Abstract
Studies on cellular drug interactions with antiretroviral agents prior to clinical trials are critical to detect possible drug interactions. Herein, we demonstrated that two 2′-deoxycytidine antiretroviral agents, dexelvucitabine (known as β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine, DFC, d-d4FC, or RVT) and lamivudine (3TC), combined in primary human peripheral blood mononuclear (PBM) cells infected with human immunodeficiency virus 1 strain LAI (HIV-1LAI), resulted in additive-to-synergistic effects. The cellular metabolism of DFC and 3TC was studied in human T-cell lymphoma (CEM) and in primary human PBM cells to determine whether this combination caused any reduction in active nucleoside triphosphate (NTP) levels, which could decrease with their antiviral potency. Competition studies were conducted by coincubation of either radiolabeled DFC with different concentrations of 3TC or radiolabeled 3TC with different concentrations of DFC. Coincubation of radiolabeled 3TC with DFC at concentrations up to 33.3 μM did not cause any marked reduction in 3TC-triphosphate (TP) or any 3TC metabolites. However, a reduction in the level of DFC metabolites was noted at high concentrations of 3TC with radiolabeled DFC. DFC-TP levels in CEM and primary human PBM cells decreased by 88% and 94%, respectively, when high concentrations of 3TC (33.3 and 100 μM) were added, which may influence the effectiveness of DFC-5′-TP on the HIV-1 polymerase. The NTP levels remained well above the median (50%) inhibitory concentration for HIV-1 reverse transcriptase. These results suggest that both β-d- and β-l-2′-deoxycytidine analogs, DFC and 3TC, respectively, substrates of 2′-deoxycytidine kinase, could be used in a combined therapeutic modality. However, it may be necessary to decrease the dose of 3TC for this combination to prove effective.
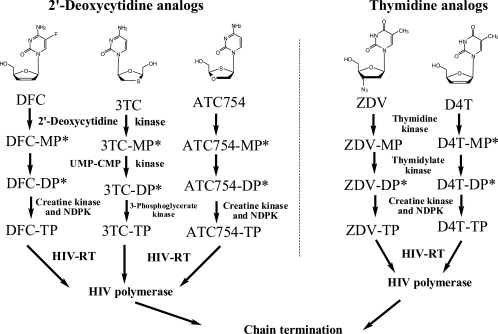
The primary goal of antiretroviral therapy for the treatment of human immunodeficiency virus (HIV) infections is suppression of viral replication to undetectable levels. This goal can be achieved by a combination of highly active antiretroviral therapy, involving the use of agents from at least two distinct classes such as two nucleoside reverse transcriptase inhibitors (NRTI) and either a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (2, 9). However, to date, the combination of two NRTI has been restricted to those nucleosides that are activated by different kinases in their first phosphorylation step (Fig. 1). Nucleoside combinations approved by the U.S. Food and Drug Administration (FDA) include the following: (i) lamivudine (3TC) and zidovudine (ZDV), (ii) emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF), (iii) abacavir (ABC) and 3TC, (iv) ABC, 3TC, and ZDV, and (v) FTC, TDF, and efavirenz.
FIG. 1.
Intracellular phosphorylation of nucleosides and their incorporation into HIV-1 RT. ATC754 is also known as SPD754 or (−)-dOTC. NDPK, nucleoside diphosphate kinase; D4T, stavudine; *, the nucleoside analog is likely a substrate for this enzyme.
Few combination studies have been conducted using nucleoside analogs that share the same phosphorylation enzyme. Previous in vitro studies with 3TC or FTC with apricitabine (ATC or AVX754), a 2′-deoxycytidine analog formerly known as BCH-10618, (−)-dOTC, or SPD754, demonstrated a significant reduction in the active nucleoside triphosphate (NTP) levels of ATC-triphosphate (ATC-TP) in primary human peripheral blood mononuclear (PBM) cells (12). Interestingly, the intracellular levels of 3TC-TP in humans were unaffected by coadministration of ATC, but the levels of ATC-TP were reduced by approximately sixfold in the presence of 3TC (4, 5). These data reinforce the necessity of ascertaining intracellular NTP levels when assessing nucleoside analog interactions. Similarly, the combination of two thymidine analogs, ZDV and stavudine, is contraindicated in the clinic, since they both use thymidine kinase for activation to their corresponding nucleotides (14).
