Abstract
T Mizuochi,dH Asakura,bM Fujiwaraa
Background—Murine leukemia virus, LP-BM5, induces severe immunodeficiency with abnormal lymphoproliferation in susceptible C57BL/6 mice. In a previous study, it was shown that a Sjögren's syndrome-like systemic exocrinopathy is induced in the virus infected mice. Aims—To examine lymphocyte functions of the virus infected mice. Methods—Four-week old mice were inoculated with the virus and their spleen cells were transferred into syngeneic nu/nu mice. Their organs were examined by light and electron microscopy. Phenotypes of the colon infiltrating cells were examined by flow cytometry. Results—All nu/nu recipients had died by six weeks after cell transfer, showing runting disease like cachexia with diarrhoea and anal bleeding. Histopathological examination revealed that systemic exocrinopathy was adoptively transferable and that the colon became thickened due to mononuclear cell infiltration into the mucosal and submucosal layer with hyperplasia of intestinal epithelial cells. No virus particles were found in the colon. Flow cytometric analyses revealed that most of the infiltrating CD4+ T cells showed CD45RBlow. No intestinal lesions were observed in the virus infected mice nor in nu/nu mice inoculated with normal lymphocytes. Conclusion—Lymphocytes of the virus infected mice induced colitis and hyperplasia of intestinal epithelial cells as well as systemic exocrinopathy in nu/nu mice. Our experimental system may give some insight into intestinal lesions associated with virus infection.
Keywords: murine leukemia virus; nude mice; enterocolitis; colitogenic cells
Full Text
The Full Text of this article is available as a PDF (452.2 KB).
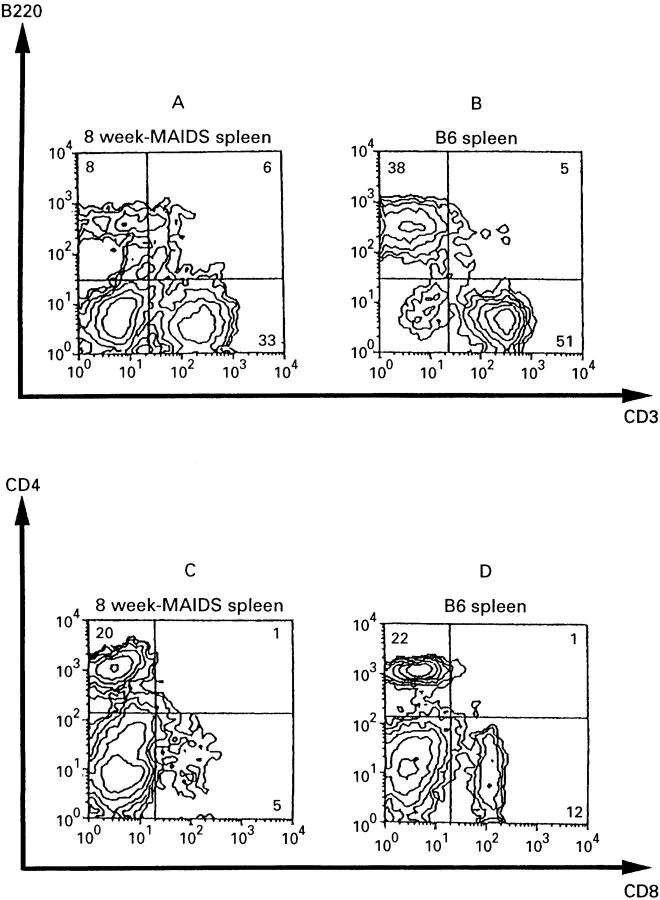
Figure 1 .
: Flow cytometric analyses of spleen cells. A and C: spleen cells of mice infected with LP-BM5 eight weeks previously; B and D: untreated B6 spleen cells. Spleen cells were doubly stained with either FITC-anti-CD3 and PE-anti-B220 (A,B) or FITC-anti-CD4 and PE-anti-CD8 (C,D).
