Abstract
Antifungal efficacies of the echinocandin drugs caspofungin, micafungin, and anidulafungin were reduced significantly in the presence of 50% human serum, which yielded nearly equivalent MICs or minimum effective concentrations against diverse Candida spp. and Aspergillus spp. Consistent with a direct drug interaction, serum decreased the sensitivity of glucan synthase to echinocandin drugs.
The echinocandin drugs caspofungin, micafungin, and anidulafungin inhibit the fungal β-1,3-glucan synthase enzyme, which blocks the formation of glucan polymers, thereby disrupting fungal cell wall integrity (2). Animal and human studies indicate that echinocandin drugs are extensively bound to serum proteins (1, 4, 5, 15), and serum was shown to reduce the antifungal properties of micafungin with some Candida spp. (3), yet little is known about the influence of serum on antifungal efficacy with the different echinocandin drugs.
Echinocandin susceptibility in the presence or absence of 50% human serum (Sigma) was evaluated with a diverse collection of clinical isolates, laboratory strains, and reference strains of Candida spp. and Aspergillus spp., according to the guidelines in CLSI documents M27-A2 (9) and M38-A (8), respectively. Abnormal colony morphology was used to establish a minimum effective concentration (MEC) for Aspergillus spp. after 48 h of incubation at 35°C (6). Glucan synthase (GS) isolation and 50% inhibitory concentration (IC50) inhibition kinetics were performed as described previously (11). A murine candidiasis model utilizing female BALB/c mice (age, 10 to 12 weeks; weight, 20 to 25 g) was used to assess the relative in vivo efficacies of echinocandin drugs (13).
Serum increased caspofungin MICs an average of 2-fold, with a range of 1- to 16-fold, while it had a more pronounced effect on the other drugs, increasing the MIC an average of 16-fold with a range of 8- to 256-fold for anidulafungin and an average of 64-fold with a range of 32- to 128-fold for micafungin (Table 1). The effects of serum on MICs were assessed for other non-Candida albicans spp. The largest MIC shift for caspofungin (eightfold) was with Candida krusei, while Candida tropicalis strains showed the most significant shifts (128-fold) for both micafungin and anidulafungin. These drugs consistently showed pronounced shifts, which reflected their greater antifungal potencies in the absence of serum. These differences disappeared in the presence of 50% serum, where all three drugs showed comparable MICs.
TABLE 1.
Effect of 50% serum on echinocandin MICsa of Candida spp.
| Candida sp. (nb) | Result for drug
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Caspofungin
|
Anidulafungin
|
Micafungin
|
|||||||
| MIC50
|
Fold change | MIC50
|
Fold change | MIC50
|
Fold change | ||||
| −Serum | +50% serum | −Serum | +50% serum | −Serum | +50% serum | ||||
| C. albicans (47) | ≤0.25 (0.0156-0.5) | 0.5 (0.03-2.0) | 2 (1-16) | 0.0156 (0.0156) | 0.25 (0.125-4.0) | 16 (8-256) | 0.0156 (0.0156) | 1 (0.5-2.0) | 64 (32-128) |
| C. glabratac | 0.5 (0.25-0.5) | 1 (1.0-2.0) | 4 (2.0-4.0) | 0.0156 (0.0156-0.125) | 1 (0.5-2) | 64 (16-64) | 0.0156 (0.015) | 1 (1) | 64 (64) |
| C. krusei (10) | ≤1 (0.25-2.0) | 8 (0.5-16.0) | 8 (2.0-16.0) | 0.25 (0.0156-0.25) | 8 (2.0-16.0) | 32 (16-256) | 0.06 (0.015-0.06) | 8 (2.0-16.0) | 133 (8-267) |
| C. parapsilosis (10) | 1 (0.5-2.0) | 4 (2-16.0) | 4 (2-8) | 2 (1-4) | 16 (16) | 8 (4-16) | 1 (0.125-2.0) | 16 (16) | 16-128 (8-128) |
| C. tropicalis (10) | 0.125 (0.015-0.25) | 0.5 (0.125-0.5) | 4 (1-33) | 0.0156 (0.0156-0.031) | 2 (0.125-2.0) | 128 (8-128) | 0.0156 (0.0156) | 2 (0.5-4.0) | 128 (8-256) |
All values represent averages for triplicate experiments with less than 15% variance. Numbers in parentheses represent n-fold changes relative to values for the zero-serum control.
n, no. of isolates.