Dexelvucitabine (known as β-d-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine, RVT, DFC, or d-d4FC) is currently in Phase 2b clinical trials for the treatment of HIV infections (http://www.aidsmeds.com/drugs/reverset) (8). Preclinical studies indicate that DFC-TP has a long intracellular half-life and inhibits replication of both wild-type and mutant strains of HIV commonly observed during treatment with ZDV, 3TC, and other NRTI (19).
3TC is a (−)-β-2′-deoxycytidine analog approved by the FDA for the treatment of HIV and hepatitis B virus infections and is presently one of the most widely used nucleoside analogs in highly active antiretroviral therapy regimens (11). Since 3TC and DFC are both phosphorylated by 2′-deoxycytidine kinase, it was anticipated that they might interact with each other (19). However, cellular antiviral assays reported herein by our group demonstrated mostly synergistic or additive antiviral interactions at low concentrations of 3TC relative to DFC. Based on these observations, the cellular metabolism of the combination of these two potent 2′-deoxycytidine analogs was studied, in order to determine whether any reduction in active NTP levels occurs.
(Parts of this paper were presented at national and international meetings prior to the release of clinical data with the combination of 3TC and DFC [15].)
MATERIALS AND METHODS
Chemicals.
[5-3H]3TC (specific activity = 8 Ci/mmol) and [6-3H]DFC (specific activity = 1 Ci/mmol) were synthesized by Moravek Biochemicals, Inc. (Brea, CA). Tetrabutylammonium phosphate (TBAP) was purchased from Alltech Associates, Inc. (Deerfield, IL). Scintillation liquid, EcoLite, was obtained from Valeant Pharmaceuticals (Costa Mesa, CA). The chemical purity of each compound, as determined by high-performance liquid chromatography (HPLC) and spectral analysis, was greater than 98%. All other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO).
Cell culture systems.
Human T-cell lymphoma (CEM) cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD) and were maintained in suspension cultures in RPMI 1640 medium (GIBCO Laboratories, Grand Island, NY), supplemented with 1 mM sodium pyruvate, 10% (vol/vol) fetal bovine serum, and 1 mM penicillin G/streptomycin sulfate. CEM cells were grown at 37°C in a 5% CO2, 95% air atmosphere. The media were replenished every 3 days, and cells were subcultured once a week. Primary human PBM cells were isolated using a Histopaque technique from buffy coats derived from healthy donors, obtained from the American Red Cross (Atlanta, GA). After processing, the PBM cells were stimulated by incubating cells for 3 days in medium containing 10 μl/ml phytohemagglutinin (PHA) before use. All the experiments reported in this paper were performed using PHA-stimulated primary human PBM cells and not resting cells, except where indicated.
Competition studies.
CEM and primary human PBM cells (2 × 106 cells/per time point) were exposed to either 10 μM [3H]3TC with unlabeled DFC (1, 33.3, and 100 μM) or 10 μM [3H]DFC with unlabeled 3TC (1, 33.3, and 100 μM) for 4 h. Radiolabeled 3TC and radiolabeled DFC (10 μM) with 50 or 100 μM 2′-deoxycytidine was used as a positive control. All combination studies were performed in triplicate.
At selected times, the cells were centrifuged for 10 min at 350 × g at 4°C, and the pellet was resuspended and washed three times with cold phosphate-buffered saline. Viable cells were counted using a hemocytometer, and the viability was assessed by trypan blue exclusion (viability > 98%). Intracellular DFC, 3TC, and their respective metabolites were extracted by incubation overnight at −20°C with 60% methanol/water (1 ml); the supernatants were then collected and centrifuged at 14,000 rpm (Eppendorf centrifuge model 5415C) for 5 min. The extracts were dried under a gentle filtered airflow and stored at −20°C, until they were assayed. The residues were resuspended in 100 μl of water, and aliquots were injected into the HPLC system.
