Abstract
Background—Populations at low risk of colonic cancer consume large amounts of fibre and starch and pass acid, bulky stools. One short chain fatty acid (SCFA), butyrate, is the colon's main energy source and inhibits malignant transformation in vitro. Aim—To test the hypothesis that altering colonic transit rate alters colonic pH and the SCFA content of the stools. Patients—Thirteen healthy adults recruited by advertisement. Methods—Volunteers consumed, in turn, wheat bran, senna and loperamide, each for nine days with a two week washout period between study periods, dietary intake being unchanged. Before, and in the last four days of each intervention, whole gut transit time (WGTT), defaecation frequency, stool form, stool β-glucuronidase activity, stool pH, stool SCFA concentrations and intracolonic pH (using a radiotelemetry capsule for continuous monitoring) were assessed. Results—WGTT decreased, stool output and frequency increased with wheat bran and senna, vice versa with loperamide. The pH was similar in the distal colon and stool. Distal colonic pH fell with wheat bran and senna and tended to increase with loperamide. Faecal SCFA concentrations, including butyrate, increased with senna and fell with loperamide. With wheat bran the changes were non-significant, possibly because of the short duration of the study. Baseline WGTT correlated with faecal SCFA concentration (r=−0.511, p=0.001), with faecal butyrate (r=−0.577, p<0.001) and with distal colonic pH (r=0.359, p=0.029). Conclusion—Bowel transit rate is a determinant of stool SCFA concentration including butyrate and distal colonic pH. This may explain the inter-relations between colonic cancer, dietary fibre intake, stool output, and stool pH.
Keywords: bowel cancer; colonic pH; fibre; intestinal transit; pH; short chain fatty acids
Full Text
The Full Text of this article is available as a PDF (154.2 KB).
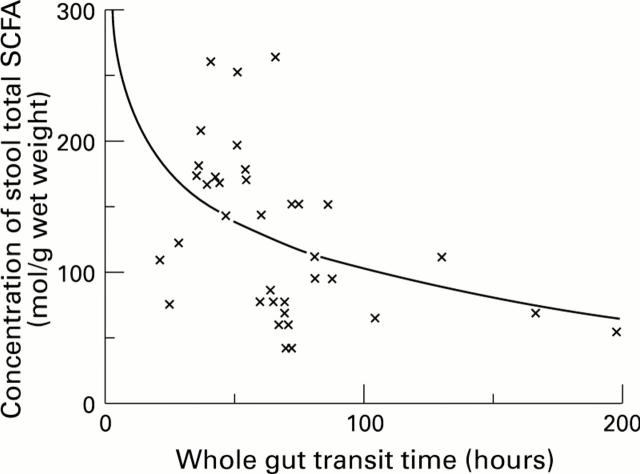
Figure 1 .
: The relation between whole gut transit time off-treatment and total stool SCFA concentration.
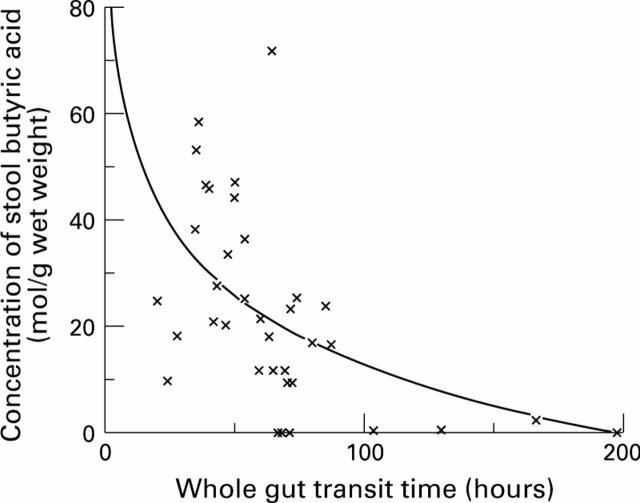
Figure 2 .
: The relation between whole gut transit time off-treatment and stool butyrate concentration.
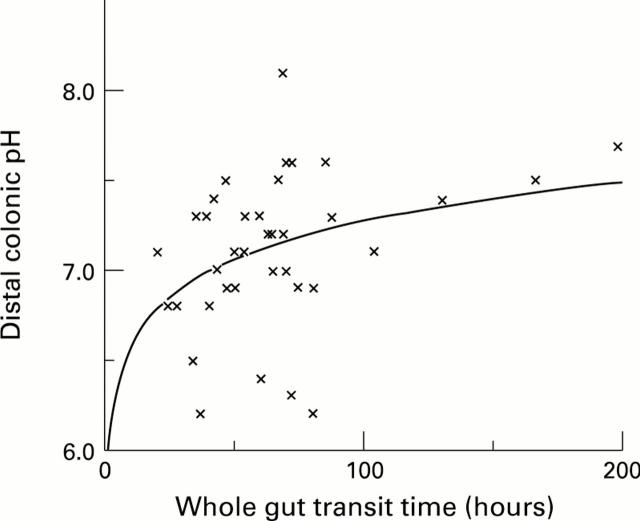
Figure 3 .