C. glabrata strains grew poorly in human serum. Therefore, mouse serum (Pel-Freez, Rogers, AR) was used. −Serum, no serum.
Similar serum-induced effects were observed with a collection of Aspergillus spp., where microscopically observed MECs for the three drugs shifted higher in the presence of serum. Micafungin and anidulafungin again showed the most pronounced antifungal shifts, 32- to 133-fold and 16- to 32-fold, respectively, reflecting their more active behavior in the absence of serum (Table 2). The three drugs displayed nearly equivalent MECs in the presence of 50% serum.
TABLE 2.
Effect of 50% serum on caspofungin and micafungin MECsa of Aspergillus spp.
| Aspergillus sp. (nb) | Result for drug
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Caspofungin
|
Anidulafungin
|
Micafungin
|
|||||||
| MIC50
|
Fold change | MIC50
|
Fold change | MIC50
|
Fold change | ||||
| −Serum | +50% serum | −Serum | +50% serum | −Serum | +50% serum | ||||
| A. fumigatus (10) | <0.25 (0.016-0.25) | 1 (0.5-2.0) | 11 (2-67) | 0.0156 (0.016) | 0.5 (0.25-2.0) | 32 (32-128) | 0.0156 (0.0156) | 1 (0.5-2.0) | 64 (33-133) |
| A. flavus (7) | 0.03 (0.016-0.06) | 0.5 (0.125-0.5) | 8 (4-17) | 0.0156 (0.016) | 0.5 (0.125-1.0) | 32 (16-64) | 0.0156 (0.0156) | 0.5 (0.5-2.0) | 33 (17-33) |
| A. niger (8) | 0.06 (0.06-0.25) | 1.0 (0.5-1.0) | 8 (4-17) | 0.0156 (0.0156) | 0.5 (0.25-0.5) | 32 (16-32) | 0.0156 (0.0156) | 2 (0.5-2.0) | 133 (33-133) |
| A. terreus (9) | 0.125 (0.03-0.5) | 0.5 (0.25-0.5) | 4 (1-8) | 0.0156 (0.0156) | 0.25 (0.125-0.25) | 16 (8-16) | 0.0156 (0.0156) | 0.5 (0.125-0.5) | 32 (4-32) |
MEC is defined as the lowest concentration of drug producing a macroscopically visible morphological change. For an explanation of values, see footnote a of Table 1.
n, no. of isolates.
The reduced antifungal properties of echinocandin drugs in the presence of serum suggested that protein binding was having a direct effect on the drugs, perhaps by altering their ability to inhibit glucan synthase. This possibility was investigated by examining direct effects of serum in inhibition of glucan synthase from C. albicans and Aspergillus fumigatus. As illustrated for anidulafungin and caspofungin, increasing amounts of serum (0 to 50%) decreased drug effectiveness (higher IC50s) (Fig. 1). Similar behavior was observed with enzymes isolated from three different strains of A. fumigatus and C. albicans (Table 3). The average IC50 n-fold shifts for the enzymes at maximum serum levels for caspofungin, micafungin, and anidulafungin were 12.8 ± 6.3, 4.6 ± 0.7 and 11.3 ± 9.5 ng/ml, respectively, for A. fumigatus and 5.2 ± 0.9, 4.9 ± 1.1 and 10.5 ± 4.2 ng/ml, respectively, for C. albicans. The data suggest that serum exerts its effect through a direct drug interaction. The GS IC50 shift for caspofungin was consistent with serum-induced shifts in the MIC or MEC (Tables 1 and 2). The larger MIC shifts for micafungin and anidulafungin suggested a secondary factor might play a role, such as drug transport into the cell. This suggestion is supported by data showing that all three drugs inhibited GS in the same drug range (IC50) (Table 3). In the absence of serum, the enhanced antifungal potencies of micafungin and anidulafungin would reflect better drug penetration into the cell and access to the target. Efficient uptake for these drugs would be expected given their highly hydrophobic tail structures. Furthermore, the drugs bound to serum proteins may be presented as altered substrates for a high-affinity transporter operating at or near the MIC (10).