Separation of DFC and 3TC metabolites was performed by ion-pairing reverse-phase HPLC on a Columbus 5-μm C18 column (250 mm by 4.6 mm) (Phenomenex, Torrance, CA) using a Varian Pro Star HPLC model 210 with manual injection (Walnut Creek, CA). The mobile phase consisted of buffer A (25 mM ammonium acetate with 5 mM TBAP, pH 7.0) and buffer B (methanol). Elution was performed using a multistage linear gradient of buffer B from 0 to 50%. Retention times were as follows: DFC-diphosphate (DP)-choline, 6 min; DFC, 20 min; DFC-monophosphate (MP), 30 min; DFC-DP, 40 min; DFC-TP, 48 min; 3TC-DP-choline, 6 min; 3TC, 25 min; 3TC-MP, 32 min; 3TC-DP, 44 min; and 3TC-TP, 52 min. The limit of detection was approximately 0.01 pmol/106 cells. Radioactivity was quantified using a 2500 TR liquid scintillation analyzer (Perkin Elmer, Life and Analytical Sciences, Wellesley, MA). Based on previous work with other cytidine analogs, incubation with alkaline phosphatase, and authentic standards, we identified DFC metabolites (13, 17).
Drug interaction studies in HIV-infected primary human PBM cells.
The combined antiviral effect of DFC and 3TC was tested against HIV-1 strain LAI (HIV-1LAI) in 3-day primary human PBM cells after 5 days in culture. The concentration of the drugs used was based initially on the ratios of their respective 50% effective concentrations (EC50) in human lymphocytes (10:1, DFC:3TC). Studies were also conducted at ratios favoring DFC (3:1, 25:1, and 100:1), since in this cell culture system 3TC is more potent than DFC. The medium contained human recombinant interleukin 2 (26.5 units/ml). Virus was added to the cell suspensions 1 h prior to the addition of drugs. The assays were performed in T25 flasks. One milliliter of supernatant was centrifuged at 12,000 rpm for 2 h at 4°C in a Jouan Br43i centrifuge (Thermo Electron Corp., Marietta, OH). Ten microliters of the resuspended virus pellet extract was used to determine the amount of HIV-1 reverse transcriptase (RT) present, as previously described (10). A semiautomated radioactive detection method was performed using a Packard Filter Mate harvester (Perkin Elmer, Life and Sciences, Wellesley, MA) and a matrix 9600 direct beta counter (Perkin Elmer, Life and Sciences). The data were analyzed as previously described (10). The cytotoxicities of the drugs alone and in combinations at different ratios were determined in primary human PBM cells using a commercial colorimetric [3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide] (MTT) assay as described previously (19, 21).
Statistical analyses for the combination antiviral studies.
A robust computer algorithm, previously developed to determine synergy, additivity, and antagonism between drugs, was used (3, 7). This method calculated the combination index (CI) for each drug combination together with confidence intervals. Based on this method, a CI of <1, equal to 1, or >1 indicates synergy, additivity, or antagonism, respectively. This analysis has been used by numerous investigators working on antiviral combinations (6, 18). A t test (two-sample test assuming equal variance) was used to determine statistical significance (P < 0.05).
RESULTS
Anti-HIV-1 combination studies between DFC and 3TC in primary human PBM cells demonstrated additivity or synergy at ratios of 10:1, 25:1, and 100:1 (Table 1). Additive-to-weak antagonism was noted with DFC:3TC only at high-effect levels (90 to 95% inhibition) for the combination at a 3:1 ratio (Table 1). No cytotoxicity was observed for any combination used at the highest concentration, as determined by tetrazolium reduction assays (data not shown). Therefore, a cellular metabolism study was performed to determine whether these two potent 2′-deoxycytidine analogs interfered with phosphorylation to active NTP in CEM and primary human PBM cells.
TABLE 1.
Effect of 3TC and DFC combinations against HIV-1LAI-infected human PBM cells
| Treatment (ratio) | EC50a | EC90a | CI ± SE at Fab of:
|
|||
|---|---|---|---|---|---|---|
| 0.50 | 0.75 | 0.90 | 0.95 | |||
| Expt 1 | ||||||
| 3TC | 0.06 | 0.32 | ||||
| DFC | 0.35 | 2.22 | ||||
| DFC + 3TC (100:1) | 0.26 | 1.99 | 0.80 (0.77) | 0.90 ± 0.63 (0.86 ± 0.58) | 1.00 ± 0.43 (0.95 ± 0.41) | 1.08 ± 0.39 (1.02 ± 0.32) |
| DFC + 3TC (25:1) | 0.29 | 1.32 | 1.15 ± 0.65 (1.00 ± 0.72) | 0.97 ± 0.46 (0.85 ± 0.53) | 0.82 ± 0.32 (0.73 ± 0.39) | 0.73 ± 0.25 (0.65 ± 0.31) |
| Expt 2 | ||||||
| 3TC | 0.026 | 0.12 | ||||
| DFC | 0.075 | 0.23 | ||||
| DFC + 3TC (10:1) | 0.003 | 0.092 | 0.05 ± 0.04 (0.05 ± 0.04) | 1.14 ± 0.08 (0.14 ± 0.07) | 0.46 ± 0.22 (0.43 ± 0.17) | 1.04 ± 0.61 (0.93 ± 0.40) |
| DFC + 3TC (3:1) | 0.059 | 0.28 | 1.48 ± 0.68 (1.15 ± 0.44) | 1.72 ± 0.71 (1.30 ± 0.42) | 2.02 ± 0.81 (1.50 ± 0.50) | 2.27 ± 0.95 (1.65 ± 0.57) |
In μM as determined from the median effect plot.
