Abstract
The nitrothiazole analogue nitazoxanide [NTZ; 2-acetolyloxy-N-(5-nitro-2-thiazolyl)benzamide] represents the parent compound of a class of drugs referred to as thiazolides and exhibits a broad spectrum of activities against a wide variety of helminths, protozoa, and enteric bacteria infecting animals and humans. NTZ and other thiazolides are active against a wide range of other intracellular and extracellular protozoan parasites in vitro and in vivo, but their mode of action and respective subcellular target(s) have only recently been investigated. In order to identify potential targets of NTZ and other thiazolides in Giardia lamblia trophozoites, we have developed an affinity chromatography system using the deacetylated derivative of NTZ, tizoxanide (TIZ), as a ligand. Affinity chromatography on TIZ-agarose using cell extracts of G. lamblia trophozoites resulted in the isolation of an approximately 35-kDa polypeptide, which was identified by mass spectrometry as a nitroreductase (NR) homologue (EAA43030.1). NR was overexpressed as a six-histidine-tagged recombinant protein in Escherichia coli, purified, and then characterized using an assay for oxygen-insensitive NRs with dinitrotoluene as a substrate. This demonstrated that the NR was functionally active, and the protein was designated GlNR1. In this assay system, NR activity was severely inhibited by NTZ and other thiazolides, demonstrating that the antigiardial activity of these drugs could be, at least partially, mediated through inhibition of GlNR1.
Giardia lamblia (synonymous with Giardia duodenalis and Giardia intestinalis), a flagellated protozoan, is the most common causative agent of persistent diarrhea worldwide. The life cycle includes motile, flagellated trophozoites parasitizing the upper intestine and thick-walled cysts forming in the lower intestine that are shed with the feces (43). Antigiardial chemotherapy is directed against the trophozoites. Metronidazole (MET) and other nitroimidazoles have been used since the late 1950s as the therapy of choice against giardiasis (14, 43, 46).
Nitazoxanide (NTZ) [2-acetolyloxy-N-(5-nitro-2-thiazolyl)benzamide] was originally developed as a veterinary antihelminthic (36). The drug has been shown to exhibit a broad spectrum of activity in vitro and in vivo against intestinal pathogens such as G. lamblia (1, 32), Entamoeba histolytica (1), the apicomplexan parasite Cryptosporidium parvum (13), and a range of anaerobic bacteria infecting animals and humans (15, 18, 45). In addition, NTZ has been shown to act in vitro against Neospora caninum (11, 12). The drug is currently marketed in the United States for the treatment of equine myeloencephalitis caused by Sarcocystis neurona (29) and the treatment of persistent diarrhea caused by G. lamblia and Cryptosporidium parvum (13, 15, 45).
In vivo, NTZ is rapidly deacetylated to tizoxanide (TIZ [5]), a compound with equal effectiveness (1, 32). In the liver, TIZ is then transformed to tizoxanide glucuronide (TIG) and excreted via bile or urine (5).
NTZ has been postulated to exhibit a mode of action similar to that of MET. Following drug administration, MET is transformed into an active compound after reduction of its nitro group by nitroreductases (NRs) including the pyruvate ferredoxin oxidoreductase (POR) system present in many anaerobic microorganisms (20). MET resistance is correlated with significantly lower POR activities in bacteria (34) and parasites such as G. lamblia (9) or Trichomonas vaginalis (10, 35; see also reference 37). Some of these MET-resistant strains, however, are still sensitive to other nitroimidazoles (30, 41, 42) or to NTZ (1). Sisson et al. (38) have described POR as a major target of NTZ by showing that NTZ inhibits Helicobacter pylori POR in vitro, and POR has been identified as a major target also in Giardia and other parasites (19). The same group shows that NTZ is not reduced by ferredoxin reduced by POR but rather inhibits POR activity in its protonated form by interfering with its cofactor thiamine pyrophosphate (19). G. lamblia shares many metabolic and genetic attributes of anaerobic bacteria. Pyruvate decarboxylation and subsequent electron transport to anaerobic electron acceptors rely on a eubacterium-like POR and an archaebacterium/eubacterium-like ferredoxin (6) and are thus different from those of higher eukaryotes.
Besides POR, molecular targets or binding proteins for thiazolides are unknown. A successful approach to isolate such binding proteins is affinity chromatography on an inert matrix with a covalently bound ligand followed by mass spectrometry-based sequencing of proteins eluted with free ligand in excess (see, e.g., references 3, 8, and 47). Here, we present a TIZ affinity chromatography approach in order to identify TIZ binding proteins in G. lamblia and report on the initial characterization of one major binding protein, namely, an NR (GlNR1).
MATERIALS AND METHODS
Tissue culture media, biochemicals, and drugs.
If not otherwise stated, all biochemical reagents were from Sigma (St. Louis, MO). The thiazolides (Table 1) were synthesized at the Department of Chemistry, University of Liverpool, or at the Department of Biochemistry and Chemistry, University of Berne. They were kept as 100 mM stock solutions in dimethyl sulfoxide (DMSO) at −20°C.
TABLE 1.
Overview of compounds used in this studya
The numeration and the two structural motifs, i.e., the benzene ring (B) and the thiazole moiety (T), are indicated on the NTZ formula.
b Activity (growth inhibition) against G. lamblia.
