Abstract
An experimental study has been performed to compare the in vitro activity and the in vivo efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. In vitro experiments included MIC determination, time-kill, and synergy studies. For in vivo studies, a mouse model of sepsis has been used. The main outcome measures were bacterial lethality, quantitative blood cultures, and plasma levels of lipopolysaccharide, tumor necrosis factor alpha, and interleukin-6. The combination of tachyplesin III or colistin with imipenem showed in vitro synergistic interaction. A significant increase in efficacy was also observed in vivo: combination-treated groups had significantly lower levels of bacteremia than did groups treated with a single agent. Tachyplesin III combined with imipenem exhibited the highest efficacy on all main outcome measurements. These results highlight the potential usefulness of these combinations and provide therapeutic alternatives for serious infections caused by gram-negative bacteria in the coming years.
Resistance among bacteria is on the rise, both in the hospital and in the community (3, 22). In comparison with that in gram-positive cocci, for which resistance to a single antibiotic indicates the antibiotic resistance phenotype of interest, multidrug resistance in gram-negative bacilli is difficult to define. Among these resistant bacteria, Pseudomonas aeruginosa represents a pathogen with notable virulence characteristics and the ability to exhibit antibiotic resistance. Multidrug resistance in P. aeruginosa strains has been defined as resistance to two or more of various antibiotics typically used to treat infections with these organisms. This variable definition is clearly arbitrary and may be of questionable practical value to a clinician (21, 23). Of greater relevance may be a definition of “panresistance,” because the complete or almost complete lack of treatment options, now primarily beta-lactams, especially carbapenem, and fluoroquinolones, is an increasingly common and desperate occurrence in the hospital setting (23).
Antimicrobial therapy for infections due to these multiresistant organisms remains a clinical dilemma with hospitalized patients. Strategies for prevention and therapy include both infection control and modifications in antibiotic use, such as antibiotic cycling or rotation, combination therapies, and the use of “old” and new drugs for the management of infections with gram-negative bacilli resistant to all other alternatives (4, 16).
Since the discovery of polymyxins, a number of cationic peptides have been isolated from a wide range of bacterial, plant, and animal species (2, 12, 14). They are a recently emerged class of antibiotics with therapeutic potential. These molecules are important components of the innate immune response in most multicellular organisms, used by animals to effectively deal with pathogenic microorganisms in their environments (12, 14, 18). In mammals, these peptides are found in circulating phagocytes, where they contribute to the killing of engulfed microorganisms, and in epithelial surfaces, where they act as a local defense mechanism that protects anatomical compartments from microbial invasion.
Tachyplesins are a group of antimicrobial peptides isolated from horseshoe crabs. Tachyplesin III (KWCFRVCYRGICYRKCR-NH2), isolated from the lymph hemolysate of Southeast Asian horseshoe crabs Tachypleus gigas and Carcinoscorpius rotundicauda and consisting of 17 amino acids with two disulfide bridges, is a representative antimicrobial peptide with a cyclic β-sheet. It is similar in structure and activity profile to the protegrins, a family of peptides found in pig intestines. Because of its potency and relatively small size, this peptide is an attractive target for structure/activity studies that may lead to therapeutics to treat infections (13, 19). Tachyplesin III exhibits broad-spectrum activity against gram-negative and -positive bacteria, fungi, and even enveloped viruses at low concentrations. Furthermore, it has also been shown to play a role in the proinflammatory response because it forms complexes with bacterial lipopolysaccharides (LPS) that neutralize the factor C-activating activity of LPS in a manner similar to that of anti-LPS factor (19). It was reported, even though the precise action mechanism of these cationic peptides remains to be determined, that they rapidly perturbed the membrane function of pathogenic microorganisms.
Recent reports have shown that a synergistic effect was observed in several clinically isolated bacterial strains when some antimicrobial peptides were combined with several clinically used antibiotics. Therefore, the presence of this synergistic effect makes the cationic peptides potentially valuable as an adjuvant for antimicrobial chemotherapy against antibiotic-resistant bacterial strains (9, 25).
In order to broaden our knowledge of this role, we evaluated the activities of the combination of tachyplesin III or colistin and imipenem in vitro and in vivo using two P. aeruginosa strains.