A CI of <1, equal to 1, or >1 indicates synergy, additivity, or antagonism, respectively. Fa is a component of the median effect equation referring to the fraction of the system (e.g., 0.50 means the CI at a 50% reduction of RT activity). CI values were determined for a mutually nonexclusive interaction (values in italics are for mutually exclusive interaction, which is less rigorous).
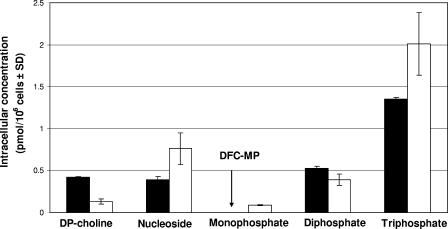
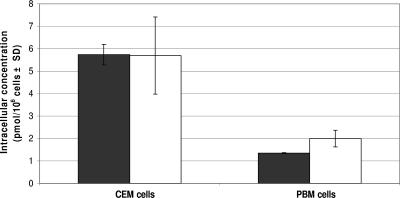
DFC (10 μM) and 3TC (10 μM) were rapidly bioconverted to their MP, DP, and TP forms and to the DP-choline derivative in CEM and primary human PBM cells (Fig. 2). DFC-TP and 3TC-TP reached levels of 5.74 ± 0.46 and 5.70 ± 1.72 pmol/106 cells, respectively, in CEM cells after 4 h (Fig. 3). Intracellular concentrations in primary human PBM cells of DFC-TP and 3TC-TP after 4 h of incubation were 1.35 ± 0.02 and 2.01 ± 0.37 pmol/106 cells, respectively (Fig. 3). The natural nucleoside 2′-deoxycytidine was used as a positive control, since it has been previously shown to inhibit the phosphorylation and anti-HIV activity of 3TC (23). As expected, coincubation of radiolabeled 3TC with 2′-deoxycytidine resulted in no detectable 3TC metabolites (Fig. 4).
FIG. 2.
Intracellular concentrations of [3H]DFC or [3H]3TC and their respective metabolites after 4 h of incubation in primary human PBM cells (DFC metabolites, solid bars; 3TC metabolites, white bars). Metabolites were separated by ion-pairing reverse-phase HPLC with a Columbus 5-μm C18 column (Phenomenex, Torrance, CA) using a Pro Star model (Varian, Walnut Creek, CA) with manual injection. The mobile phase consisted of buffer A (25 mM ammonium acetate with 5 mM TBAP, pH 7.0) and buffer B (methanol). Elution was performed using a multistage linear gradient of buffer B. DFC-MP was below the limit of detection.
FIG. 3.
Intracellular concentrations of 10 μM [3H]DFC-TP or 10 μM [3H]3TC-TP in CEM and primary human PBM cells at 4 h (DFC-TP, solid bars; 3TC-TP, white bars). Metabolites were separated as described in Fig. 2. The standard deviations (SD) for DFC-TP in primary human PBM cells is 0.02 pmol/106 cells.
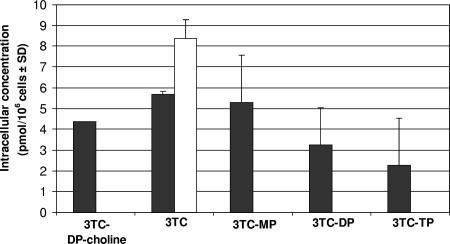
FIG. 4.