FIG. 1.
Serum alters the IC50 for glucan synthase from C. albicans by anidulafungin and caspofungin. Drug inhibition kinetics for glucan synthase from C. albicans strain 36082 were determined by monitoring the incorporation of [3H]uridine diphosphoglucose (CPM) as a function of anidulafungin (AFG) (A) or caspofungin (CAS) (B) concentration in the presence of increasing amounts of serum, as indicated. The calculated IC50s are shown for each drug at a given serum level. All measurements were performed in triplicate.
TABLE 3.
IC50 for glucan synthase from Candida and Aspergillus
| Strain | EC50 of drug for glucan synthase (ng/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspofungin
|
Micafungin
|
Anidulafungin
|
||||||||||
| 0 | 10 | 20 | 50 | 0 | 10 | 20 | 50 | 0 | 10 | 20 | 50 | |
| A. fumigatus | ||||||||||||
| R21 | 1.6 | 2.7 | 3.6 | 10.3 | 3.7 | 6.2 | 10.3 | 23.2 | 0.75 | 1.9 | 3.1 | 11.9 |
| MF5668 | 1.1 | 1.0 | 3.2 | 4.8 | 2.2 | 3.2 | 3.3 | 7.6 | 0.29 | 5.5 | 6.9 | 28.1 |
| ATCC 13073 | 2.0 | 2.8 | 5.9 | 9.4 | 2.0 | 4.7 | 7.5 | 9.8 | 13.3 | 21.4 | 77.4 | |
| C. albicans | ||||||||||||
| ATCC 90028 | 0.3 | 1.3 | 2.1 | 6.3 | 18.0 | 28.4 | 50.1 | 90.2 | 1.00 | 4.72 | 18.6 | 24.7 |
| ATCC 36082 | 0.9 | 2.8 | 5.3 | 8.7 | 10.8 | 23.7 | 31.4 | 57.0 | 18.5 | 24.0 | 71.4 | |
| SC5314 | 14.7 | 27.0 | 36.9 | 80.5 | 26.9 | 32.6 | 50.6 | 89.7 | 17.1 | 15.3 | 74.7 | 89.0 |
EC50s (50% effective concentrations) of each drug are sorted by percentage of serum used (given below drug name).
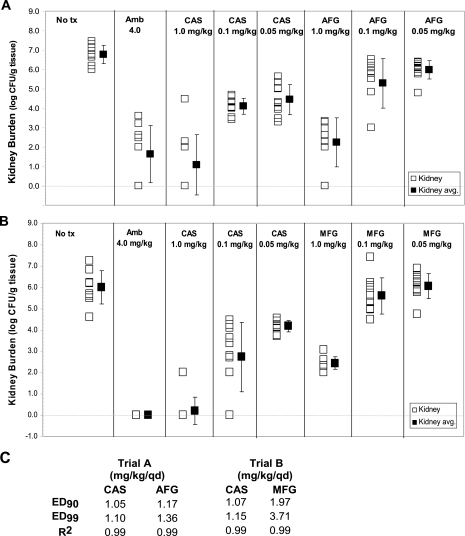
The effect of serum in neutralizing MIC differences was also consistent with results of animal model studies in which caspofungin and anidulafungin were comparable in efficacy while micafungin was only somewhat less effective over the dose range (0.05 to 1 mg/kg of body weight/day) in reducing kidney fungal burdens (99% effective dose) in a candidiasis model (Fig. 2).
FIG. 2.
