 | 120 ± 52 | 1.3 ± 4.0* |
| Saline | 3/3 | 4.0 ± 2.6 | 2/10 | 6.8 ± 1.0 | 168 ± 115 | −4.9 ± 6.9 |
| Normal control | 3/3 | 6.7 ± 4.0 | — | — | — | — |
Treated b.i.d. for 7 days beginning at −4 h.
Jimenez strain (10 CCID50/animal).
***, P ≤ 0.001, **, P ≤ 0.01, and *, P < 0.05, compared with saline-treated controls.
Difference between weights on 3 and 6 dpi.
Standard deviation (SD) was derived from a single experiment with the number of animals indicated in “No. alive/total” column.
MDD, mean day to death of mice dying prior to 21 dpi.
—, not applicable.
Treatment with 32 mg/kg/day under the same treatment regimen described above resulted in complete survival of all animals and significantly improved serum ALT levels and weight gain (Table 1). None of the hamsters treated with 100 or 32 mg/kg/day had icteric serum, which is a disease sign commonly seen in YFV-infected animals. Treatment with 10 mg/kg/day resulted in a survival rate of 80%, but this was not significantly higher than survival observed in saline-treated controls (50%). None of the other disease parameters were significantly improved in animals treated with 10 mg/kg/day of T-1106. Half of the serum samples from the group of hamsters treated with 10 mg/kg/day were icteric and had a statistically higher average serum ALT of 253 ± 64 IU/liter (n = 5), as compared with the nonicteric serum samples from the same group that had an average of 94 ± 36 IU/liter (n = 5) (P < 0.01). Oral treatment with T-1106 at 100 or 32 mg/kg/day, administered as described above, resulted in significant improvement of disease parameters and appeared to be as effective as i.p. treatment at the same dose (Table 1). Ribavirin treatment at a dose of 50 mg/kg/day, given at the same treatment schedule as T-1106, resulted in a significant improvement in survival and weight change.
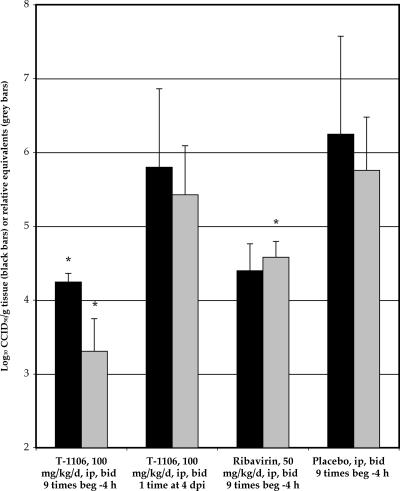
Liver samples were taken 4 dpi to determine the effect of T-1106 treatment on YFV titer. A significant decrease in liver titer, as determined by QRT-PCR and infectious cell culture assay, was observed in animals treated with T-1106, beginning 4 h before virus challenge and continuing with twice-daily treatment through 4 dpi (Fig. 2). Treatment with T-1106 beginning 4 dpi did not result in a reduction of liver virus, but this was not surprising, since animals had been treated only once with T-1106 before liver samples were taken. A significant reduction of YFV titer was also observed with ribavirin treatment beginning before virus challenge by QRT-PCR, but not by infectious cell culture assay, the latter because of more sample variation (Fig. 2).
FIG. 2.
Infectious cell culture assay (black bars) or QRT-PCR (gray bars) detection of virus titer from liver samples taken 4 dpi from Syrian golden hamsters infected with YFV and treated with T-1106, with treatment beginning (beg.) −4 h (9 treatments prior to necropsy) or 4 dpi (1 treatment prior to necropsy), or ribavirin initiated −4 h (9 treatments prior to necropsy) (*, P < 0.05 compared with respective placebo treatment group). Data are from one experiment (n = 5).