MATERIALS AND METHODS
Organisms.
The commercially available quality control strain of P. aeruginosa, ATCC 27853, and one clinical multiresistant P. aeruginosa strain were used in this study. The clinical strain was isolated from a specimen submitted for routine bacteriological investigation to the Institute of Infectious Diseases and Public Health, Polytechnic University of Marche, Ancona, Italy. Its susceptibility pattern indicates resistance to aminopenicillins, aztreonam, cephalosporins, carbapenems, quinolones, and aminoglycosides.
Synthetic peptide.
Tachyplesin III (molecular mass, 2,495.5 Da) was synthesized by 9-fluorenylmethoxycarbonyl solid-phase chemistry (5, 8). The protected peptidyl resin was treated with 92% trifluoroacetic acid, 2% ethanedithiol, 2% water, and 2% triisopropylosilane for 2 h. After cleavage, the solid support was removed by filtration, and the filtrate was concentrated under reduced pressure. The cleaved peptide was precipitated with diethyl ether, dissolved in 20% acetic acid, and oxidized with 0.1 M iodine in methanol. The tachyplesin III was purified and analyzed by high-performance liquid chromatography. The resulting fractions with purity greater than 94 to 95% were tested by high-performance liquid chromatography. The peptide was analyzed by matrix-assisted laser desorption ionization-time of flight mass spectrometry. The peptide was solubilized in phosphate-buffered saline (PBS) (pH 7.2), yielding 1 mg/ml stock solution. Solutions of drugs were made fresh on the day of assay or stored at −80°C in the dark for short periods.
MIC determination and bacterial killing assay.
Tachyplesin III, colistin (Sigma-Aldrich, Milan, Italy), and imipenem (Merck, Sharp & Dohme, Milan, Italy) powders were diluted in accordance with the manufacturers’ recommendations. Solutions of drugs were made fresh on the day of assay or stored at −80°C in the dark until 20 days. The MICs were determined using a broth microdilution method with Mueller-Hinton (MH) broth (Becton Dickinson Italia, Milan, Italy) and initial inocula of 5 × 105 CFU/ml, according to the procedures outlined by CLSI (formerly NCCLS) (20). Polypropylene 96-well plates (Becton Dickinson and Co., Franklin Lakes, NJ) were incubated for 18 h at 37°C in air. When the peptide tachyplesin III was tested, the plates were shaken throughout the study, since this compound has a tendency to precipitate. The MIC was taken as the lowest drug concentration at which observable growth was inhibited. Experiments were performed in triplicate. The minimum bactericidal concentration was taken as the lowest concentration of each drug that resulted in a more than 99.9% reduction of the initial inoculum. Experiments were performed in triplicate.
Both strains were grown at 37°C in MH broth. Aliquots of exponentially growing bacteria were resuspended in fresh MH broth at approximately 107 cells/ml and separately exposed to each peptide at 2× MIC for 0, 2, 5, 10, 20, 30, 40, 50, and 60 min at 37°C. After these times, 0.1-ml samples were serially diluted and plated onto MH agar plates to obtain viable colonies. The limit of detection for this method was approximately 10 CFU/ml. In preliminary experiments, antibiotic carryover was ruled out by plating samples of bacterial suspensions. Killing was also investigated in combination studies, to evaluate the activity of imipenem at a concentration of 4 mg/liter when combined with each peptide at 0.5× MIC and 1× MIC. In all tubes, a 5 × 105 CFU/ml log-phase inoculum was added along with MH broth to give a final volume of 10 ml. All tubes were incubated overnight at 35°C, and the bacterial growth in each tube was determined by performing consecutive 1:10 (vol/vol) dilutions of a 0.1-ml aliquot from each tube in MH broth and by plating a 0.1-ml volume of each dilution onto MH agar. Experiments were performed in triplicate. If a combination caused a decrease in viable cell count of ≥log102 compared with the most active single agents, the effects of the combination were considered to be synergic. If the decrease in viable cell count was log101 to log102, the effects of the combination were considered to be additive.
Synergy studies.