Relative efficacies of caspofungin and micafungin in a murine candidiasis model. BALB/c mice (10 to 12 weeks; weight, 20 to 25 g) were infected by intravenous injection in the tail vein with 5 × 105 cells of C. albicans strain 36082. After 24 h, they were treated daily for 14 days at 0.05, 0.1, or 1 mg/kg/day with either caspofungin (CAS) and anidulafungin (AFG) (A) or CAS and micafungin (MFG) (B). The mice were evaluated for fungal kidney burdens, expressed as log CFU. Negative sham (no drug [No tx control]) and positive ambisome (Amb) (4 mg/kg/day) controls are indicated. Panel C shows 90 and 99% effective doses (ED90 and ED99, respectively) obtained for the two drug trials (panels A and B). qd, once a day.
The effect of serum on echinocandin efficacy reflects known serum binding properties of echinocandin drugs. Micafungin is 99% protein bound, primarily to albumin and alpha-1-acid glycoprotein (1). Caspofungin is 96.5% protein bound, primarily to albumin (14, 15), and protein-bound drug can be recovered from target tissues (4). Anidulafungin was reported to be >84% protein bound (15), but it may be >98% bound (D. Sheehan, personal communication). It is not clear whether echinocandin drugs are active in the presence of bound serum protein or if a small proportion of free biologically active drug is available in the presence of serum.
In summary, human serum shifts the apparent antifungal potencies of echinocandin drugs in in vitro growth assays with diverse Candida spp. and Aspergillus spp., yielding nearly equivalent MICs or MECs, respectively. This behavior effectively negates any in vitro differences in potency between the drugs observed in standard testing medium. The primary effect of serum binding to this class of drugs appears to be a concomitant decrease in inhibition of glucan synthase.
Acknowledgments
We thank Svetlana Senina and Rema Suresh for expert technical assistance with the murine model.
Footnotes
Published ahead of print on 9 April 2007.
REFERENCES
- 1.Carver, P. L. 2004. Micafungin. Ann. Pharmacother. 38:1707-1721. [DOI] [PubMed] [Google Scholar]
- 2.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 3.Ernst, E. J., E. E. Roling, C. R. Petzold, D. J. Keele, and M. E. Klepser. 2002. In vitro activity of micafungin (FK-463) against Candida spp.: microdilution, time-kill, and postantifungal-effect studies. Antimicrob. Agents Chemother. 46:3846-3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajdu, R., R. Thompson, J. G. Sundelof, B. A. Pelak, F. A. Bouffard, J. F. Dropinski, and H. Kropp. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoang, A. 2001. Caspofungin acetate: an antifungal agent. Am. J. Health Syst. Pharm. 58:1206-1217. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-beta-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reference deleted.
- 8.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 9.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 2nd ed. NCCLS document M27-A2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 10.Paderu, P., S. Park, and D. S. Perlin. 2004. Caspofungin uptake is mediated by a high-affinity transporter in Candida albicans. Antimicrob. Agents Chemother. 48:3845-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park, S., R. Kelly, J. N. Kahn, J. Robles, M. J. Hsu, E. Register, W. Li, V. Vyas, H. Fan, G. Abruzzo, A. Flattery, C. Gill, G. Chrebet, S. A. Parent, M. Kurtz, H. Teppler, C. M. Douglas, and D. S. Perlin. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Santangelo, R., P. Paderu, G. Delmas, Z. W. Chen, R. Mannino, L. Zarif, and D. S. Perlin. 2000. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob. Agents Chemother. 44:2356-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stone, J. A., X. Xu, G. A. Winchell, P. J. Deutsch, P. G. Pearson, E. M. Migoya, G. C. Mistry, L. Xi, A. Miller, P. Sandhu, R. Singh, F. deLuna, S. C. Dilzer, and K. C. Lasseter. 2004. Disposition of caspofungin: role of distribution in determining pharmacokinetics in plasma. Antimicrob. Agents Chemother. 48:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiederhold, N. P., and R. E. Lewis. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313-1333. [DOI] [PubMed] [Google Scholar]