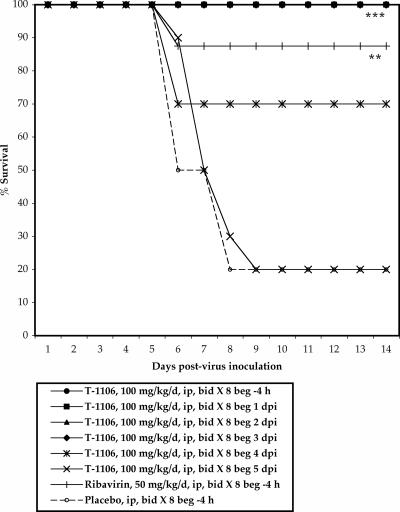
Animals were treated twice daily with 100 mg/kg/day of T-1106 beginning at various times after virus challenge, administered through i.p. injection. Significant improvement in survival, mean day to death, and weight change was observed in animals treated up to 3 dpi with T-1106 (Fig. 3 and Table 2). Serum ALT levels were reduced in groups with treatment initiated 1, 2, or 3 dpi, but this reduction was not statistically significant in the group where treatment was initiated 3 dpi. A significant improvement in survival was also observed with T-1106 treatment initiated 4 dpi, although other disease parameters were not significantly improved. There was a slight reduction in serum ALT in animals treated with T-1106 beginning 4 dpi as compared with placebo treatment, although this reduction was not statistically significant (Table 2). Treatment initiated 5 dpi with T-1106 did not significantly improve any disease parameters.
FIG. 3.
Survival of Syrian golden hamsters infected with yellow fever virus and treated i.p. with 100 mg/kg/day of T-1106 at various times after virus challenge (***, P < 0.001, and **, P < 0.01, compared with placebo). beg, beginning of treatment. Data were compiled from two separate experiments (Table 2, experiments 3 and 4). T-1106 groups, n = 10 animals/group; ribavirin group, n = 18 animals; placebo group, n = 20 animals.
TABLE 2.
Effect of post-virus-exposure, i.p. T-1106 treatment on Syrian golden hamsters challenged with yellow fever virusa
| Treatment | Beginning of treatment schedule (b.i.d, 8 days) | Result forb:
|
|||||
|---|---|---|---|---|---|---|---|
| Toxicity controls
|
Infected and treated
|
||||||
| No. alive/total | Mean wt. changec (g) ± SDd | No. alive/total | MDDe ± SD | Mean serum ALT concn (IU/liter) ± SD | Mean wt changec (g) ± SD | ||
| Expt 3 | |||||||
| T-1106 (100 mg/kg/day) | −4 h | 3/3 | 8.0 ± 2.8 | 10/10*** | >21 ± 0.0*** | 58 ± 2.6*** | 6.0 ± 4.0** |
| 1 dpi | —f | — | 10/10*** | >21 ± 0.0*** | 56 ± 5.7*** | 6.1 ± 5.4** | |
| 2 dpi | — | — | 10/10*** | >21 ± 0.0*** | 57 ± 7.4*** | 6.3 ± 2.2*** | |
| 3 dpi | — | — | 10/10*** | >21 ± 0.0*** | 79 ± 23 | 2.9 ± 4.5** | |
| Ribavirin (50 mg/kg/day) | −4 h | — | — | 7/8** | 6.0 ± 0.0 | 120 ± 52 | 1.3 ± 4.0* |
| Saline | −4 h | 3/3 | 4.0 ± 2.6 | 2/10 | 6.8 ± 1.0 | 168 ± 115 | −4.9 ± 6.9 |
| Normal control | — | 3/3 | 6.7 ± 4.0 | — | — | — | — |
| Expt 4 | |||||||
| T-1106 (100 mg/kg/day) | −4 h | — | — | 10/10*** | >21 ± 0.0*** | 55 ± 8.1** | 7.2 ± 2.1** |
| 4 dpi | — | — | 7/10** | 6.0 ± 0.0 | 108 ± 42 | −4.7 ± 8.8 | |
| 5 dpi | — | — | 2/10 | 7.4 ± 0.9 | 205 ± 81 | −6.9 ± 4.2 | |
| Ribavirin (50 mg/kg/day) | −4 h | — | — | 9/10*** | 9.0 ± 0.0 | 98 ± 17** | 4.1 ± 2.5** |
| Saline | −4 h | — | — | 1/10 | 7.0 ± 1.2 | 183 ± 69 | −5.2 ± 5.0 |
| Normal control | — | 3/3 | 7.7 ± 1.2 | — | — | — | — |
Jimenez strain (10 CCID50/animal).
***, P ≤ 0.001, **, P ≤ 0.01, and *, P < 0.05, compared with saline-treated controls.
Difference between weights on 3 and 6 dpi.
Standard deviation was derived from a single experiment with the number of animals indicated in “No. alive/total” column.
MDD, mean day to death of hamsters dying prior to 21 dpi.
—, not applicable.