: The relation between whole gut transit time off-treatment and distal colonic pH.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardon T., Fioramonti J. Nature of the effects of bran on digestive transit time in pigs. Br J Nutr. 1983 Nov;50(3):685–690. doi: 10.1079/bjn19830140. [DOI] [PubMed] [Google Scholar]
- Bingham S. A. Mechanisms and experimental and epidemiological evidence relating dietary fibre (non-starch polysaccharides) and starch to protection against large bowel cancer. Proc Nutr Soc. 1990 Jul;49(2):153–171. doi: 10.1079/pns19900021. [DOI] [PubMed] [Google Scholar]
- Bown R. L., Gibson J. A., Sladen G. E., Hicks B., Dawson A. M. Effects of lactulose and other laxatives on ileal and colonic pH as measured by a radiotelemetry device. Gut. 1974 Dec;15(12):999–1004. doi: 10.1136/gut.15.12.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt D. P., Walker A. R., Painter N. S. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972 Dec 30;2(7792):1408–1412. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Bingham S. A., Heaton K. W., Eastwood M. A. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber) Gastroenterology. 1992 Dec;103(6):1783–1789. doi: 10.1016/0016-5085(92)91435-7. [DOI] [PubMed] [Google Scholar]
- Eastwood M. A., Kirkpatrick J. R., Mitchell W. D., Bone A., Hamilton T. Effects of dietary supplements of wheat bran and cellulose on faeces and bowel function. Br Med J. 1973 Nov 17;4(5889):392–394. doi: 10.1136/bmj.4.5889.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehle F. R., Robertson J. B., Van Soest P. J. Influence of dietary fibers on fermentation in the human large intestine. J Nutr. 1982 Jan;112(1):158–166. doi: 10.1093/jn/112.1.158. [DOI] [PubMed] [Google Scholar]
- El Oufir L., Flourié B., Bruley des Varannes S., Barry J. L., Cloarec D., Bornet F., Galmiche J. P. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996 Jun;38(6):870–877. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. F., Pye G., Bramley R., Clark A. G., Dyson T. J., Hardcastle J. D. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988 Aug;29(8):1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin B. R., Adlercreutz H., Gorbach S. L., Warram J. H., Dwyer J. T., Swenson L., Woods M. N. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med. 1982 Dec 16;307(25):1542–1547. doi: 10.1056/NEJM198212163072502. [DOI] [PubMed] [Google Scholar]
- Goldin B. R., Gorbach S. L. The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J Natl Cancer Inst. 1976 Aug;57(2):371–375. doi: 10.1093/jnci/57.2.371. [DOI] [PubMed] [Google Scholar]
- Hague A., Manning A. M., Hanlon K. A., Huschtscha L. I., Hart D., Paraskeva C. Sodium butyrate induces apoptosis in human colonic tumour cell lines in a p53-independent pathway: implications for the possible role of dietary fibre in the prevention of large-bowel cancer. Int J Cancer. 1993 Sep 30;55(3):498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- Heaton K. W., O'Donnell L. J. An office guide to whole-gut transit time. Patients' recollection of their stool form. J Clin Gastroenterol. 1994 Jul;19(1):28–30. doi: 10.1097/00004836-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Heaton K. W., Radvan J., Cripps H., Mountford R. A., Braddon F. E., Hughes A. O. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992 Jun;33(6):818–824. doi: 10.1136/gut.33.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman L., Peters S., Fisher A., Pomare E. W. Differing effects of pectin, cellulose and lignin on stool pH, transit time and weight. Br J Nutr. 1983 Sep;50(2):189–195. doi: 10.1079/bjn19830088. [DOI] [PubMed] [Google Scholar]
- Hove H., Rye Clausen M., Brøbech Mortensen P. Lactate and pH in faeces from patients with colonic adenomas or cancer. Gut. 1993 May;34(5):625–629. doi: 10.1136/gut.34.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Kang H. J., Kim S. W., Kobashi K. pH-inducible beta-glucosidase and beta-glucuronidase of intestinal bacteria. Chem Pharm Bull (Tokyo) 1992 Jun;40(6):1667–1669. doi: 10.1248/cpb.40.1667. [DOI] [PubMed] [Google Scholar]
- Lampe J. W., Fredstrom S. B., Slavin J. L., Potter J. D. Sex differences in colonic function: a randomised trial. Gut. 1993 Apr;34(4):531–536. doi: 10.1136/gut.34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald I. A., Webb G. R., Mahony D. E. Fecal hydroxysteroid dehydrogenase activities in vegetarian Seventh-Day Adventists, control subjects, and bowel cancer patients. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S233–S238. doi: 10.1093/ajcn/31.10.S233. [DOI] [PubMed] [Google Scholar]