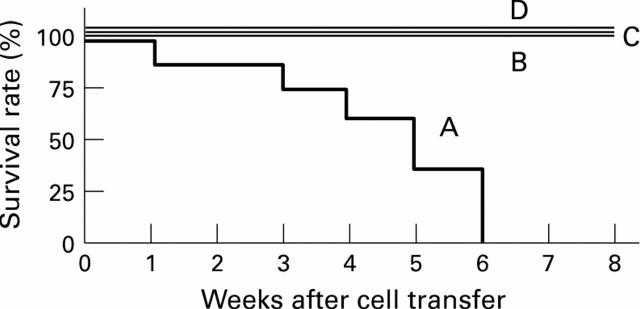
Figure 2 .
: Survival rate of B6 nu/nu mice inoculated with MAIDS spleen cells and control groups of mice. A: B6 nu/nu mice inoculated with MAIDS spleen cells; B: B6 nu/nu mice inoculated with untreated B6 spleen cells; C: B6 nu/nu mice infected with LP-BM5; D: untreated B6 nu/nu mice. Seven mice were used in each group and data were collected from two or three repeated experiments.
Figure 3 .
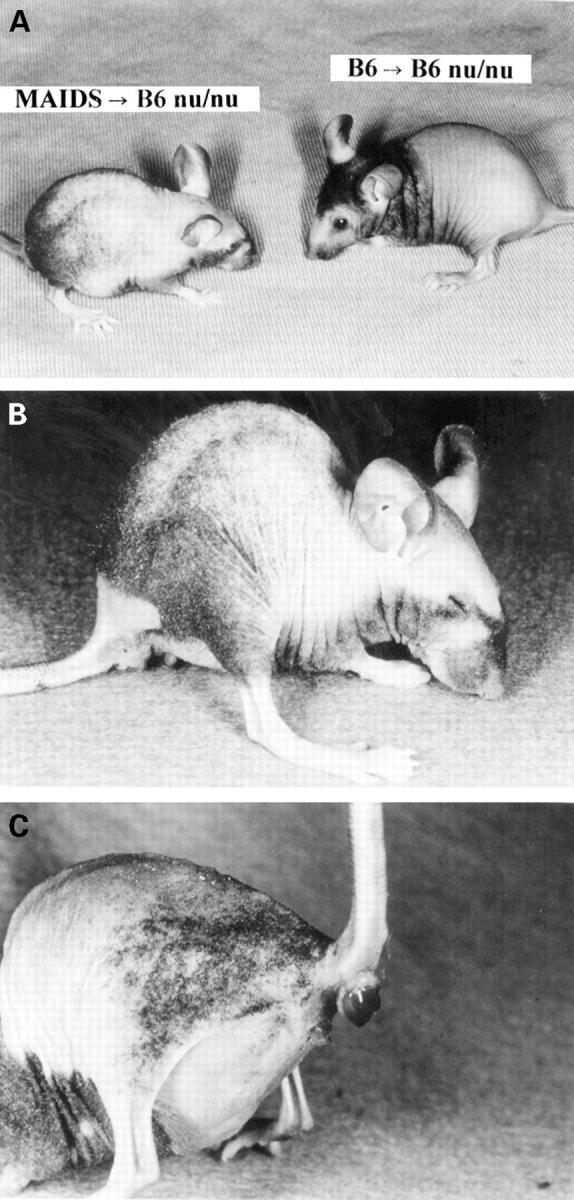
: Runting disease-like appearance of B6 nu/nu mice. A: gross appearance of a nu/nu mouse which received MAIDS spleen cells compared with B6 nu/nu mice which received syngeneic spleen cells; B: typical hunchback of a nu/nu mouse which received MAIDS spleen cells; C: mouse which shows anal prolapse and anal bleeding.
Figure 4 .