In interaction studies, the strains were tested against the antibiotic combinations by using a checkerboard titration method using 96-well polypropylene microtiter plates. The ranges of drug dilutions used were 0.125 to 64 mg/liter for tachyplesin III and 0.25 to 256 mg/liter for colistin and imipenem. The fractionary inhibitory concentration (FIC) index for combinations of two antimicrobials was calculated according to the equation FIC index = FICA + FICB = A/MICA + B/MICB, where A and B are the MICs of drug A and drug B, respectively, in the combination, MICA and MICB are the MICs of drug A and drug B, respectively, alone, and FICA and FICB are the FICs of drug A and drug B, respectively. The FIC indexes were interpreted as follows: <0.5, synergy; 0.5 to 4.0, indifference; and >4.0, antagonism (7). In addition, time-kill synergy studies were performed at recommended subinhibitory concentrations (one-fourth and one-half the MIC). Synergy or antagonism was defined as a 100-fold increase or decrease and indifference was defined as a <10-fold increase or decrease in killing after incubation with the combination compared to killing with the most active single agent.
Hemolysis of human red blood cells and cytotoxicity assay.
Fresh human red blood cells (hRBC) with EDTA were rinsed three times with PBS (35 mM phosphate buffer-0.15 M NaCl [pH 7.3]) by centrifugation for 10 min at 800 × g at 35°C and resuspended in PBS. Tachyplesin dissolved in PBS was then added to 50 μl of a solution of the stock hRBC in PBS to reach a final volume of 100 μl (final erythrocyte concentration, 4% vol/vol). The resulting suspension was incubated with agitation for 60 min at 37°C. The samples were then centrifuged at 800 × g for 10 min. The release of hemoglobin was monitored by measuring the absorbance of the supernatant at 540 nm. Controls for 0% hemolysis (blank) and 100% hemolysis consisted of hRBC suspended in PBS and 1% Triton X-100, respectively.
For the cytotoxicity assay, A-549 cells from human lung carcinoma (BioWhittaker Inc., Walkersville, MD) were cultured in 25-cm2 tissue culture flasks. The medium consisted of Dulbecco's modified Eagle's medium with 10% fetal calf serum (Bio-Whittaker). The cytotoxicities of tachyplesin III A at 1× minimum bactericidal concentration were determined by using a CellTiter 96 AQ cell proliferation assay (Promega Corp., Lyon, France).
Animals.
BALB/c male mice weighting 25 to 30 g were used for all the experiments. Each mouse was housed in an individual cage under constant temperature (22°C) and humidity with a 12-h light/dark cycle and had access to chow and water ad libitum throughout the study. The study was approved by the animal research ethics committee of the I.N.R.C.A. I.R.R.C.S., Polytechnic University of Marche, Ancona, Italy.
Preparation and implantation of the inoculum and antibiotic therapy.
P. aeruginosa ATCC 27853 and the clinical isolate strain were grown overnight at 37°C in brain heart infusion broth. When bacteria were in the log phase of growth, the suspension was centrifuged at 1,000 × g for 15 min, the supernatant was discarded, and the bacteria were resuspended in sterile saline to achieve a concentration of approximately 1 × 108 CFU/ml. All animals were anesthetized by an intramuscular injection of ketamine (30 mg/kg of body weight). The mice were injected intravenously via the tail vein with 0.2 ml of one of the above-mentioned bacterial suspensions (2.0 × 107 CFU) on day 0 and monitored for 72 h.
Immediately after bacterial challenge, the mice were randomly chosen to receive, intravenously, isotonic sodium chloride solution (control group), 20 mg/kg imipenem, 1 mg/kg tachyplesin III, 1 mg/kg colistin, or 20 mg/kg imipenem combined with 1 mg/kg tachyplesin III or 1 mg/kg colistin. Each group included 20 mice. The animals were returned to individual cages and monitored for the subsequent 72 h. The end points of the study were lethality rates, quantitative blood cultures, and endotoxin, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6) plasma levels. Toxicity was evaluated on the basis of the presence of drug-related adverse effects (local signs of inflammation, weight loss, vomiting, diarrhea, and fever) in the supplementary tachyplesin III-treated group without challenge.
Evaluation of treatment.