Axenic culture of Giardia trophozoites.
Trophozoites from G. lamblia WB clone C6 were grown under anaerobic conditions in 10-ml culture tubes (Nunc, Roskilde, Denmark) containing modified TYI-S-33 medium as described previously (23). In order to initiate subcultures, flasks containing confluent trophozoite lawns were incubated on ice for 15 min. Suspended motile trophozoites were counted (Neubauer chamber, 200×). Subcultures were initiated by adding 104 trophozoites to a new culture tube. Trophozoites were grown to confluence and harvested by centrifugation (1,300 × g, 13 min, 4°C). Trophozoite pellets were transferred to Eppendorf tubes, washed three times with phosphate-buffered saline (PBS) followed by centrifugation (5,000 × g, 8 min, 4°C), and stored at −20°C.
Protein extraction.
For protein extraction, frozen trophozoite pellets corresponding to ca. 3 × 108 cells were resuspended in ice-cold extraction buffer, i.e., PBS containing 1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride. Suspensions were vortexed thoroughly and centrifuged (13,000 rpm, 10 min, 4°C). Extraction of pellets was repeated twice. For 3 × 108 trophozoites, 5 ml of extraction buffer was used in total. Supernatants were combined (approximately 5 mg of total protein) and subjected to TIZ-agarose affinity chromatography.
Affinity chromatography using TIZ-agarose.
In order to produce TIZ-agarose, 1 g lyophilized epoxy-agarose with a C12 spacer was suspended in 15 ml H2O and centrifuged at 300 × g for 5 min. Washes in water were repeated twice, and one wash used coupling buffer (0.1 M NaHCO3, pH 9.5). After the last wash, 20 mg TIZ was added and coupling buffer was added to a maximum volume of 5 ml. The mixture was incubated for 3 days at 37°C under slow but continuous shaking in order to allow coupling of the epoxy group to TIZ via the OH- group in position 2 of the C6 ring (Table 1). The resulting column medium (approximately 3 ml) was then transferred to a chromatography column (Novagen, Merck, Darmstadt, Germany), and the column was washed with coupling buffer (20 ml). This was followed by a wash with ethanolamine (1 M, pH 9.5) for 4 h at 20°C in the absence of light in order to block residual reactive groups. Finally, the column was extensively washed with PBS and PBS-DMSO (1:1) in order to remove unbound TIZ. The orange TIZ column was stored in PBS containing 0.02% NaN3 at 4°C.
Prior to affinity chromatography, the TIZ column was washed with 50 ml PBS equilibrated at 20°C. Crude extracts (5 ml) of Giardia trophozoites prepared as described above were loaded with a flow rate of ca. 0.25 ml/min. The column was washed with PBS until the baseline was flat (8 column volumes, corresponding to about 24 ml). Proteins binding to the TIZ column were eluted with 1 mM NTZ in PBS followed by elution with a pH shift (100 mM glycine Cl−, pH 2.9) in order to remove nonspecifically bound proteins. Moreover, fractions were taken before elutions with NTZ (pre-NTZ) or pH shift (pre-pH shift). The sizes of these fractions ranged between 3 and 5 ml. From all fractions, 0.05- to 0.2-ml aliquots were taken for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). SDS-PAGE was performed according to the method of Laemmli (25) using a Hoefer Minigel 250 apparatus (Amersham, GE Healthcare, Little Chalfont, United Kingdom). Silver staining was performed according to the method of Blum et al. (4).
Protein sequencing by mass spectrometry.
For protein sequencing, the NTZ eluates with the highest amounts of binding protein were pooled and dialyzed against 1 g/liter of ammonium bicarbonate for 4 h and then against 0.4 g/liter of ammonium bicarbonate overnight at 4°C in the dark. The dialyzed fraction was then lyophilized. Aliquots of the lyophilized binding protein (ca. 200 ng) were suspended in SDS-polyacrylamide sample buffer, loaded on a 12% acrylamide gel, and subjected to electrophoresis. After staining with colloidal Coomassie blue (0.1% Coomassie brilliant blue G-250 in 34% methanol with 0.5% acetic acid and 17% ammonium sulfate), a band of ca. 35 kDa was excised and processed for mass spectrometry analysis.
Mass spectrometry identification of proteins was performed as previously described (40). Liquid chromatography-tandem mass spectrometry data were acquired using an LTQ ion-trap mass spectrometer (Thermo-Electron, Hemel Hempstead, United Kingdom) coupled online to a Thermo-Finnigan surveyor high-pressure liquid chromatography system equipped with a BioBasic C18 reversed-phase column (100 × 0.18 mm) and submitted to MASCOT to search predicted open reading frames from GiardiaDB (http://gmod.mbl.edu/perl/site/giardia?page=intro) and MSDB. Database search parameters included fixed carbamidomethyl modification of cysteine residues; variable oxidation of methionine; a peptide tolerance of up to ±2 Da; a tandem mass spectrometry tolerance of ±0.8 Da; a +1, +2, +3 peptide charge state; and a single missed trypsin cleavage.
Cloning and heterologous expression of GlNR1 in Escherichia coli.