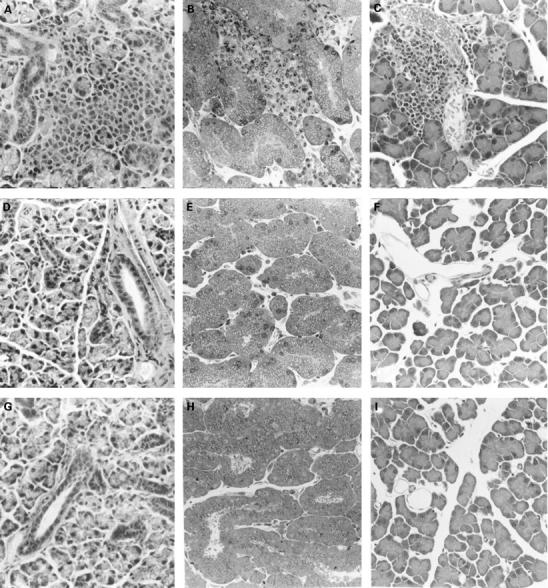
: Sjögren's syndrome-like exocrinopathy in B6 nu/nu mice inoculated with MAIDS spleen cells. A-C: Sjögren's syndrome-like exocrinopathy in salivary gland (A), lacrimal gland (B), and pancreas (C); D-I: controls; no pathological lesions are observed in these organs of nu/nu mice inoculated with untreated B6 spleen cells (D, salivary gland; E, lacrimal gland; F, pancreas) nor in uninoculated mice (G, salivary gland; H, lacrimal gland; I, pancreas). (Haematoxylin and eosin; original magnification: ×160.)
Figure 5 .
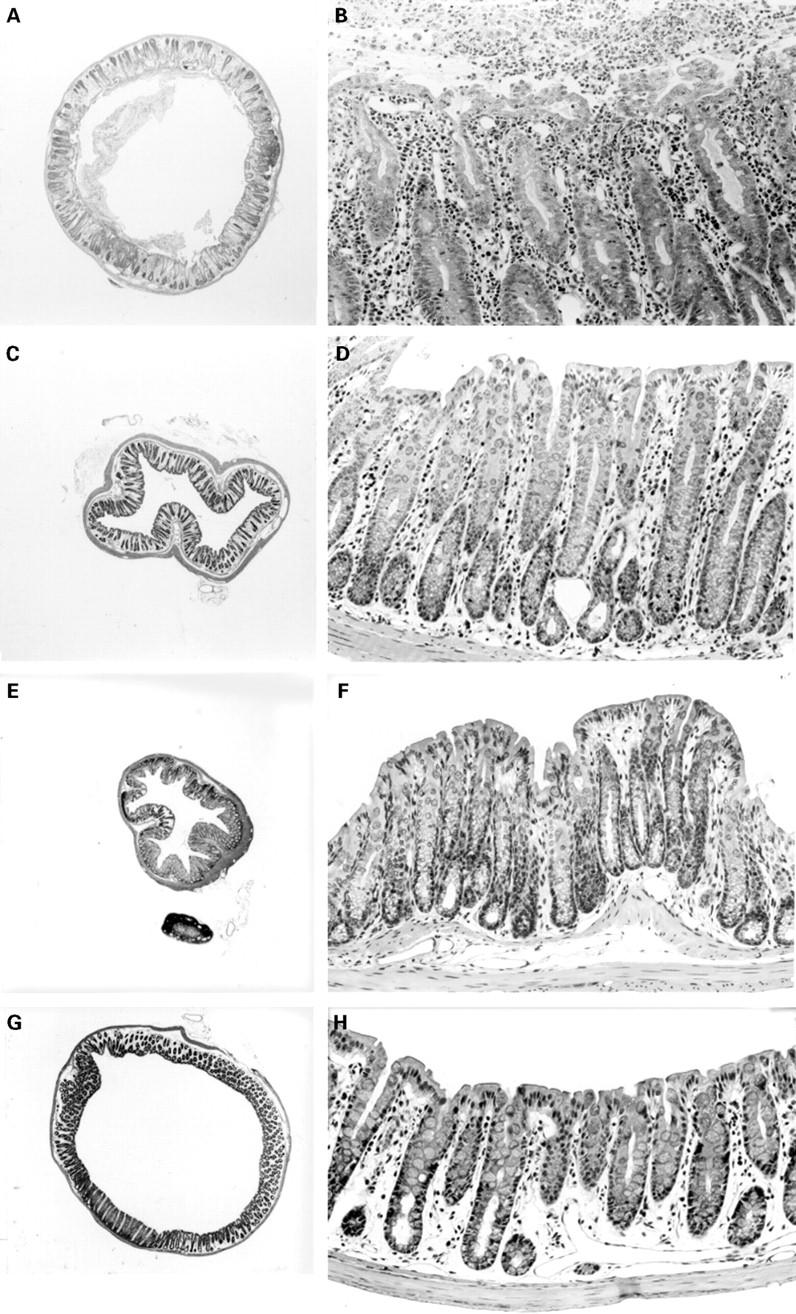
: Occurrence of severe colitis in B6 nu/nu mice inoculated with MAIDS spleen cells. A,B: B6 nu/nu mice inoculated with MAIDS spleen cells. Colitis with thickened walls and hyperplasia of the epithelial cells are observed; C-H: controls; colons of B6 nu/nu mice inoculated with B6 spleen cells (C,D), those of untreated B6 nu/nu mice (E,F), and B6 nu/nu mice infected with LP-BM5 (G,H). (Haematoxylin and eosin; original magnifications: A,C,E,G: ×16; B,D,F,H: ×160.)