Competition study: radiolabeled 10 μM 3TC with 100 μM 2′-deoxycytidine in CEM cells after 1 h of coincubation (3TC at 10 μM, solid bars; 3TC at 10 μM plus 2′-deoxycytidine at 100 μM, white bar). No nucleoside MP, DP, TP, or DP-choline metabolites were detected.
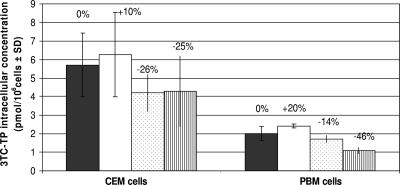
Competition studies in CEM cells between [3H]3TC and several concentrations of DFC resulted in no significant reduction (P > 0.05) of 3TC-TP levels up to 100 μM DFC (Fig. 5). Even in the presence of nonphysiological 100 μM DFC, [3H]3TC-TP levels were reduced by 25% from 5.70 ± 1.72 pmol/106 cells (no DFC added) to 4.28 ± 1.90 pmol/106 cells (Fig. 5). Similar results were observed in primary human PBM cells at concentrations up to 33.3 μM DFC. However, a significant 46% inhibition of 3TC-TP levels (P < 0.05) was observed with 100 μM DFC in primary human PBM cells, from 2.01 ± 0.37 to 1.09 ± 0.16 pmol/106 cells.
FIG. 5.
Competition study. Intracellular concentrations of [3H]3TC-TP after coincubation with radiolabeled 10 μM 3TC (solid bars) and DFC (1 μM, white bars; 33 μM, dotted bars; or 100 μM, striped bars) for 4 h in CEM and primary human PBM cells.
No reductions in any of the 3TC metabolites were observed when 1 μM DFC was added, a reduction between 10 to 26% was observed with 33.3 μM DFC, and 13 to 46% inhibition was observed with 100 μM DFC. 3TC-DP-choline levels did not change with the addition of DFC in any of the cell systems used in this study.
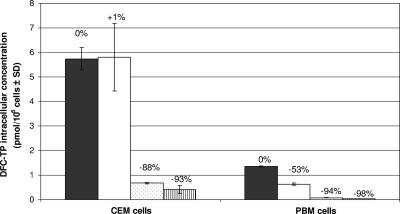
In primary human PBM cells, the levels of [3H]DFC-TP at 4 h were significantly lower than those found in CEM cells (Fig. 6). However, no reduction in [3H]DFC-TP was noted with 1 μM 3TC in CEM cells. Significant reductions in [3H]DFC-TP levels from 5.74 ± 0.46 pmol/106 cells to 0.67 ± 0.02 and 0.41 ± 0.16 pmol/106 cells were noted in the presence of 33.3 and 100 μM 3TC, respectively, in CEM cells (Fig. 6). Similar reductions in DFC-TP levels were observed in primary human PBM cells, at all concentrations tested (P < 0.05). Whereas DFC-TP levels in CEM cells remained above the 50% inhibitory concentration (IC50, 0.18 μM) for the HIV-1 RT, in primary human PBM cells, these levels remained below the IC50 for the HIV-1 RT when 33.3 or 100 μM 3TC was present (Fig. 6) (19). Interestingly, in CEM cells, an addition of 3TC as low as 1 μM decreased the formation of the liponucleotide DFC-DP-choline by 74% (P < 0.05; data not shown).
FIG. 6.
Competition study. Intracellular concentrations of [3H]DFC-TP after coincubation with radiolabeled 10 μM DFC (solid bars) and 3TC (1 μM, white bars; 33.3 μM, dotted bars; or 100 μM, striped bars) for 4 h in CEM and primary human PBM cells.
All DFC metabolite levels decreased to the same range in primary human PBM and CEM cells with 33.3 μM 3TC (83 to 94%) and 100 μM 3TC (92% to below the limit of detection). At 1 μM 3TC, we observed a significant decrease of the DFC-DP-choline: 74% inhibition in CEM cells, but only 48% inhibition in primary human PBM cells.
Similar results were observed in resting primary human PBM cells when DFC was incubated with 33.3 and 100 μM 3TC (data not shown). The only difference was observed when 1 μM 3TC was added. In resting PBM cells, 1 μM 3TC did not significantly reduce the level of DFC-TP, while in the PHA-stimulated primary human PBM cells, a decrease in the DFC-TP levels was observed (data not shown). This is consistent with the fact that cytidine analogs such as DFC and 3TC are cell cycle independent (19, 20).