In order to clone GlNR1 into the His tag expression vector pET151 directional TOPO (Invitrogen, Carlsbad, California), the primers pNRforward and pNRreverse (Table 2) were created for the amplification of a 795-bp product encoding the GlNR1 (EAA43030.1) polypeptide (MWG Biotech, Ebersberg, Germany).
TABLE 2.
Overview of primers used in this study
| Gene | Accession no. | Region of CDSa or rRNA | Primer name and sequence (5′→3′) |
|---|---|---|---|
| GlACT1 | EAA39190 | CDS bp 715-933 | ACTquantF, ACATATGAGCTGCCAGATGG |
| ACTquantR, TCGGGGAGGCCTGCAAAC | |||
| GlGDH | XM_773614 | CDS bp 761-893 | GDH-F, AGGTCCTCACCTTCTCAGACT |
| GDH-R, GGATACTTGTCCTTGAACTCGG | |||
| GlNR1 | EAA43030.1 | Full length (794 bp) | NRfullF, CACCATGGTTGAAG GTTATCCTG |
| NRfullR, TTACTTAAATGTAATGTCGAC | |||
| CDS bp 526-794 plus 8 bp of 3′-flanking sequence | NRquantF, CCTGCTGACAAGGCCGCA | ||
| NRquantR, AACACCAATTACTTAAATGTAATG | |||
| 16S rRNA | M54878.1 | bp 79-300 | Gl16SquantF, GACGGCTCAGGACAACGG |
| Gl16SquantR, CTCTCCGGAGTCGAACCC |
CDS, coding sequence.
For amplification by PCR, genomic DNA was extracted from 2 × 107 trophozoites using the Dneasy kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The resulting DNA was diluted 10 times in ultrapure water. The PCR was performed using 0.6 U of Pfu polymerase (Promega, Madison, WI), 2 μl 10× Pfu buffer (Promega), 20 pmol of each primer, and 0.16 mM deoxynucleoside triphosphates (Promega) in a total volume of 20 μl. The annealing temperature was 54°C. The resulting 795-bp product was inserted into pET151 vector using the respective cloning kit according to the directions of the manufacturer, and the vector was transformed into E. coli TOP 10 cells (Invitrogen).
For heterologous expression of GlNR1 in E. coli, minipreparation DNA from E. coli TOP 10 containing the plasmid with the insert in the correct orientation (pETGlNR1) was batch transformed into E. coli BL21 Star (Invitrogen). The batch was grown overnight in 2 ml LB with 100 ppm carbenicillin and then transferred to a 500-ml Erlenmeyer flask containing 50 ml LB with 100 ppm carbenicillin and grown until the absorbance at 600 nm was 0.5. Expression of recombinant GlNR1 was induced by addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). As a negative control, 10 ml of culture was withdrawn prior to induction and cultivated in a 100-ml Erlenmeyer flask. Cells were harvested after 3.5 h, chilled down, pelleted by centrifugation (4,000 × g, 20 min, 4°C), and stored at −20°C.
For His tag purification, pellets from induced E. coli cells were suspended in 0.5 ml LEW buffer (Macherey-Nagel, Düren, Germany) containing 0.5% Triton X-100 and 10 μl protease inhibitor cocktail suitable for His tag purification (P8849; Sigma). The suspension was freeze-thawed three times on dry ice and then centrifuged for 15 min at 13,000 rpm and 4°C. The extraction was repeated once. Supernatants were combined and loaded on Protino Ni-TED 150 columns (Macherey-Nagel) in tandem. Columns were washed with 1× LEW buffer, and bound protein was eluted with elution buffer according to the manufacturer's instructions. Eluted protein (recombinant GlNR1 [recGlNR1]) was stored in 50% glycerol in elution buffer at −20°C.
NR enzyme assay.
NR activity was measured by a photometric assay based on the reduction of dinitrotoluene (DNT) by NADH (24). The assay was performed in 96-well microtiter plates (Nunc) with 174 μl assay buffer (50 mM Tris-Cl−, pH 7.0) per well containing DNT or other compounds (0 to 50 μM in 100 mM stocks in DMSO) and recGlNR1 (1 to 2 μl) or buffer (enzyme blank). The reaction was started by addition of 6 μl NADH (4.5 mM in assay buffer; 0.15 mM as final concentration). After 5 min of preincubation, the absorbance at 340 nm was read at various time points (0 to 20 min) on a 96-well plate spectrophotometer (Versamax; Molecular Devices, Sunnyvale, CA). Enzyme activity was calculated from the linear decrease of absorption over time and expressed in μkat per g recombinant protein, 1 kat being 1 mol of substrate (NADH; extinction coefficient, 6,220 M−1 cm−1) oxidized per s.
The inhibition constants (Ki) for NTZ were measured at variable concentrations of DNT and 0.15 mM NADH or variable concentrations of NADH and 15 μM DNT and variable concentrations of inhibitor. The results were fitted to the equation Ki = I/{(S/Km)[(Vm/v) − 1] −1}, assuming a competitive inhibition, S being the DNT concentration, or to the equation Ki′ = I/[(Vm/v) − (Km/s) − 1], assuming a noncompetitive inhibition, S being the NADH concentration, v being the initial rate of product formation, Vm being the maximum catalytic rate, and Km being the Michaelis constant.
Quantification of GlNR1 expression by real-time RT-PCR.