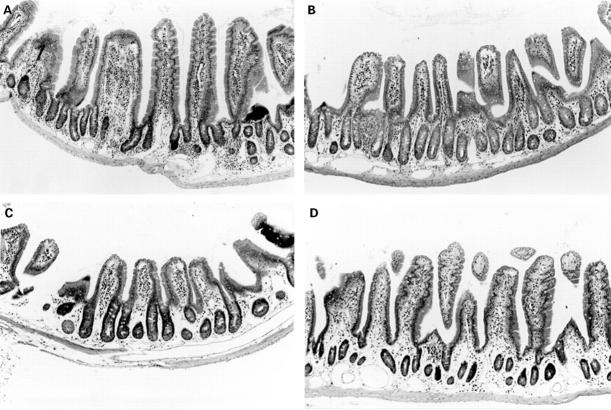
Figure 6 .
: Occurrence of intestinal lesions in B6 nu/nu mice inoculated with MAIDS spleen cells. A: thickened intestinal wall in B6 nu/nu mice inoculated with MAIDS spleen cells. Cellular infiltration is localised in the lamina propria and submucosa of the small intestine. Epithelial cell hyperplasia is also observed but neither erosion nor ulcer is observed; B-D: controls; small intestines of B6 nu/nu mice inoculated with B6 spleen cells (B), untreated B6 nu/nu mice (C), and B6 nu/nu mice infected with LP-BM5 (D). (Haematoxylin and eosin; original magnification: ×120.)
Figure 7 .
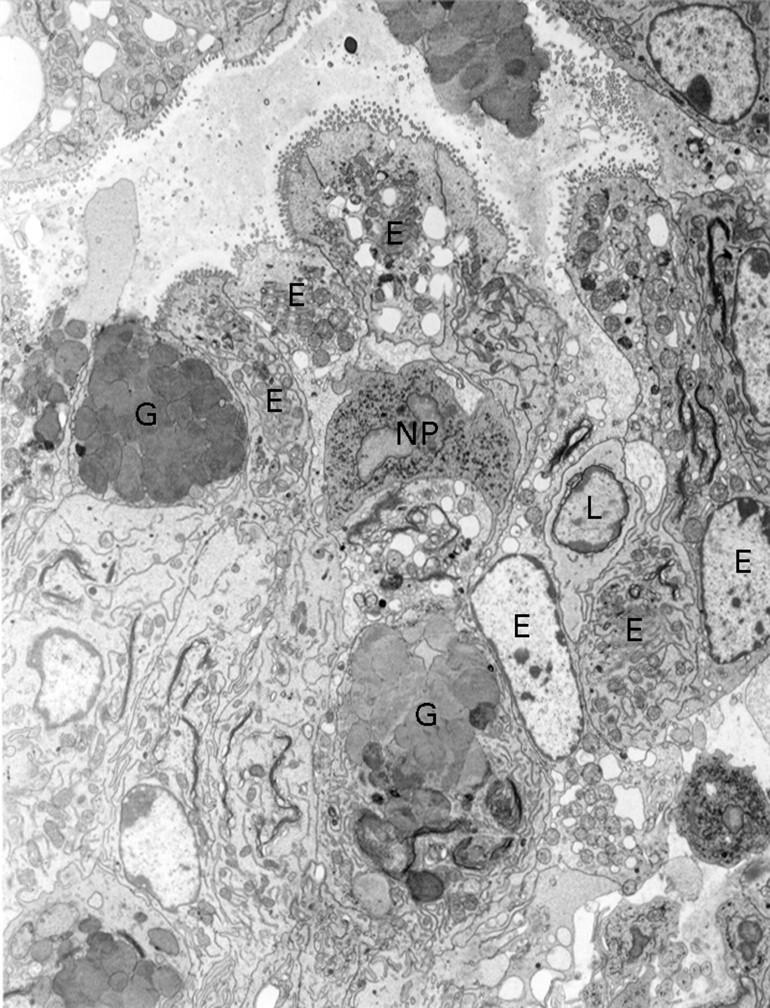
: Electron microscopy findings of colitis in a B6 nu/nu mouse inoculated with MAIDS spleen cells. Colonic epithelial cells are injured by infiltrating lymphocytes, macrophages, and neutrophils. No virus particles are detected. Original magnification: ×3000. E, epithelial cells; G, goblet cells; L, lymphocyte; NP, neutrophil.