DISCUSSION
Clinical studies involving combinations of NRTI that share the same activating enzymes have been contraindicated because of potential antiviral antagonism. Since 3TC (and FTC) is widely used for first-line therapy and DFC is being considered for salvage therapy, the potential drug interaction between these two 2′-deoxycytidine analogs was investigated.
Antiviral assays of the combination of DFC and 3TC in infected human PBM cells with HIV-1LAI demonstrated additive or synergistic effects at relevant ratios close to their respective EC50 values or when the ratio favored DFC (10:1, 25:1, and 100:1), suggesting a possible benefit of coadministration for the treatment of HIV infections (Table 1). However, at levels of 3TC close to that for DFC (3:1 ratio of DFC:3TC), additive-to-moderate antagonism was noted. Therefore, a study of the cellular metabolism of these drugs alone and in combination was warranted.
Both nucleoside analogs were metabolized to their MP, DP, and TP metabolites and to the DP-choline derivative in CEM and primary human PBM cells. 3TC and DFC were phosphorylated to their respective 5′-TP metabolites at equivalent concentrations in CEM cells (Fig. 3).
After coincubation of radiolabeled 3TC with different concentrations of DFC for 4 h, a modest reduction (P > 0.05) in 3TC metabolites was noted (Fig. 5). Even at 100 μM DFC, a nonphysiological concentration (expected maximum concentration of drug in serum in elimination of 7.70 ± 1.58 μM; median concentration of 2.15 ± 0.38 μM), only a 25% reduction in 3TC-TP concentration was noted in CEM cells, but a 46% reduction was detected in primary human PBM cells (Fig. 5) (22). DFC had no marked effect on intracellular 3TC-TP levels at physiological concentrations.
When [3H]DFC was coincubated in CEM cells with 33.3 or 100 μM 3TC, a marked reduction (≥88%) of DFC-TP levels was noted. Similar results were observed in PBM cells (Fig. 6). Liponucleotide metabolites have previously been described for other cytidine analogs, including d- and l-2′,3′-dideoxycytidine (ddC) and its 5-fluorinated derivative d- and l-FddC, following incubation in cell culture (1, 17). 5′-Diphosphoethanolamine and diphosphocholine liponucleotides may be associated with peripheral neuropathy observed in individuals treated with ddC, although this has not been confirmed (13). These liponucleotides could also serve as a depot form of the drug, with a long intracellular half-life. Even at 1 μM 3TC, a significant reduction in DFC-DP-choline derivative was noted in CEM cells.
Although 3TC reduced intracellular DFC-TP levels in a concentration-dependent manner, DFC-TP levels in CEM cells remained above the IC50 for the HIV-1 RT. However, in primary human PBM cells, the levels of the DFC-TP were six times lower than the IC50 for the HIV-1 RT. Consistent with the combination anti-HIV assays, 3TC partially prevented the phosphorylation of DFC, but only at high nonphysiological concentrations (>1 to 3 μM).
These findings are consistent with a previous report by Erickson-Viitanen et al. and support the hypothesis that enzymes involved in the activation of DFC and 3TC are not rate limiting for the production of their 5′-TP metabolites (9). It is likely that this drug interaction occurs at the NTP levels with the HIV RT. Furthermore, it is known that d- and l-nucleoside analogs can be phosphorylated by different enzymes at the nucleoside DP level (NDP to NTP) (16). Thus, the lack of drug interaction could also result from affinities to different kinases.
A recent Phase 2b clinical study demonstrated that DFC is a powerful drug against HIV-1-resistant viruses containing a thymidine analog and/or M184V mutation in the viral polymerase (8). Interestingly, DFC at 200 mg orally once a day was highly effective in drug-experienced individuals who were not taking 3TC or FTC (mean reduction in viral load at week 16 was 1.4 and 1.5 log10 copies/ml in optimized and nonoptimized regimens, respectively), but it was less effective when these oxathiolane nucleoside analogs were administered, which supports the results presented herein. Therefore, in vitro competition studies between nucleoside analogs can provide information that may be extrapolated to humans, especially when physiologically relevant concentrations are used.