For quantification of GlNR1 expression by real-time reverse transcription-PCR (RT-PCR), trophozoites were grown until near confluence as described above. NTZ, MET (25 μM), or DMSO (control) was added to the culture tubes, and cells were incubated for 3 h. At this time point, cells were still motile. Cells were harvested as described above, and RNA was extracted using the QIAGEN RNeasy kit including DNase I digestion (to remove residual genomic DNA) according to the instructions provided by the manufacturer. RNA was eluted with 50 μl RNase-free water and stored at −80°C.
First-strand cDNA was synthesized using the QIAGEN OmniscriptRT kit as described by the manufacturer. After quantitative RT-PCR, expression levels were given as relative values in arbitrary units relative to the amount of 16S rRNA (M54878.1 [39]). The primers NRquantF and NRquantR were used for the quantification of GlNR1 (EAA43030.1) expression, the primers GDH-F and GDH-R were used for the quantification of G. lamblia glutamate dehydrogenase (GlGDH; XM_773614) constitutively expressed at high levels (28), and the primers ACTquantF and ACTquantR were used for the quantification of another constitutively expressed gene, namely, the G. lamblia actin (GlACT1) (EAA39190; Table 2) transcript. Quantitative PCR was performed as described previously (44). From the quantitative RT-PCR data, mean values (±standard errors [SE]) from triplicate determinations were assessed and expression levels of the genes summarized in Table 2 were given as values in arbitrary units relative to the amount of 16S rRNA.
RESULTS
An NR homologue is a major TIZ binding protein in G. lamblia.
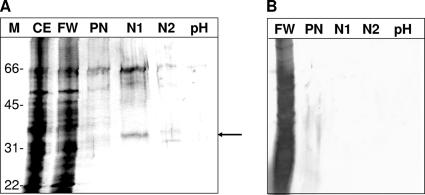
Following TIZ affinity chromatography, a major Giardia protein of approximately 35 kDa was eluted with 1 mM NTZ. The fractions before elution and pH shift after elution did not yield any further major bands. The protein smear appearing at 66 kDa and below was identified as a medium contaminant, bovine serum albumin (data not shown). When elution was performed with the bromothiazolide RM4819, which was ineffective on G. lamblia (see the work of Müller et al. [32]), instead of NTZ, no band was eluted (Fig. 1).
FIG. 1.
Affinity chromatography of cell extract from 3 × 108 G. lamblia trophozoites. Aliquots of fractions were separated by SDS-PAGE. Bands were visualized by silver staining. Lanes: M, sizes of marker proteins (kDa); CE, crude extract; FW, flowthrough plus wash; PN, pre-NTZ (1 mM in PBS); N1, NTZ; N2, NTZ pre-pH shift (pH 2.9); pH, pH shift. The 35-kDa protein is marked with an arrow. (A) TIZ-agarose as matrix; (B) RM4819-agarose as matrix.
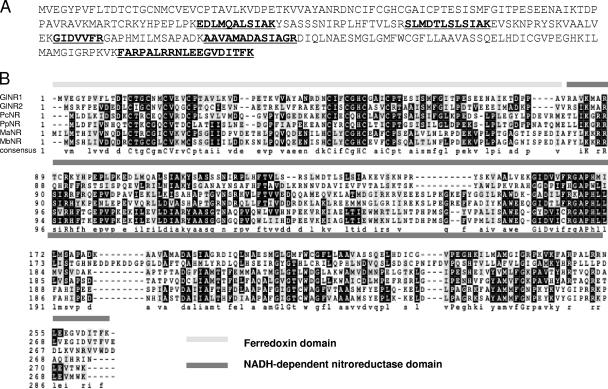
The 35-kDa protein eluted from TIZ columns by NTZ was subjected to mass spectrometry. Six peptides were identified (MASCOT probability score, 313) as belonging to the NR homologue EAA43030.1 (Fig. 2A). Blast analysis (2) revealed that the closest homologues were XM_764091.1 from G. lamblia, NC_007498.2 from Pelobacter carbinolicus, NZ_AAJH01000004.1 from Pelobacter propionicus, C2A NC_003552.1 from Methanosarcina acetivorans, and NC_007355.1 from Methanosarcina barkeri, all with probability scores of 2 × 10−25 or less. All these proteins exhibit similar overall structures, with an N-terminal ferredoxin domain of about 80 amino acids, similar to the N terminus of a prokaryotic acetyl coenzyme A synthase, followed by a highly conserved NADH-dependent NR domain (Fig. 2Β). The protein from an organism known to be sensitive to NTZ (19) with the closest similarity to EAA43030.1 was NZ_AAML03000013.1 from Clostridium difficile with 23% sequence identity. Serial analysis of gene expression (Giardia lamblia Genome Database [www.mbl.edu/Giardia]) of the two G. lamblia NR homologues revealed that 0.034% of all transcripts in trophozoites code for EAA43030.1, while 0.0026% of transcripts code for XM_764091.1. Thus, EAA43030.1 seems to be expressed at a much higher level than XM_764091.1. Both proteins lack a signal peptide at their N terminus and are therefore considered cytosolic.
FIG. 2.