Figure 8 .
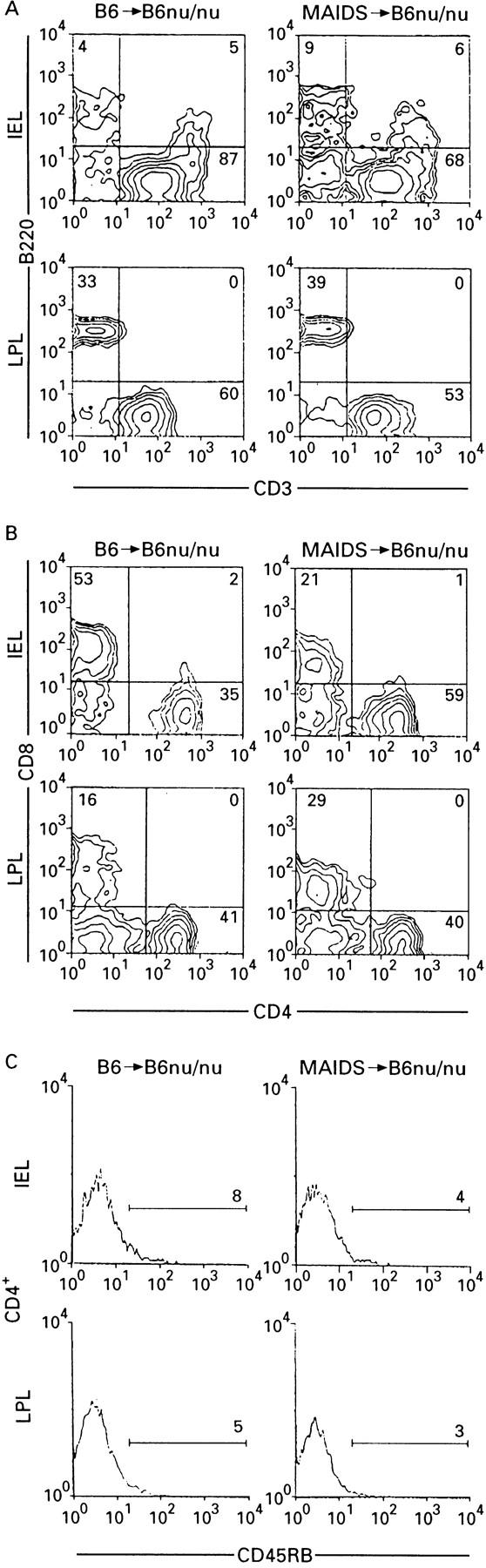
: Flow cytometry analyses of iIEL and LPL of colon of B6 nu/nu mice inoculated with MAIDS spleen cells. iIEL and LPL of B6 nu/nu mice inoculated with either MAIDS or untreated B6 spleen cells were stained with the following fluorescent dye labelled antibodies: A: FITC- anti-CD3 and PE-anti-B220; B: FITC-anti-CD4 and PE-anti-CD8; C: PE-anti-CD4 and FITC-anti-CD45RB.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birkeland M. L., Johnson P., Trowbridge I. S., Puré E. Changes in CD45 isoform expression accompany antigen-induced murine T-cell activation. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6734–6738. doi: 10.1073/pnas.86.17.6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. D., Parrott D. M. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981 Jun;22(6):481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988 Feb 5;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., Taguchi T., Aicher W. K., McGhee J. R., Bluestone J. A., Eldridge J. H., Kiyono H. Immunoregulatory functions for murine intraepithelial lymphocytes: gamma/delta T cell receptor-positive (TCR+) T cells abrogate oral tolerance, while alpha/beta TCR+ T cells provide B cell help. J Exp Med. 1992 Mar 1;175(3):695–707. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991 Jul;5(10):2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- Klinken S. P., Fredrickson T. N., Hartley J. W., Yetter R. A., Morse H. C., 3rd Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988 Feb 15;140(4):1123–1131. [PubMed] [Google Scholar]
- Kopelman R. G., Zolla-Pazner S. Association of human immunodeficiency virus infection and autoimmune phenomena. Am J Med. 1988 Jan;84(1):82–88. doi: 10.1016/0002-9343(88)90012-5. [DOI] [PubMed] [Google Scholar]
- Morrissey P. J., Charrier K., Braddy S., Liggitt D., Watson J. D. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993 Jul 1;178(1):237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS). J Exp Med. 1987 Jun 1;165(6):1737–1742. doi: 10.1084/jem.165.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar G., Koch S., Haas M., Swain S. L. CD4 T cells in murine acquired immunodeficiency syndrome: polyclonal progression to anergy. J Exp Med. 1992 Jun 1;175(6):1589–1599. doi: 10.1084/jem.175.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka K., Iiai T., Watanabe H., Tanaka T., Miyasaka M., Sato K., Asakura H., Abo T. Similarities and differences between extrathymic T cells residing in mouse liver and intestine. Cell Immunol. 1994 Jan;153(1):52–66. doi: 10.1006/cimm.1994.1005. [DOI] [PubMed] [Google Scholar]