Blood samples for culture were obtained from the tail vein by aseptic percutaneous puncture 24 h after bacterial challenge. The animals that died before this time were not tested. To perform quantitative bacterial cultures, blood samples were serially diluted and a 0.1-ml volume of each dilution was spread on blood agar plates and cultured at 35°C for 48 h and the CFU were counted. The limit of detection was 10 CFU/ml.
For determination of endotoxin, TNF-α, and IL-6 levels in plasma, blood samples (0.2 ml for each animal) were collected from the tail vein at 0, 6, 12, 24, and 48 h postinjection. Endotoxin concentrations were measured by using the commercially available Limulus amebocyte lysate test (E-TOXATE; Sigma-Aldrich). Endotoxin standards (0, 0.015, 0.03, 0.06, 0.125, 0.25, and 0.5 EU/ml) were tested in each run, and the concentration of endotoxin in the tested samples was calculated by comparison to the standard curve.
IL-6 and TNF-α levels were measured by enzyme-linked immunosorbent assay according to the manufacturer's specifications (Walter Occhiena srl, Turin, Italy). The lower limits of sensitivity for IL-6 and TNF-α were 12 pg/ml and 0.05 ng/ml, respectively. The assays were performed in duplicate.
Statistical analysis.
Lethality rates were compared between groups by use of Fisher's exact test. Data from quantitative blood cultures are presented as means ± standard deviations of the means; statistical comparisons between groups were made by analysis of variance. Post hoc comparisons were performed by Bonferroni's test. Plasma endotoxin, IL-6, and TNF-α mean values were compared between groups by analysis of variance. Significance was accepted when the P value was ≤0.05.
RESULTS
In vitro studies.
According to the results of the broth microdilution method recommended by CLSI, imipenem exhibited MICs of 0.50 and 32 mg/liter for P. aeruginosa ATCC 27853 and the clinical isolate (data not shown). Colistin and tachyplesin III exhibited MICs of 4 and 4 mg/liter for P. aeruginosa ATCC 27853 and 8 and 4 mg/liter for the multiresistant strain, respectively. For both the strains, killing by tachyplesin III was shown to be very rapid: its activity against the organism was complete after a 15-min exposure period at a concentration of 2× MIC. Colistin showed the same activity, while imipenem exhibited a slower bactericidal effect on P. aeruginosa. Interestingly, an increase of greater than 100-fold in killing at 60 min was observed when the peptides were combined with ipiminem (data not shown). In the combination studies, synergy was observed between tachyplesin III and imipenem for both P. aeruginosa ATCC 27853 (FIC index, 0.312; MICs, 2 and 0.12 mg/liter for tachyplesin III and imipenem, respectively) and the clinical isolate (FIC index, 0.312; MICs, 2 and 8 mg/liter for tachyplesin III and imipenem, respectively). Similarly, synergy was also observed between colistin and imipenem for both P. aeruginosa ATCC 27853 (FIC index, 0.385; MICs, 2 and 0.25 mg/liter for tachyplesin III and imipenem, respectively) and the clinical isolate (FIC index, 0.458; MICs, 4 and 8 mg/liter for tachyplesin III and imipenem, respectively). These data were confirmed by the time-kill synergy studies (data not shown).
The cytotoxic effect was practically absent at the concentrations tested. Our data revealed that tachyplesin showed low hemolytic activity in spite of its high activity against the organism. In fact, its hemolytic activity was observed at concentrations higher than the MICs (4.5%, 12.6%, and 26.7% at concentrations of 8, 16, and 32 mg/liter, respectively).
In vivo studies.