(A) Protein sequence of GlNR1 (EAA43030.1). Peptides identified by mass spectrometry are shown by underlining and boldface. (B) Alignment of GlNR1 with five of the most closely related oxygen-insensitive NRs, namely, the hypothetical Giardia lamblia NR XM_764091.1 (GlNR2), the Pelobacter carbinolicus (DSM 2380) NR family protein NC_007498.2 (PcNR), the Pelobacter propionicus DSM 2379 NADH NR NZ_AAJH01000004.1 (PpNR), the Methanosarcina acetivorans C2A NADH NR NC_003552.1 (MaNR), and the Methanosarcina barkeri strain Fusaro NR NC_007355.1 (MbNR). The alignment was produced with ClustalW (21). The conserved ferredoxin- and NAD(P)H-dependent NR domains are highlighted.
EAA43030.1 exhibits NR activity and is named GlNR1.
The coding sequence of EAA43030.1 was amplified by PCR and cloned and expressed in E. coli. By SDS-PAGE, the resulting product migrated at around 35 kDa. The recombinant protein was the major protein in IPTG-induced E. coli and could further be purified by His tag affinity chromatography (Fig. 3A). The purified protein was subjected to an NR assay based on the oxidation of NADH in the presence of nitro compounds (Fig. 3B). The 35-kDa recombinant protein readily reduced DNT, but no reductase activity could be measured when NTZ or MET was offered as a substrate (Fig. 3B). Thus, we propose that EAA43030.1 be named Giardia lamblia NR 1 (GlNR1).
FIG. 3.
(A) SDS-PAGE of recGlNR1 in noninduced (C) or induced (I) (3.5 h, 1 mM IPTG) E. coli and after His tag purification (HT). Lane M, sizes in kilodaltons. (B) Activity of recGlNR1 with DNT, NTZ, or MET as a substrate (50 μM). Assays were run in triplicate. Mean values ± SE are given.
The NR activity of recGlNR1 is inhibited by a number of thiazolides.
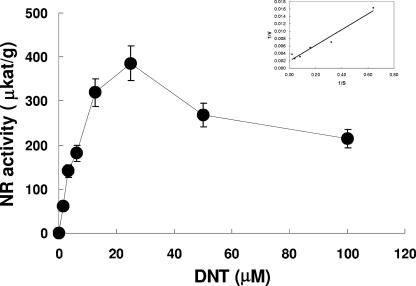
recGlNR1 exhibited a peak activity at around 25 μM DNT, followed by a decrease of specific activity at higher DNT concentrations. The Km, calculated after Lineweaver-Burk transformation, was 11 ± 1.3 μM (Fig. 4) for DNT and 650 μM ± 108 μM for NADH (not shown). Both values were in good agreement with previously published data from other NRs (24).
FIG. 4.
Activity of recGlNR1 with DNT as substrate. The inset contains the Lineweaver-Burk plot used for the calculation of the Km value for DNT. Assays were run in triplicate. Mean values ± SE are given.
In the presence of NTZ, specific activity decreased in a concentration-dependent matter (Fig. 5A and B) in competition with DNT with a Ki of 0.3 ± 0.1 μM. When NADH was varied and DNT kept constant, a noncompetitive Ki′ of 0.5 ± 0.11 μM was found.
FIG. 5.
Activity of recGlNR1 with DNT as substrate in the presence of NTZ (2 to 10 μM) or DMSO (0 μM). Assays were run in triplicate. Mean values ± SE are given. (A) The NADH concentration was 0.15 mM, and the DNT concentration varied. (B) The DNT concentration was kept constant at 15 μM, and the NADH concentration varied.
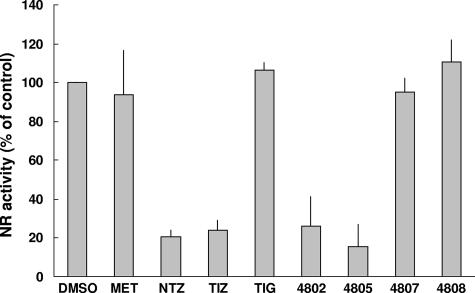
MET and a range of thiazolides (NTZ derivatives) were assessed for functional inhibition of recGlNR1 at a concentration of 5 μM. All nitrothiazolides earlier shown to kill Giardia during axenic culture (32), namely, NTZ, TIZ, RM4802, and RM4805, also severely impaired GlNR1 NR activity by more than 75% (Fig. 6). RM4805 was the strongest inhibitor. TIG and MET did not inhibit NR activity (Fig. 6), nor did the nitro compounds RM4807 and RM4808, which were earlier shown to exhibit no activity against G. lamblia (32).
FIG. 6.
Activities of recGlNR1 with DNT as substrate (25 μM) in the presence of DMSO (solvent control), MET, and a number of nitrothiazolides (NTZ, TIZ, TIG, 4802, 4805, 4807, and 4808, all at 5 μM). Assays were run in triplicate. Mean values ± SE are given.
NR expression in NTZ-treated Giardia trophozoites.
In order to quantify NR expression, we have developed a quantitative real-time RT-PCR assay using 16S rRNA as a reference for the amount of total cDNA. In order to compare the expression level of GlNR1 to those of genes considered constitutive and expressed in large amounts, GlACT1 and GlGDH have been included besides GlNR1 in our analysis. In untreated trophozoites, the expression level of GlNR1 is approximately 100 times lower than the GlGDH1 expression level and 10 times lower than the GlACT1 expression level. Upon treatments with NTZ or MET (25 μM for 3 h), the relative amounts of cDNAs of all three genes were decreased. A pronounced decrease of GlGDH to ca. 50% of the NTZ levels was observed in MET-treated trophozoites. GlNR1 expression levels were decreased in the same proportion as the levels of the two other genes (Fig. 7).
