Abstract
Background—The pathology of non-alcoholic chronic pancreatitis has not yet been sufficiently studied. Aims—To identify the major changes of pancreatic tissue in patients surgically treated for non-alcoholic chronic pancreatitis. Patients—Pancreatectomy specimens from 12 patients with non-alcoholic chronic pancreatitis, including four patients with autoimmune or related diseases (Sjögren's syndrome, primary sclerosing cholangitis, ulcerative colitis, and Crohn's disease), were reviewed. Methods—Morphological changes were studied histologically and immunohistochemically (to type inflammatory cells) and compared with the pancreatic alterations found in 12 patients with alcoholic chronic pancreatitis. Results—In patients with non-alcoholic chronic pancreatitis, with or without associated autoimmune or related diseases, pancreatic inflammation particularly involved the ducts, commonly resulting in duct obstruction and occasionally duct destruction. None of these features was seen in alcoholic chronic pancreatitis which, however, showed pseudocysts and calcifications. Conclusion—The pancreatic changes in patients with non-alcoholic chronic pancreatitis clearly differ from those with alcoholic chronic pancreatitis. The term chronic duct destructive pancreatitis is suggested for this type of pancreatic disease.
Keywords: non-alcoholic chronic pancreatitis; duct destructive pancreatitis
Full Text
The Full Text of this article is available as a PDF (260.5 KB).
Figure 1 .
: Gross appearance of non-alcoholic chronic pancreatitis mimicking pancreatic carcinoma (duodeno-hemipancreatectomy specimen from patient 8). On sectioning the head of the pancreas appears indurated and pale. The intrapancreatic segment of the distal bile duct is narrowed (arrows).
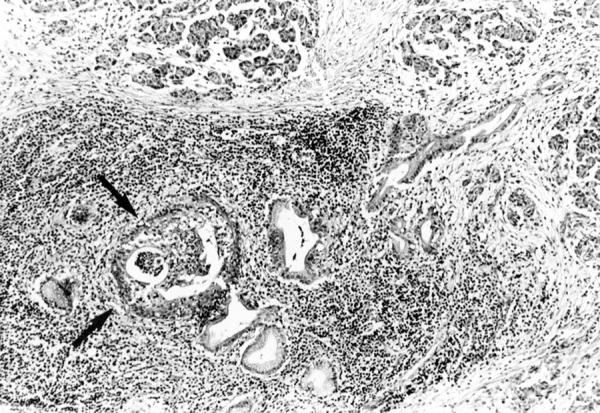
Figure 2 .

: Non-alcoholic duct destructive chronic pancreatitis: large interlobular duct surrounded by a dense inflammatory infiltrate composed of mononuclear cells. Haematoxylin and eosin; original magnification ×60.
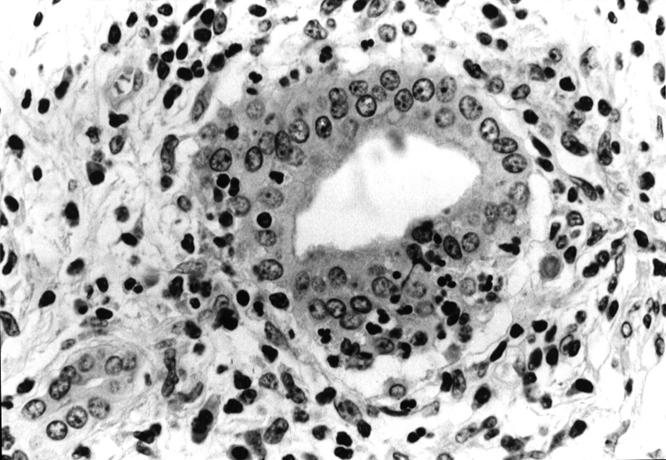
Figure 3 .
: Non-alcoholic duct destructive chronic pancreatitis: inflammatory process destroying the epithelium (arrows) of some interlobular ducts. Haematoxylin and eosin; original magnification ×120.
Figure 4 .
: Non-alcoholic duct destructive chronic pancreatitis: a small interlobular duct embedded in a dense inflammatory infiltrate. The epithelium has been invaded by lymphocytes and granulocytes. Haematoxylin and eosin; original magnification ×240.
Figure 5 .