As shown in Table 1, the control groups showed 100% bacterial lethality rates within 72 h. In contrast, for both the bacterial strains, immediate treatment with drugs demonstrated efficacies significantly higher than that of the control treatment (P < 0.05). For P. aeruginosa ATCC 27853, lethality rates of 30%, 30%, and 40% were observed for groups treated with imipenem, tachyplesin III, and colistin A, respectively. For the clinical strain, imipenem showed a higher lethality rate (80%), while the same lethality rates were observed for peptide-treated groups (30%). The combination of tachyplesin III or colistin and imipenem showed the lowest lethality rates, 5% and 10% for the control strain and 10% and 15% for the clinical isolate, respectively. Quantitative blood culture showed high bacterial numbers both in the ATCC 27853 control group (5.8 × 107 ± 0.8 × 107 CFU/ml) and in the clinical isolate control group (7.9 × 107 ± 2.2 × 107 CFU/ml), as shown in Table 1. Tachyplesin III demonstrated a good antibacterial activity against the ATCC strain, reducing the bacterial growth to 3.6 × 103 ± 0.6 × 103 CFU/ml, comparable to the activities of colistin and imipenem (6.0 × 104 ± 1.7 × 104 CFU/ml and 2.8 × 103 ± 0.4 × 103 CFU/ml, respectively). When imipenem was combined with tachyplesin III and colistin, the positive interactions produced the lowest bacterial counts (1.1 × 101 ± 0.1 × 101 CFU/ml for tachyplesin III plus imipenem and 4.6 × 101 ± 0.5 × 101 for colistin plus imipenem). Against the panresistant strain, tachyplesin III and colistin showed antibacterial activities comparable to those against the ATCC strain, while imipenem showed weak activity. Similar to the results of the previous experiment, when imipenem was combined with tachyplesin III or colistin, the positive interaction produced the lowest bacterial counts. Overall, any combination-treated group had significantly lower bacterial counts than the groups treated with a single agent (P < 0.05).
TABLE 1.
Effects of tachyplesin III, colistin, and imipenem in a mouse model of P. aeruginosa-induced sepsis
| Treatmenta (mg/kg) | Bacterial lethalityb
|
Blood culture result (24 h postchallenge)
|
||||||
|---|---|---|---|---|---|---|---|---|
|
P. aeruginosa ATCC 27853
|
P. aeruginosa clinical isolate
|
No. positive/total no. of mice
|
CFU/ml (mean± SD) of:
|
|||||
| No. of mouse deaths/total no. of mice (%) | No. of mouse deaths within 24 h/total no. of mice | No. of mouse deaths/total no. of mice | No. of mouse deaths within 24 h/total no. of mice | P. aeruginosa ATCC 27853 | P. aeruginosa clinical isolate | P. aeruginosa ATCC 27853 | P. aeruginosa clinical isolate | |
| Sodium chloride control | 20/20 (100) | 8/20 | 20/20 (100) | 7/20 | 20/20 | 20/20 | 5.8 × 107 ± 0.8 × 107 | 7.9 × 107 ± 2.2 × 107 |
| Tachyplesin III (1) | 6/20c (30) | 2/20 | 6/20c (30) | 1/20 | 7/20c | 10/20c | 3.6 × 103 ± 0.6 × 103c | 5.0 × 103 ± 0.9 × 103c |
| Colistin (1) | 8/20c (40) | 2/20 | 6/20c (30) | 1/20 | 8/20c | 13/20c | 6.0 × 104 ± 1.7 × 104c | 1.4 × 104 ± 8.6 × 103c |
| Imipenem (20) | 6/20c (30) | 1/20 | 16/20 (80) | 4/20 | 6/20c | 10/20 | 2.8 × 103 ± 0.4 × 103c | 8.7 × 106 ± 2.9 × 106 |
| Tachyplesin III (1) and imipenem (20) | 1/20c,d (5) | 0/20 | 2/20c,d (10) | 0/20 | 1/20c,d | 3/20c,d | 1.1 × 101 ± 0.1 × 101c,d | 1.6 × 101 ± 0.2 × 101c,d |
| Colistin (1) and imipenem (20) | 2/20c,d (10) | 0/20 | 3/20c,d (15) | 1/20 | 2/20c,d | 3/20c,d | 4.6 × 101 ± 0.5 × 101c,d | 5.2 × 101 ± 1.5 × 101c,d |
Treatment was carried out immediately postchallenge.
Mortality was monitored for 72 h following the challenge.
P < 0.05 (Fisher's test) or P < 0.05 (Bonferroni's test) versus the control group.
P < 0.05 (Fisher's test) or P < 0.05 (Bonferroni's test) versus the singly treated group.