FIG. 7.
Quantification of GlNR1 expression by real-time RT-PCR. Tachyzoites were grown for 3 h in the presence of NTZ or MET (25 μM) prior to harvest. RNA was extracted and reverse transcribed to cDNA. Transcripts of GlACT1 (ACT), GlGDH (GDH), and GlNR1 (NR) were quantified in relation to 16S rRNA. The relation is expressed in arbitrary units (AU). Mean values ± SE are given. Note that, for reasons of scale, NR values were multiplied by 100 and ACT values by 10.
DISCUSSION
In this study we have shown that the ferredoxin NR GlNR1 is a major thiazolide binding protein in G. lamblia and that its DNT NR activity can be inhibited by NTZ with a Ki lower than 1 μM, thus in a range comparable to 50% inhibitory concentration values for trophozoite growth (32). GlNR1 is expressed in trophozoites, and transcriptional expression of GlNR1 relative to the constitutively expressed GlGDH and GlACT1 genes is not affected by drug treatment. The observed overall decrease of transcription relative to 16S rRNA seems to be an early consequence of drug toxicity prior to loss of cell motility. Very little is known about ferredoxin NRs, and this work is one of the first studies. The mechanism is not understood. It could be that NAD(P)H reduces the ferredoxin moiety, which in turn reduces the substrate. This would explain the relatively high Km for NADH. We did not obtain any evidence that either NTZ or MET was reduced by recGlNR1 as measured by oxidation of NADH. Moreover, concerning NTZ, a change in absorption at 416 nm, the maximum of the NTZ spectrum (see reference 38), due to protonation of the anion was not seen either in the presence or in the absence of DNT. The presence of a nitro group seems, however, to be required for binding of GlNR1 to thiazolides, since affinity chromatography carried out under identical conditions with epoxy-agarose coupled to a bromo derivative lacking the nitrothiazole did not produce any detectable bound protein. The fact that the glucuronated version of TIZ (TIG) did not inhibit NR activity indicates that this nitrothiazole compound may be ineffective in vivo not only due to impaired uptake due to the attached glucuronide but possibly also due to impaired inhibition of the respective target enzyme(s).
Related ferredoxin NRs with a ferredoxin domain at their N terminus like GlNR1 and GlNR2 are found in anaerobic prokaryotes such as Clostridium, Methanosarcina, Pelobacter, and others. Oxygen-insensitive NRs without ferredoxin domains are present in many archaebacteria and eubacteria (33), where they allow the assimilation of nitro compounds as C sources (22, 27). In H. pylori, an NR of this type encoded by the gene rdxA has been shown to be responsible for resistance to MET (16). RdxA NR is not very similar to classical NRs of E. coli and other enteric bacteria. It is unique in its ability to reduce MET to a toxic radical, whereas another related enzyme, FrxA (see also reference 31), has no activity. Both enzymes, however, have shown activities on NTZ (35). Eukaryotic parasites such as Entamoeba histolytica and G. lamblia may have acquired these NRs by independent lateral transfer (6, 33), since E. histolytica NR shows poor relatedness to GlNR1. Therefore, inhibition of NR by a given drug in one species cannot be generalized to others. While it has been demonstrated that the expression of MET-reducing enzymes is downregulated in MET-resistant bacteria and MET-resistant Giardia strains, we have no evidence, so far, that GlNR1 expression is substantially affected in NTZ- or MET-resistant Giardia clones (data not shown; unpublished data).
Neither MET nor NTZ appears to be reduced by GlNR1, and the actual physiological substrate of this enzyme is unknown. MET may be reduced by POR or GlNR2, neither of which has yet been characterized with respect to its biochemical properties in Giardia. NTZ and related thiazolides can act, however, as GlNR1 inhibitors (Fig. 6). Interestingly, protonated NTZ has been shown to inhibit POR activity by interfering with its cofactor thiamine pyrophosphate (19).
The functional relevance of GlNR1 is still unknown and needs further investigation. The simplest explanation would be that GlNR1 does not represent the main NTZ target but binds thiazolides due to structural similarities with the target protein(s). GlNR1 could thus act as an internal buffer for the drug and could thus be lowering its availability for target proteins. By inhibiting GlNR1 function, however, the enzyme cannot transfer its electrons to an (unknown) endogenous or exogenous substrate as in anaerobic prokaryotes (20, 22). Such an impairment of intermediary metabolism may enhance other effects of the drug such as POR inhibition. In the presence of nitro drugs, inhibition of GlNR1 would thus further disturb the redox balance of the cell (see reference 7), leading to damage of the membrane (26) and the secretory apparatus (17) followed by cell death.
In conclusion, an NR, GlNR1, was isolated from a G. lamblia extract by TIZ affinity chromatography. So far it is unclear whether GlNR1 is the sole, or main, target for thiazolides or whether it has a more indirect role in the thiazolide mode of action. Further ongoing studies, including the detailed analysis of thiazolide- and MET-resistant Giardia, as well as overexpression and/or knockout studies, will shed light on the functional relevance of GlNR1 and its role as a target for NTZ and NTZ derivatives.