Taken together, these studies suggest that although 3TC and DFC are cytidine analogs activated by 2′-deoxycytidine kinase, they may be considered for combination therapy for the treatment of HIV infections, although a dose reduction for 3TC may be needed (9, 20). It should be noted that FTC, a related nucleoside, is approved at a dose of 200 mg, which is 33% and 50% lower than the approved dose of 3TC for HIV and hepatitis B virus, respectively. Alternatively, since both 3TC and DFC can be administered once a day, a future salvage therapy clinical trial should be considered where the drugs are given at different times of the day (e.g., 9 a.m. and 9 p.m.). Similar intracellular pharmacokinetic drug interaction studies should be considered with other novel nucleoside analogs to maximize the understanding of potential interactions before the drugs are administered to HIV-1-infected individuals.
Acknowledgments
B.I.H.-S. was supported by a supplement of NIH grant 5R37-AI-41980 as a minority supplement. This work was supported in part by CFAR grant 5P30-AI-50409 and the Department of Veterans Affairs.
R.F.S. receives or will receive royalties from the sale of 3TC and DFC.
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Arner, E. S. J., and S. Eriksson. 1993. Deoxycytidine and 2′,3′-dideoxycytidine metabolism in human monocyte-derived macrophages. A study of both anabolic and catabolic pathways. Biochem. Biophys. Res. Commun. 197:1499-1504. [DOI] [PubMed] [Google Scholar]
- 2.Barbaro, G., A. Scozzafava, A. Mastrolorenzo, and C. T. Supuran. 2005. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr. Pharm. Des. 11:1805-1843. [DOI] [PubMed] [Google Scholar]
- 3.Belen'kii, M. S., and R. F. Schinazi. 1994. Multiple drug effect analysis with confidence interval. Antivir. Res. 25:1-11. [DOI] [PubMed] [Google Scholar]
- 4.Bethell, R., J. Adams, J. De Mys, J. Lippens, A. Richard, C. Ren, P. Collins, C. Struthers-Semple, T. Holdich, and J. Sawyer. 2004. Pharmacological evaluation of a dual deoxycytidine analogue combination: 3TC and SPD754, abstr. 138. 11th Conf. Retrovir. Opportun. Infect., San Francisco, CA, 8 to 11 February 2004.
- 5.Bethell, R. C., Y. S. Lie, and N. T. Parkin. 2005. In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs. Antivir. Chem. Chemother. 16:295-302. [DOI] [PubMed] [Google Scholar]
- 6.Chou, T.-C. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharm. Rev. 58:621-681. [DOI] [PubMed] [Google Scholar]
- 7.Chou, T.-C., and P. Talalay. 1984. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 22:27-55. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, C., C. Katlama, R. Murphy, J. Gathe, C. Brinson, G. Richmond, P.-M. Girad, J. Fessel, A. Liappis, E. Puglia, B. Rodwick, J. Nadler, W. O'Brien, K. Arasteh, M. J. Otto, S. Erickson-Viitanen, and R. Levy. 2005. Antiretroviral activity and tolerability of reverset (D-d4FC), a new fluorocytidine nucleoside analog, when used in combination therapy in treatment-experienced patients: results of phase IIb study RVT-203, abstr. WeOaLB103. Third IAS Conf. HIV Pathog. Treatm., Rio de Janeiro, Brazil, 24 to 27 July 2005.
- 9.Erickson-Viitanen, S., J. T. Wu, G. Shi, S. Unger, R. W. King, B. Fish, R. Klabe, R. Gelenziunas, K. Gallagher, M. J. Otto, and R. F. Schinazi. 2003. Cellular pharmacology of D-d4FC, a nucleoside analog active against drug-resistant HIV. Antivir. Chem. Chemother. 14:39-47. [DOI] [PubMed] [Google Scholar]
- 10.Ericksson, B. F., and R. F. Schinazi. 1989. Combinations of 3′-azido-3′-deoxythymidine (zidovudine) and phosphonoformate (foscarnet) against human immunodeficiency virus type 1 and cytomegalovirus replication in vitro. Antimicrob. Agents Chemother. 33:663-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin, D., A. S. Roemer, and L. Placidi. 2004. The global antiviral therapeutics market, p. 159-184. In R. F. Schinazi and D. C. Liotta (ed.), Frontiers in nucleosides and nucleic acids. IHL Press, Tucker, GA.