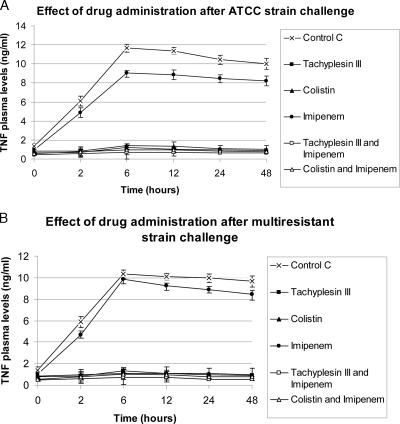
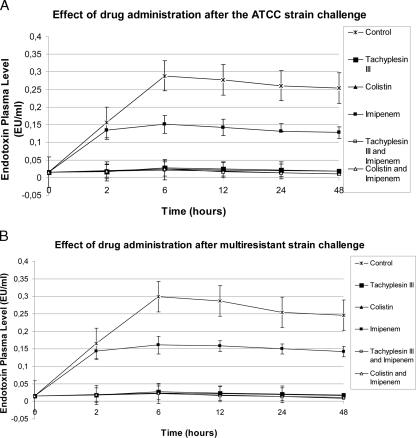
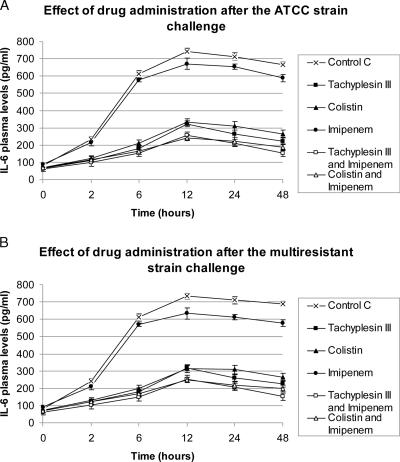
For both the strains, plasma peak levels of endotoxin, TNF-α, and IL-6 were observed 6 and 12 h after bacterial challenge. Tachyplesin III and colistin treatments (alone or the combinations) resulted in marked decreases (P < 0.05) of endotoxin, TNF-α, and IL-6 plasma levels compared to those of control and vancomycin-treated groups. (Fig. 1 to 3). The strongest reduction in endotoxin and cytokine plasma levels was observed in the groups treated with the combination of tachyplesin III and imipenem.
FIG. 1.
Effects on TNF-α levels of drugs alone or combined after bacterial challenge.
FIG. 3.
Effects on endotoxin levels of drugs alone or combined after bacterial challenge.
None of the animals had clinical evidence of drug-related adverse effects or hypersensitivity reactions, and no changes in physiological parameters were observed in the supplementary peptide-treated groups without previous infection.
DISCUSSION
It is well known that P. aeruginosa may become resistant to the antibiotic being used to treat an infection and that prior use of a particular antibiotic predicts that P. aeruginosa will develop resistance to that antibiotic. The main reasons for this bacterial resistance are thought to be the organism's low outer membrane permeability to antimicrobial agents, genetic elements capable of carrying and transferring diverse antibiotic resistance determinants, and finally, efflux pumps. Due to its potent inflammatory activity and association with sepsis, LPS can be an important virulence factor in Pseudomonas infections (1, 11).
In the present study, we evaluated the efficacy of the combination of tachyplesin or colistin and imipenem against two strains of P. aeruginosa with different patterns of susceptibility, first in vitro and then in vivo. We used one susceptible and one multiresistant strain that can be associated with clinical failure following antibiotic therapy. In time-killing curves and with the checkerboard titration method, a synergistic effect between the peptides and imipenem was observed. This synergistic pattern was also clearly observed in the in vivo setting. Previous studies have reported contradictory results for interactions among peptides and antibiotics (7, 9, 25, 26). The peptide acts by inserting into the cytoplasmic membrane and triggering the activity of bacterial murein hydrolases, resulting in damage to or degradation of bacterial membranes and lysis of the cell. In addition, antimicrobial peptides can induce, by the loss of effective outer membrane porin channels, a membrane permeability that facilitates the penetration of antibiotics and the consequent enhancement of their activity (6, 17, 25, 27).