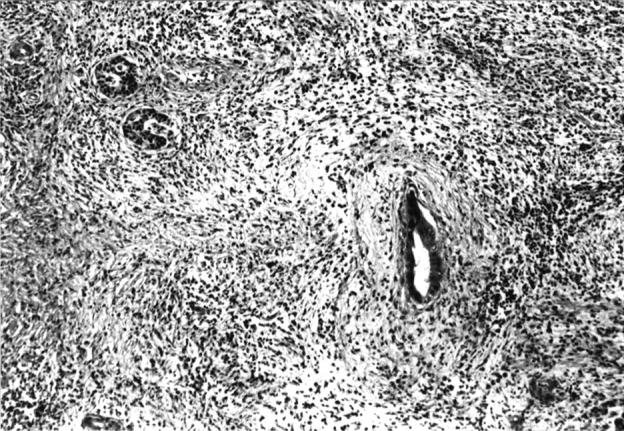
: Non-alcoholic duct destructive chronic pancreatitis: advanced changes in the pancreas, characterised by fibrotic replacement of acinar tissue and severe duct obliteration. Haematoxylin and eosin; original magnification ×120.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann R. W., Heitz P. U., Klöppel G. Course of alcoholic chronic pancreatitis: a prospective clinicomorphological long-term study. Gastroenterology. 1996 Jul;111(1):224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- Axon A. T., Ashton M. G., Lintott D. J. Chronic pancreatitis and inflammatory bowel disease. Clin Radiol. 1979 Mar;30(2):179–182. doi: 10.1016/s0009-9260(79)80146-4. [DOI] [PubMed] [Google Scholar]
- BALL W. P., BAGGENSTOSS A. H., BARGEN J. A. Pancreatic lesions associated with chronic ulcerative colitis. Arch Pathol (Chic) 1950 Sep;50(3):347–358. [PubMed] [Google Scholar]
- Bedossa P., Bacci J., Lemaigre G., Martin E. Lymphocyte subsets and HLA-DR expression in normal pancreas and chronic pancreatitis. Pancreas. 1990 Jul;5(4):415–420. doi: 10.1097/00006676-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Borum M., Steinberg W., Steer M., Freedman S., White P. Chronic pancreatitis: a complication of systemic lupus erythematosus. Gastroenterology. 1993 Feb;104(2):613–615. doi: 10.1016/0016-5085(93)90434-e. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 6-1982. A 55-year-old man with eight months of obstructive jaundice. N Engl J Med. 1982 Feb 11;306(6):349–358. doi: 10.1056/NEJM198202113060608. [DOI] [PubMed] [Google Scholar]
- Epstein O., Chapman R. W., Lake-Bakaar G., Foo A. Y., Rosalki S. B., Sherlock S. The pancreas in primary biliary cirrhosis and primary sclerosing cholangitis. Gastroenterology. 1982 Dec;83(6):1177–1182. [PubMed] [Google Scholar]
- Guisset M., Klotz F., Debonne J. M., Vitte S. Syndrome sec et hépatite virale chronique C de bas grade. A propos d'une série de 50 cas. Rev Med Interne. 1993;14(10):1006–1006. doi: 10.1016/s0248-8663(05)80124-9. [DOI] [PubMed] [Google Scholar]
- Gurian L. E., Keeffe E. B. Pancreatic insufficiency associated with ulcerative colitis and pericholangitis. Gastroenterology. 1982 Mar;82(3):581–585. [PubMed] [Google Scholar]
- Haddad J., Deny P., Munz-Gotheil C., Ambrosini J. C., Trinchet J. C., Pateron D., Mal F., Callard P., Beaugrand M. Lymphocytic sialadenitis of Sjögren's syndrome associated with chronic hepatitis C virus liver disease. Lancet. 1992 Feb 8;339(8789):321–323. doi: 10.1016/0140-6736(92)91645-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalleh R. P., Gilbertson J. A., Williamson R. C., Slater S. D., Foster C. S. Expression of major histocompatibility antigens in human chronic pancreatitis. Gut. 1993 Oct;34(10):1452–1457. doi: 10.1136/gut.34.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Koike M., Tsuruta K., Okamoto A., Tabata I., Fujita N. Lymphoplasmacytic sclerosing pancreatitis with cholangitis: a variant of primary sclerosing cholangitis extensively involving pancreas. Hum Pathol. 1991 Apr;22(4):387–395. doi: 10.1016/0046-8177(91)90087-6. [DOI] [PubMed] [Google Scholar]