Acknowledgments
We are indebted to Andrew Stachulski (Department of Chemistry, University of Liverpool) and Christian Leumann (Department of Biochemistry and Chemistry, University of Berne) for the synthesis of the thiazolides.
This study was supported by Swiss National Science Foundation grant no. 3100A0-112532/1 (A.H.) and a grant from the Swiss Secretariat for Education and Science (SBF COST Action B22; grant no. C05.0104, J.M. and N.M.), and J.M. was partially financially supported by the University of Berne. J.M.W. gratefully acknowledges the receipt of funding from Romark Laboratories.
Footnotes
Published ahead of print on 16 April 2007.
REFERENCES
- 1.Adagu, I. S., D. Nolder, D. Warhurst, and J.-F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. L. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andon, N., D. Eckert, J. R. Yates, and P. Haynes. 2003. High-throughput functional affinity purification of mannose binding proteins from Oryza sativa. Proteomics 3:1270-1278. [DOI] [PubMed] [Google Scholar]
- 4.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 5.Broekhuysen, J., A. Stockts, R. L. Lins, J. De Graeve, and J.-F. Rossignol. 2000. Nitazoxanide: pharmacokinetics and metabolism in man. Int. J. Clin. Pharmacol. Ther. 38:387-394. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. M., J. A. Upcroft, M. R. Edwards, and P. Upcroft. 1998. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int. J. Parasitol. 28:149-164. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan, B. B., and Y. Balmer. 2005. Redox regulation: a broadening horizon. Annu. Rev. Plant Biol. 56:187-220. [DOI] [PubMed] [Google Scholar]
- 8.Curtis, S. J., and J. L. Strominger. 1981. Purification of penicillin-binding protein 2 of Escherichia coli. J. Bacteriol. 145:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan, M., A. Wang, and C. Wang. 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol. 36:447-456. [DOI] [PubMed] [Google Scholar]
- 10.Dunne, R. L., L. A. Dunn, P. Upcroft, P. J. O'Donoghue, and J. A. Upcroft. 2003. Drug resistance in the sexually transmitted protozoan Trichomonas vaginalis. Cell Res. 13:239-249. [DOI] [PubMed] [Google Scholar]
- 11.Esposito, M., R. Stettler, S. L. Moores, C. Pidathala, N. Müller, A. Stachulski, N. G. Berry, J. F. Rossignol, and A. Hemphill. 2005. In vitro efficacies of nitazoxanide and other thiazolides against Neospora caninum tachyzoites reveal antiparasitic activity independent of the nitro group. Antimicrob. Agents Chemother. 49:3715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esposito, M., N. Müller, and A. Hemphill. 2007. Structure-activity relationships from in vitro efficacies of the thiazolide series against the intracellular apicomplexan protozoan Neospora caninum. Int. J. Parasitol. 37:183-190. [DOI] [PubMed] [Google Scholar]
- 13.Fox, L. M., and L. D. Saravolatz. 2005. Nitazoxanide: a new thiazolide antiparasitic agent. Rev. Anti-Infect. Agents 40:1173-1180. [DOI] [PubMed] [Google Scholar]
- 14.Gardner, T. B., and D. R. Hill. 2001. Treatment of giardiasis. Clin. Microbiol. Rev. 14:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilles, H. M., and P. S. Hoffman. 2002. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends Parasitol. 18:95-97. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin, A., D. Kersulyte, G. Sisson, S. J. O. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 17.Hehl, A. B., and M. Marti. 2004. Secretory protein trafficking in Giardia intestinalis. Mol. Microbiol. 53:19-28. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill, A., J. Müller, and M. Esposito. 2006. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin. Pharmacother. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman, P. S., G. Sisson, M. A. Croxen, K. Welch, W. D. Harman, N. Cremades, and M. G. Morash. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 51:868-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horner, D. S., R. P. Hirt, and T. M. Embley. 1999. A single eubacterial origin of eukaryotic pyruvate:ferredoxin oxidoreductase genes: implications for the evolution of anaerobic eukaryotes. Mol. Biol. Evol. 16:1280-1291. [DOI] [PubMed] [Google Scholar]
- 21.Huang, X., and W. Miller. 1991. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 12:337-357. [Google Scholar]
- 22.Johnson, G. R., and J. C. Spain. 2003. Evolution of catabolic pathways for synthetic compounds: bacterial pathways for degradation of 2,4-dinitrotoluene and nitrobenzene. Appl. Microbiol. Biotechnol. 62:110-123. [DOI] [PubMed] [Google Scholar]
- 23.Keister, D. B. 1983. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487-488. [DOI] [PubMed] [Google Scholar]