As has been described previously, a sepsis mouse model can be used to compare the efficacies of different antibiotic therapies and also the efficacy of a therapy for strains with different susceptibilities. We selected as the main outcome measures lethality, quantitative blood cultures, and detection of endotoxin, TNF-α, and IL-6 plasma levels, to have different parameters and better define the efficacies of the treatments. We used a short period of treatment of 24 h in order to increase the relevance of the model, since this is the critical period of such an acute infection (15). In our model, tachyplesin III and colistin showed antimicrobial activities superior (clinical isolate) or similar (ATCC control strain) and lethality rates comparable to those of imipenem. They demonstrated a greater ability than imipenem to inhibit the effects of endotoxin and, consequently, the release of cytokines.
Finally, the best results for mortality rates and bacteremia were obtained when tachyplesin III was combined with imipenem. This combination was also most effective in decreasing the levels of cytokines, confirming the capacity of the peptides to neutralize membrane components that are the inducers of cytokine activation. Several studies have shown that exposure of gram-negative organisms to bactericidal agents such as carbapenems can initially result in endotoxin release, even though the subsequent decrease in endotoxin release due to the inhibitory effect on the bacterial growth appears more relevant (24). Therefore, new drugs able to neutralize molecules released by gram-negative organisms and block their interaction with specific receptors on immune cells at the beginning of antimicrobial treatment can be an attractive concept. Today, the clinically used antimicrobial peptides are the microbial cyclic molecules such as colistin and polymyxin B. Our results emphasized that tachyplesin III, a linear invertebrate peptide, has an in vitro activity and a systemic in vivo efficacy equivalent to those of colistin.
Few treatment options remain for serious infections caused by multidrug-resistant bacteria. Carbapenems are active against some isolates of P. aeruginosa, and although the polymyxins remain the most consistently effective agents in vitro against this organism, a growing number of reports have documented resistance to these antibacterials (10). In this situation, novel compounds and antibiotic combinations will be necessary to treat infections caused by these multidrug-resistant organisms and to reduce the increasing selection pressure by antibiotics on gram-negative pathogens. One way to overcome the problems of emergence of resistance is the correct use of new antimicrobial compounds and/or combination therapies. In fact, infections with isolates that are susceptible to only one or a few agents may be treated with a single drug, but the risk of progressive resistance must be considered. Specific combination therapies can be used to increase the in vivo activity, to prevent the emergence of drug resistance, and to broaden the antimicrobial spectrum. In particular, sublethal alterations of the gram-negative bacterial outer membrane in combination with the use of antibiotics that, because of resistance, are now ineffective alone may further extend therapeutic opportunities. The good antimicrobial activity toward gram-negative bacteria with high levels of antibiotic resistance and the synergistic interaction with imipenem illustrate additional important attributes that may support tachyplesin III as a promising candidate for an adjuvant in antimicrobial therapy against multiresistant organisms. Further studies should be performed to address the development of tachyplesins for this use.
FIG. 2.
Effects on IL-6 levels of drugs alone or combined after bacterial challenge.
Acknowledgments
We thank Silvana Esposito for technical assistance.
This work was supported by a grant from the Italian Ministry of Education, University, and Research (PRIN 2005).
Footnotes
Published ahead of print on 2 April 2007.
REFERENCES
- 1.Bone, R. C. 1991. Pathophysiology of sepsis. Ann. Intern. Med. 115:457-469. [DOI] [PubMed] [Google Scholar]
- 2.Cannon, M. 1987. Antimicrobial peptides. A family of wound healers. Nature 328:478. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli, Y., N. Troillet, G. M. Eliopoulos, and M. H. Samore. 1999. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 43:1379-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamot, E., E. B. El Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen, T. 1979. Qualitative test for monitoring coupling completeness in solid phase peptide synthesis using chloranil. Acta Chem. Scand. B 33:763-766. [Google Scholar]
- 6.Darveau, R. P., M. D. Cunningham, C. L. Seachord, L. Cassiano-Clough, W. L. Cosand, J. Blake, and C. S. Watkins. 1991. β-Lactam antibiotics potentiate magainin 2 antimicrobial activity in vitro and in vivo. Antimicrob. Agents Chemother. 35:1153-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-393. In V. Lorian (ed.), Antibiotics in laboratory medicine. Williams & Wilkins, Baltimore, MD.