DISCUSSION
Treatment with T-1106 is effective in improving disease parameters in hamsters infected with YFV. Prophylactic treatment with T-1106 improved survival, mean day to death, serum ALT levels, and weight change. Pretreatment also significantly reduced virus load in the liver, as determined by QRT-PCR and infectious cell culture assay, indicating that treatment with T-1106 reduces infectious virus as well as total viral RNA in the liver. T-1106 may have a direct effect on virus replication, which would be consistent with previous studies that showed efficacy of the triphosphate form of the compound in the direct inhibition of the RNA polymerase of hepatitis C virus (5). The titer reduction could also be an indirect result of an alternative, unknown mechanism of T-1106, and future experiments will be conducted to delineate the mode of antiviral action.
A correlation between high serum ALT, icteric serum, and mortality has been previously observed in this hamster model of YFV disease (6), which could be due to virus-induced cytopathogenesis in the liver (11). None of the samples from animals treated prior to virus challenge with T-1106 were icteric, and all of the ALT levels were similar to values in uninfected hamsters. Treatment initiated 4 dpi did not result in a reduction of virus in the liver, which was likely due to the single treatment received before liver samples were taken, and no improvement in weight change or serum ALT levels was observed 6 dpi, although there was a significant increase in survival. The lack of significant reduction of virus titer, weight change, and serum ALT was likely due to the short interval between treatment initiation and sample collection.
The endpoint of efficacy of T-1106 appeared to be between 32 and 10 mg/kg/day; although not statistically significant, there was a trend towards survival in hamsters treated with 10 mg/kg/day. The lack of statistical significance may be due to the number of animals used per group. Using 32 mg/kg/day as the minimal effective dose and the well-tolerated dose of 400 mg/kg/day (Toyama Chemical Company, Ltd.; personal communication) as the maximum tolerated dose, the selective index of T-1106 would be 12.5 in hamsters. It is important for an antiviral compound to be effective in treating disease after clinical symptoms are apparent. In this hamster model of YFV infection, disease symptoms such as liver pathology, including hepatocytic apoptosis and lymphocytic hyperplasia in the spleen, and elevated serum ALT are observed 3 to 4 dpi (13, 16). Virus titers also peak in different organs on 4 dpi (6). There was a slightly diminished effect of T-1106 in the reduction of serum ALT when treatment was initiated 3 dpi, although the lack of statistical significance was likely due to high variability in placebo-treated controls, which is a limitation of the model (6). These results indicate that T-1106 is effective when administered 3 dpi, which correlates with the onset of initial clinical symptoms in hamsters.
Route of administration of compounds is important in clinical studies, and it is important to determine the optimal route for treatment. Although i.p. treatment was generally used for administration of T-1106 in these studies, p.o. treatment was just as effective. Oral treatment will be important for the clinical use of T-1106 in human patients, and it is important to note that this route is also effective in this hamster model.
It is unknown why such favorable efficacy in vivo was observed in light of the lack of in vitro activity. It is possible that the CV-1 and Vero cell lines used for in vitro studies cannot convert the compound into the active form needed for antiviral activity. T-1106 has been shown to be active in cell culture against BVDV in MDBK cells with an SI of 207 (CC50, 2,900 μM; EC90, 14 μM) and is presumed to inhibit the BVDV polymerase after being converted to the triphosphate in cells (5). In light of these results, T-1106 was tested in this hamster model of YFV, where it proved to be active against the virus.
T-1106 and other chemically related compounds have broad antiviral activity, low toxicity, and favorable efficacy in vivo, and thus may be promising candidates for therapy in human patients (3, 4). The efficacy of T-1106 in hamsters infected with YFV represents an important development in the progress toward developing effective therapies for the treatment of disease in humans infected with this virus.
Acknowledgments
We thank Robert Tesh for providing the Jimenez hamster-adapted YFV strain. We also thank Luci Wandersee, Maysun Ali, and Jeremy Strange for their work with treatments and data collection.
This work was supported by contracts NO1-AI-15435 and NO1-AI-30048 from the Virology Branch, NIAID, NIH.