- 24.Kutty, R., and G. N. Bennett. 2005. Biochemical characterization of trinitrotoluene transforming oxygen-insensitive nitroreductases from Clostridium acetobutylicum ATCC 824. Arch. Microbiol. 184:158-167. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd, D., J. C. Harris, G. A. Biagini, M. R. Hughes, S. Maroulis, C. Bernard, R. B. Wadley, and M. R. Edwards. 2004. The plasma membrane of microaerophilic protists: oxidative and nitrosative stress. Microbiology 150:1183-1190. [DOI] [PubMed] [Google Scholar]
- 27.Luque-Almagro, V. M., R. Blasco, L. Paloma Sáez, M. D. Roldán, C. Moreno-Vivián, F. Castello, and M. Martínez-Luque. 2006. Interactions between nitrate assimilation and 2,4-dinitrophenol cometabolism in Rhodobacter capsulatus E1F1. Curr. Microbiol. 53:37-42. [DOI] [PubMed] [Google Scholar]
- 28.Marti, M., A. Regös, Y. Li, E. M. Schraner, P. Wild, N. Müller, L. G. Knopf, and A. B. Hehl. 2003. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J. Biol. Chem. 278:24837-24848. [DOI] [PubMed] [Google Scholar]
- 29.McClure, S., and K. Palma. 1999. Treatment of equine protozoal myeloencephalitis with nitazoxanide. J. Equine Vet. Sci. 19:639-641. [Google Scholar]
- 30.Megraud, F., A. Occhialini, and J.-F. Rossignol. 1998. Nitazoxanide: a potential drug for eradication of Helicobacter pylori with no cross-resistance to metronidazole. Antimicrob. Agents Chemother. 42:2836-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukhopadhyay, A. K., J.-Y. Jeong, D. Dailidiene, P. S. Hoffman, and D. E. Berg. 2003. The fdxA ferredoxin gene can down-regulate frxA nitroreductase gene expression and is essential in many strains of Helicobacter pylori. J. Bacteriol. 185:2927-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller, J., G. Rühle, N. Müller, J.-F. Rossignol, and A. Hemphill. 2006. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 50:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixon, J. E., A. Wang, J. Field, H. Morrison, A. G. McArthur, M. L. Sogin, B. J. Loftus, and J. Samuelson. 2002. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 1:181-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rasmussen, B. A., K. Bush, and F. P. Tally. 1997. Antimicrobial resistance in anaerobes. Clin. Infect. Dis. 24(Suppl. 1):S110-S120. [DOI] [PubMed] [Google Scholar]
- 35.Rasoloson, D., S. Vanacova, E. Tomkova, J. Razga, I. Hrdy, J. Tachezy, and J. Kulda. 2002. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology 148:2467-2477. [DOI] [PubMed] [Google Scholar]
- 36.Rossignol, J. F., and H. Maisonneuve. 1984. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana. Am. J. Trop. Med. Hyg. 33:511-512. [DOI] [PubMed] [Google Scholar]
- 37.Samuelson, J. 1999. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 3:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sisson, G., A. Goodwin, A. Raudonikiene, N. J. Hughes, A. K. Mukhopadhyay, D. A. Berg, and P. S. Hoffman. 2002. Enzymes associated with reductive activation and action of nitazoxanide, nitrofurans, and metronidazole in Helicobacter pylori. Antimicrob. Agents Chemother. 46:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sogin, M. L., J. H. Gunderson, H. J. Elwood, R. A. Alonso, and D. A. Peattie. 1989. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science 243:75-77. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan, S., T. Baszler, N. Vonlaufen, A. Leepin, S. J. Sanderson, J. M. Wastling, and A. Hemphill. 2006. Monoclonal antibody directed against Neospora caninum tachyzoite carbohydrate epitope reacts specifically with apical complex-associated sialylated beta tubulin. J. Parasitol. 92:1235-1243. [DOI] [PubMed] [Google Scholar]
- 41.Upcroft, J. A., R. W. Campbell, K. Benakli, P. Upcroft, and P. Vanelle. 1999. Efficacy of new 5-nitroimidazoles against metronidazole-susceptible and -resistant Giardia, Trichomonas, and Entamoeba spp. Antimicrob. Agents Chemother. 43:73-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upcroft, J. A., L. A. Dunn, J. M. Wright, K. Benakli, P. Upcroft, and P. Vanelle. 2006. 5-Nitroimidazole drugs effective against metronidazole-resistant Trichomonas vaginalis and Giardia duodenalis. Antimicrob. Agents Chemother. 50:344-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Upcroft, J., and P. Upcroft. 1998. My favourite cell: Giardia. Bioessays 20:256-263. [DOI] [PubMed] [Google Scholar]
- 44.Von Allmen, N., M. Bienz, A. Hemphill, and N. Müller. 2005. Quantitative assessment of sense and antisense transcripts from genes involved in antigenic variation (vsp genes) and encystation (cwp 1 gene) of Giardia lamblia clone GS/M-83-H7. Parasitology 130:389-396. [DOI] [PubMed] [Google Scholar]
- 45.White, C. A., Jr. 2004. Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev. Anti-Infect Ther. 2:43-49. [DOI] [PubMed] [Google Scholar]
- 46.Wright, J., L. Dunn, P. Upcroft, and J. Upcroft. 2003. Efficacy of antigiardial drugs. Expert Opin. Drug Saf. 2:529-541. [DOI] [PubMed] [Google Scholar]
- 47.Yingst, D. R., S.-Y. Yang, and R. Schiebinger. 1998. Purification of active Na+-K+-ATPase using a new ouabain-affinity column. Am. J. Physiol. 275(4 Pt. 1):C1167-C1177. [DOI] [PubMed] [Google Scholar]