- 8.Fields, G. B., and R. L. Noble. 1990. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 35:161-214. [DOI] [PubMed] [Google Scholar]
- 9.Giacometti, A., O. Cirioni, W. Kamysz, G. D'Amato, C. Silvestri, M. S. Del Prete, A. Licci, A. Riva, J. Łukasiak, and G. Scalise. 2005. In vitro activity of the histatin derivative P-113 against multidrug-resistant pathogens responsible for pneumonia in immunocompromised patients. Antimicrob. Agents Chemother. 49:1249-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giamarellou, H. 2006. Treatment options for multidrug-resistant bacteria. Expert Rev. Anti-Infect. Ther. 4:601-618. [DOI] [PubMed] [Google Scholar]
- 11.Glauser, M. P., G. Zanetti, J. D. Baumgartner, and J. Cohen. 1991. Septic shock: pathogenesis. Lancet 338:732-736. [DOI] [PubMed] [Google Scholar]
- 12.Hancock, R. E. W., and M. G. Scott. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. USA 97:856-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirakura, Y., S. Kobayashi, and K. Matsuzaki. 2002. Specific interactions of the antimicrobial peptides cyclic β-sheet tachyplesin I with lipopolysaccharides. Biochim. Biophys. Acta 1562:32-36. [DOI] [PubMed] [Google Scholar]
- 14.Huttner, K. M., and C. L. Bevins. 1999. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45:785-794. [DOI] [PubMed] [Google Scholar]
- 15.Klastersky, J. 2000. Empirical treatment of sepsis in neutropenic patients. Int. J. Antimicrob. Agents 16:131-133. [DOI] [PubMed] [Google Scholar]
- 16.Kollef, M. H. 2006. Is antibiotic cycling the answer to preventing the emergence of bacterial resistance in the intensive care unit? Clin. Infect. Dis. 43:S82-S88. [DOI] [PubMed] [Google Scholar]
- 17.McCafferty, D. G., P. Cudic, M. K. Yu, D. C. Behenna, and R. Kruger. 1999. Synergy and duality in peptide antibiotic mechanisms. Curr. Opin. Chem. Biol. 3:672-680. [DOI] [PubMed] [Google Scholar]
- 18.McPhee, J. B., and R. E. Hancock. 2005. Function and therapeutic potential of host defense peptides. J. Pep. Sci. 11:677-687. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura, T., H. Furunaka, T. Miyata, F. Tokunaga, T. Muta, and S. Iwanaga. 1988. Tachyplesin, a class of antimicrobial peptide from the hemocytes of horseshoe crab (Tachypleus tridentatus). J. Biol. Chem. 263:16709-16713. [PubMed] [Google Scholar]
- 20.NCCLS. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard M7-A6. NCCLS, Wayne, PA.
- 21.Nouer, S. A., M. Nucci, M. P. de-Oliveira, F. L. Pellegrino, and B. M. Moreira. 2005. Risk factors for acquisition of multidrug-resistant Pseudomonas aeruginosa producing SPM metallo-β-lactamase. Antimicrob. Agents Chemother. 49:3663-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega, B., A. B. Groeneveld, and C. Schultsz. 2004. Endemic multidrug-resistant Pseudomonas aeruginosa in critically ill patients. Infect. Control Hosp. Epidemiol. 25:825-831. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, D. L. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43:S43-S48. [DOI] [PubMed] [Google Scholar]
- 24.Prins, J. M., E. J. Kuijper, M. L. Mevissen, P. Speelman, and S. J. van Deventer. 1995. Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect. Immun. 63:2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaara, M., and M. Porro. 1996. Group of peptides that act synergistically with hydrophobic antibiotics against gram-negative enteric bacteria. Antimicrob. Agents Chemother. 40:1801-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wareham, D. W., and D. C. Bean. 2006. In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann. Clin. Microbiol. Antimicrob. 5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeaman, M. R., and N. Y. Yount. 2003. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 55:27-55. [DOI] [PubMed] [Google Scholar]