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Arroyo, J. I., S. A. Apperson, C. B. Cropp, B. J. Marafino, Jr., T. P. Monath, R. B. Tesh, R. E. Shope, and M. A. Garcia-Blanco. 1988. Effect of human gamma interferon on yellow fever virus infection. Am. J. Trop. Med. Hyg. 38:647-650. [PubMed] [Google Scholar]
- 2.Bae, H. G., C. Drosten, P. Emmerich, R. Colebunders, P. Hantson, S. Pest, M. Parent, H. Schmitz, M. A. Warnat, and M. Niedrig. 2005. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J. Clin. Virol. 33:274-280. [DOI] [PubMed] [Google Scholar]
- 3.Furuta, Y., K. Takahashi, Y. Fukuda, M. Kuno, T. Kamiyama, K. Kozaki, N. Nomura, H. Egawa, S. Minami, Y. Watanabe, H. Narita, and K. Shiraki. 2002. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 46:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furuta, Y., K. Takahashi, M. Kuno-Maekawa, H. Sangawa, S. Uehara, K. Kozaki, N. Nomura, H. Egawa, and K. Shiraki. 2005. Mechanism of action of T-705 against influenza virus. Antimicrob. Agents Chemother. 49:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furuta, Y., K. Takahashi, M. Maekawa, H. Maegawa, H. Egawa, and N. Terashima. 2004. T-1106, a novel pyrazine nucleoside, hepatitis C virus polymerase inhibitor, abstr. F-487, p. 27. Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother.
- 6.Julander, J. G., J. D. Morrey, L. M. Blatt, K. Shafer, and R. W. Sidwell. 2007. Comparison of the inhibitory effects of interferon alfacon-1 and ribavirin on yellow fever virus infection in a hamster model. Antivir. Res. 73:140-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland, J. M., L. M. Baddour, J. E. Nelson, S. K. Elkins, R. B. Craven, B. C. Cropp, G. J. Chang, A. D. Grindstaff, A. S. Craig, and R. J. Smith. 1997. Imported yellow fever in a United States citizen. Clin. Infect. Dis. 25:1143-1147. [DOI] [PubMed] [Google Scholar]
- 8.Monath, T. P. 2005. Yellow fever vaccine. Expert Rev. Vaccines 4:553-574. [DOI] [PubMed] [Google Scholar]
- 9.Monath, T. P., J. Arroyo, I. Levenbook, Z.-X. Zhang, J. Catalan, K. Draper, and F. Guirakhoo. 2002. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J. Virol. 76:1932-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen, L. R., and A. A. Marfin. 2005. Shifting epidemiology of Flaviviridae. J. Travel Med. 12(Suppl. 1):S3-S11. [DOI] [PubMed] [Google Scholar]
- 11.Quaresma, J. A., V. L. Barros, C. Pagliari, E. R. Fernandes, F. Guedes, C. F. Takakura, H. F. Andrade, Jr., P. F. Vasconcelos, and M. I. Duarte. 2006. Revisiting the liver in human yellow fever: virus-induced apoptosis in hepatocytes associated with TGF-beta, TNF-alpha and NK cells activity. Virology 345:22-30. [DOI] [PubMed] [Google Scholar]
- 12.Sbrana, E., S. Y. Xiao, H. Guzman, M. Ye, A. P. Travassos da Rosa, and R. B. Tesh. 2004. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am. J. Trop. Med. Hyg. 71:306-312. [PubMed] [Google Scholar]
- 13.Sbrana, E., S. Y. Xiao, V. L. Popov, P. C. Newman, and R. B. Tesh. 2006. Experimental yellow fever virus infection in the golden hamster (Mesocricetus auratus). III. Clinical laboratory values. Am. J. Trop. Med. Hyg. 74:1084-1089. [PubMed] [Google Scholar]
- 14.Sidwell, R. W., D. L. Barnard, C. W. Day, D. F. Smee, K. W. Bailey, M. H. Wong, J. D. Morrey, and Y. Furuta. 2007. Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice. Antimicrob. Agents Chemother. 51:845-851. (Epub 28 December 2006.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sidwell, R. W., and J. H. Huffman. 1971. Use of disposable micro tissue culture plates for antiviral and interferon induction studies. Appl. Microbiol. 22:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesh, R. B., H. Guzman, A. P. da Rosa, P. F. Vasconcelos, L. B. Dias, J. E. Bunnell, H. Zhang, and S. Y. Xiao. 2001. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 183:1431-1436. [DOI] [PubMed] [Google Scholar]
- 17.Tomori, O. 2004. Yellow fever: the recurring plague. Crit. Rev. Clin. Lab. Sci. 41:391-427. [DOI] [PubMed] [Google Scholar]
- 18.Xiao, S. Y., H. Zhang, H. Guzman, and R. B. Tesh. 2001. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J. Infect. Dis. 183:1437-1444. [DOI] [PubMed] [Google Scholar]