- Kino-Ohsaki J., Nishimori I., Morita M., Okazaki K., Yamamoto Y., Onishi S., Hollingsworth M. A. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Gastroenterology. 1996 May;110(5):1579–1586. doi: 10.1053/gast.1996.v110.pm8613065. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pathology of acute and chronic pancreatitis. Pancreas. 1993 Nov;8(6):659–670. doi: 10.1097/00006676-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Layer P., Yamamoto H., Kalthoff L., Clain J. E., Bakken L. J., DiMagno E. P. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994 Nov;107(5):1481–1487. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Lindström E., Lindström F., von Schenck H., Ihse I. Pancreatic ductal morphology and function in primary Sjögren's syndrome. Int J Pancreatol. 1991 Feb;8(2):141–149. doi: 10.1007/BF02924428. [DOI] [PubMed] [Google Scholar]
- Lysy J., Goldin E. Pancreatitis in ulcerative colitis. J Clin Gastroenterol. 1992 Dec;15(4):336–339. doi: 10.1097/00004836-199212000-00015. [DOI] [PubMed] [Google Scholar]
- Lászik G. Z., Pap A., Farkas G. A case of primary sclerosing cholangitis mimicking chronic pancreatitis. Int J Pancreatol. 1988 Dec;3(6):503–508. doi: 10.1007/BF02788209. [DOI] [PubMed] [Google Scholar]
- Marrano D., Gullo L., Casadei R., Santini D., Leone O., Campione O. An unusual case of chronic pancreatitis of possible immune origin. Pancreas. 1996 Mar;12(2):202–204. doi: 10.1097/00006676-199603000-00016. [DOI] [PubMed] [Google Scholar]
- Mulder A. H., Horst G., Haagsma E. B., Limburg P. C., Kleibeuker J. H., Kallenberg C. G. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993 Mar;17(3):411–417. [PubMed] [Google Scholar]
- Nishimori I., Yamamoto Y., Okazaki K., Morita M., Onodera M., Kino J., Tamura S., Yamamoto Y. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjögren's syndrome. Pancreas. 1994 May;9(3):374–381. doi: 10.1097/00006676-199405000-00015. [DOI] [PubMed] [Google Scholar]
- Pawlotsky J. M., Ben Yahia M., Andre C., Voisin M. C., Intrator L., Roudot-Thoraval F., Deforges L., Duvoux C., Zafrani E. S., Duval J. Immunological disorders in C virus chronic active hepatitis: a prospective case-control study. Hepatology. 1994 Apr;19(4):841–848. [PubMed] [Google Scholar]
- SMITH M. P., LOE R. H. SCLEROSING CHOLANGITIS; REVIEW OF RECENT CASE REPORTS AND ASSOCIATED DISEASES AND FOUR NEW CASES. Am J Surg. 1965 Aug;110:239–246. doi: 10.1016/0002-9610(65)90018-8. [DOI] [PubMed] [Google Scholar]
- Seyrig J. A., Jian R., Modigliani R., Golfain D., Florent C., Messing B., Bitoun A. Idiopathic pancreatitis associated with inflammatory bowel disease. Dig Dis Sci. 1985 Dec;30(12):1121–1126. doi: 10.1007/BF01314044. [DOI] [PubMed] [Google Scholar]
- Sjögren I., Wengle B., Korsgren M. Primary sclerosing cholangitis associated with fibrosis of the submandibular glands and the pancreas. Acta Med Scand. 1979;205(1-2):139–141. doi: 10.1111/j.0954-6820.1979.tb06019.x. [DOI] [PubMed] [Google Scholar]
- Sood S., Fossard D. P., Shorrock K. Chronic sclerosing pancreatitis in Sjögren's syndrome: a case report. Pancreas. 1995 May;10(4):419–421. doi: 10.1097/00006676-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Waldram R., Kopelman H., Tsantoulas D., Williams R. Chronic pancreatitis, sclerosing cholangitis, and sicca complex in two siblings. Lancet. 1975 Mar 8;1(7906):550–552. doi: 10.1016/s0140-6736(75)91560-3. [DOI] [PubMed] [Google Scholar]
- van den Oord J. J., de Wolf-Peeters C., Vanstapel M. J., Desmet V. J. Improved immunohistochemical visualization of helper/inducer T-cells by the simultaneous application of two noncrossblocking monoclonal antibodies. Stain Technol. 1985 Jan;60(1):45–49. doi: 10.3109/10520298509113890. [DOI] [PubMed] [Google Scholar]