- 12.Gu, Z., B. Allard, J. M. de Muys, J. Lippens, R. F. Rando, N. Nguyen-Ba, C. Ren, P. McKenna, D. L. Taylor, and R. C. Bethell. 2006. In vitro antiretroviral activity and in vitro toxicity profile of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor for treatment of human immunodeficiency virus infection. Antimicrob. Agents Chemother. 50:625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao, Z., E. E. Stowe, G. Ahluwalia, D. C. Baker, A. K. Hebbler, C. Chisena, S. M. Musser, J. A. Kelley, C.-F. Perno, D. G. Johns, and D. A. Cooney. 1993. Characterization of 2′,3′-dideoxycytidine diphosphocholine and 2′,3′-dideoxycytidine diphosphoethanolamine. Drug Metab. Dispos. 21:738-744. [PubMed] [Google Scholar]
- 14.Havlir, D. V., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J.-P. Sommadossi, L. Fox, H. Kesseler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Santiago, B., K. L. Rapp, J. S. Mathew, and R. F. Schinazi. 2004. Lack of antagonistic interactions for the two cytidine analogs Reverset and lamivudine in vitro, abstr. 50:48. HIV DART 2004: Front. Drug Dev. Antiretrovir. Ther., Montego Bay, Jamaica, 12 to 16 December 2004.
- 16.Krishnan, P., Q. Fu, W. Lam, J.-Y. Liou, G. E. Dutschman, and Y.-C. Cheng. 2002. Phosphorylation of pyrimidine deoxynucleoside analog diphosphates: selective phosphorylation of l-nucleoside analog diphosphates by 3-phosphoglycerate kinase. J. Biol. Chem. 277:5453-5459. [DOI] [PubMed] [Google Scholar]
- 17.Martin, L. T., A. Faraj, R. F. Schinazi, G. Gosselin, C. Mathé, J.-L. Imbach, and J.-P. Sommadossi. 1997. Effect of stereoisomerism on the cellular pharmacology of β-enantiomers of cytidine analogs in Hep-G2 cells. Biochem. Pharmacol. 53:75-87. [DOI] [PubMed] [Google Scholar]
- 18.Schinazi, R. F. 1991. Combined therapeutic modalities for viral infections—rationale and clinical potential, p. 110-181. In T. C. Chou and D. C. Rideout (ed.), Synergism and antagonism in chemotherapy. Academic Press, Orlando, FL.
- 19.Schinazi, R. F., J. Mellors, H. Bazmi, S. Diamond, S. Garber, K. Gallagher, R. Gelenziunas, R. Klabe, M. Pierce, M. Rayner, J.-T. Wu, H. Zhang, J. Hammond, L. Bacheler, D. J. Manion, M. J. Otto, L. J. Stuyver, G. Trainor, D. C. Liotta, and S. Erickson-Viitanen. 2002. DPC 817: a cytidine nucleoside analog with activity against zidovudine- and lamivudine-resistant viral variants. Antimicrob. Agents Chemother. 46:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shewach, D. S., D. C. Liotta, and R. F. Schinazi. 1993. Affinity of the antiviral enantiomers of oxathiolane cytosine nucleosides for human 2′-deoxycytidine kinase. Biochem. Pharmacol. 45:1540-1543. [DOI] [PubMed] [Google Scholar]
- 21.Stuyver, L. J., S. Lostia, M. Adams, J. S. Mathew, B. S. Pai, J. Grier, P. M. Tharnish, Y. Choi, Y. Chong, H. Choo, C. K. Chu, M. J. Otto, and R. F. Schinazi. 2002. Antiviral activities and cellular toxicities of modified 2′,3′-dideoxy-2′,3′-didehydrocytidine analogues. Antimicrob. Agents Chemother. 46:3854-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stuyver, L. J., T. R. McBrayer, D. Schurmann, I. Kravec, A. Beard, L. Cartee, R. F. Schinazi, A. de la Rosa, R. L. Murphy, and M. J. Otto. 2004. Potent antiviral effect of Reverset in HIV-1-infected adults following a single oral dose. Antivir. Ther. 9:529-536. [PubMed] [Google Scholar]
- 23.Zhu, Y.-L., G. E. Dutschman, S.-H. Liu, E. G. Bridges, and Y.-C. Cheng. 1998. Anti-hepatitis B virus activity and metabolism of 2′,3′-dideoxy-2′,3′-didehydro-β-l(−)-5-fluorocytidine. Antimicrob. Agents Chemother. 42:1805-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]