- Mallett A. K., Bearne C. A., Rowland I. R. The influence of incubation pH on the activity of rat and human gut flora enzymes. J Appl Bacteriol. 1989 May;66(5):433–437. doi: 10.1111/j.1365-2672.1989.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Mallett A. K., Rowland I. R., Bearne C. A. Modification of rat caecal microbial biotransformation activities by dietary saccharin. Toxicology. 1985 Aug;36(2-3):253–262. doi: 10.1016/0300-483x(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Marcus S. N., Heaton K. W. Effects of a new, concentrated wheat fibre preparation on intestinal transit, deoxycholic acid metabolism and the composition of bile. Gut. 1986 Aug;27(8):893–900. doi: 10.1136/gut.27.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A., Gibson P. R., Young G. P. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993 Mar;34(3):386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark H. L., Lupton J. R. Determinants and consequences of colonic luminal pH: implications for colon cancer. Nutr Cancer. 1990;14(3-4):161–173. doi: 10.1080/01635589009514091. [DOI] [PubMed] [Google Scholar]
- O'Donnell L. J., Virjee J., Heaton K. W. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. BMJ. 1990 Feb 17;300(6722):439–440. doi: 10.1136/bmj.300.6722.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert C. J., Emmett P. M., Heaton K. W. Intestinal transit time in the population calculated from self made observations of defecation. J Epidemiol Community Health. 1993 Aug;47(4):331–333. doi: 10.1136/jech.47.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probert C. S., Emmett P. M., Heaton K. W. Some determinants of whole-gut transit time: a population-based study. QJM. 1995 May;88(5):311–315. [PubMed] [Google Scholar]
- Reddy B., Engle A., Katsifis S., Simi B., Bartram H. P., Perrino P., Mahan C. Biochemical epidemiology of colon cancer: effect of types of dietary fiber on fecal mutagens, acid, and neutral sterols in healthy subjects. Cancer Res. 1989 Aug 15;49(16):4629–4635. [PubMed] [Google Scholar]
- Rowland I. The influence of the gut microflora on food toxicity. Proc Nutr Soc. 1981 Jan;40(1):67–74. doi: 10.1079/pns19810011. [DOI] [PubMed] [Google Scholar]
- Spiller G. A., Chernoff M. C., Hill R. A., Gates J. E., Nassar J. J., Shipley E. A. Effect of purified cellulose, pectin, and a low-residue diet on fecal volatile fatty acids, transit time, and fecal weight in humans. Am J Clin Nutr. 1980 Apr;33(4):754–759. doi: 10.1093/ajcn/33.4.754. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Hylemon P. B. 7alpha-Dehydroxylation of cholic acid and chenodeoxycholic acid by Clostridium leptum. J Lipid Res. 1979 Mar;20(3):325–333. [PubMed] [Google Scholar]
- Stephen A. M., Wiggins H. S., Englyst H. N., Cole T. J., Wayman B. J., Cummings J. H. The effect of age, sex and level of intake of dietary fibre from wheat on large-bowel function in thirty healthy subjects. Br J Nutr. 1986 Sep;56(2):349–361. doi: 10.1079/bjn19860116. [DOI] [PubMed] [Google Scholar]
- Thornton J. R. High colonic pH promotes colorectal cancer. Lancet. 1981 May 16;1(8229):1081–1083. doi: 10.1016/s0140-6736(81)92244-3. [DOI] [PubMed] [Google Scholar]
- Tucker D. M., Sandstead H. H., Logan G. M., Jr, Klevay L. M., Mahalko J., Johnson L. K., Inman L., Inglett G. E. Dietary fiber and personality factors as determinants of stool output. Gastroenterology. 1981 Nov;81(5):879–883. [PubMed] [Google Scholar]
- Walker A. R. Diet, bowel motility, faeces composition and colonic cancer. S Afr Med J. 1971 Apr 3;45(14):377–379. [PubMed] [Google Scholar]
- Walker A. R., Walker B. F., Segal I. Faecal pH value and its modification by dietary means in South African black and white schoolchildren. S Afr Med J. 1979 Mar 24;55(13):495–498. [PubMed] [Google Scholar]
- Wyman J. B., Heaton K. W., Manning A. P., Wicks A. C. Variability of colonic function in healthy subjects. Gut. 1978 Feb;19(2):146–150. doi: 10.1136/gut.19.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Munster I. P., Tangerman A., Nagengast F. M. Effect of resistant starch on colonic fermentation, bile acid metabolism, and mucosal proliferation. Dig Dis Sci. 1994 Apr;39(4):834–842. doi: 10.1007/BF02087431. [DOI] [PubMed] [Google Scholar]