- Powrie F., Leach M. W., Mauze S., Caddle L. B., Coffman R. L. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993 Nov;5(11):1461–1471. doi: 10.1093/intimm/5.11.1461. [DOI] [PubMed] [Google Scholar]
- Powrie F., Mason D. OX-22high CD4+ T cells induce wasting disease with multiple organ pathology: prevention by the OX-22low subset. J Exp Med. 1990 Dec 1;172(6):1701–1708. doi: 10.1084/jem.172.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Selvey L. A., Morse H. C., 3rd, Granger L. G., Hodes R. J. Preferential expansion and activation of V beta 5+ CD4+ T cells in murine acquired immunodeficiency syndrome. J Immunol. 1993 Aug 1;151(3):1712–1722. [PubMed] [Google Scholar]
- Spivak J. L., Bender B. S., Quinn T. C. Hematologic abnormalities in the acquired immune deficiency syndrome. Am J Med. 1984 Aug;77(2):224–228. doi: 10.1016/0002-9343(84)90695-8. [DOI] [PubMed] [Google Scholar]
- Strober W., Ehrhardt R. O. Chronic intestinal inflammation: an unexpected outcome in cytokine or T cell receptor mutant mice. Cell. 1993 Oct 22;75(2):203–205. doi: 10.1016/0092-8674(93)80062-j. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Makino M., Okada Y., Kinoshita J., Yui R., Kanazawa H., Asakura H., Fujiwara M., Mizuochi T., Komuro K. Exocrinopathy resembling Sjögren's syndrome induced by a murine retrovirus. Lab Invest. 1993 Oct;69(4):430–435. [PubMed] [Google Scholar]
- Van der Heijden P. J., Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987 Nov 5;103(2):161–167. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L., Stites D. P. Hypothesis: AIDS is an autoimmune disease directed at the immune system and triggered by a lymphotropic retrovirus. Clin Immunol Immunopathol. 1986 Dec;41(3):305–313. doi: 10.1016/0090-1229(86)90001-2. [DOI] [PubMed] [Google Scholar]
- de Clerck L. S., Couttenye M. M., de Broe M. E., Stevens W. J. Acquired immunodeficiency syndrome mimicking Sjögren's syndrome and systemic lupus erythematosus. Arthritis Rheum. 1988 Feb;31(2):272–275. doi: 10.1002/art.1780310216. [DOI] [PubMed] [Google Scholar]