Abstract
Background—Genetic polymorphisms in N-acetyltransferase (NAT2) can change the normally fast acetylation of substrates to slow acetylation, and have been associated with the development of some cancers. The NAT2 locus may also suffer dysregulation during cancer progression, as the gene resides on chromosome 8p22, a region which is frequently deleted in colorectal cancer. Subjects and Methods—A polymerase chain reaction based method was used to determine NAT2 genotype in 275 patients with colon cancer and 343 normal control DNAs. Within the cancer group, 65cases known to contain deletions in chromosome 8p were examined for loss of heterozygosity at the NAT2 locus. Results—Overall, there was no statistical difference in frequency or distribution of NAT2 alleles and genotype between colon cancer and control groups. There was a significant association between the slow acetylation genotype and early age of onset. NAT2 genotype did not vary with other clinical features of colon cancer, which included Dukes's stage, site of tumour, and sex. Of 48 informative cases, only three (6%) showed loss of heterozygosity, indicating that the NAT2 locus is not commonly deleted in colorectal cancer. This suggests that NAT2 is retained during the process of allele loss possibly because of its proximity to a gene necessary for cell viability. Conclusions—NAT2 does not play a major role in colorectal cancer risk, but may influence risk in some age groups. The nature of the loss of heterozygosity at the chromosome 8p site is complex and is worthy of further study.
Keywords: xenobiotic; N-acetlytransferase 2; polymorphism; colorectal cancer; loss of heterozygosity; tumour suppressor gene
Full Text
The Full Text of this article is available as a PDF (168.0 KB).
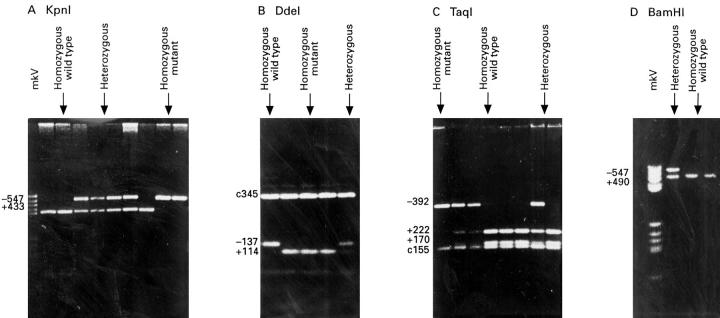
Figure 1 .
: NAT2 PCR products from constitutive DNA of patients with colorectal cancer, following digestion with restriction enzymes: (A) Kpn I; (B) DdeI; (C) TaqI; and (D) BamHI, illustrating four NAT2 polymorphic sites. Band sizes (in base pairs) are given for polymorphic sites (+ and -) and constant bands (c). Restriction patterns for each enzyme are illustrated: homozygous wild type; heterozygous; and homozygous mutant. MkV denotes molecular weight marker.
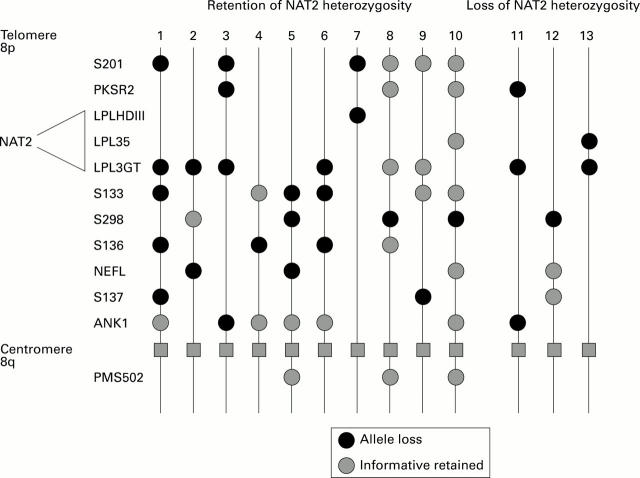
Figure 2 .
: Heterozygosity of NAT2 was retained in 94% of cancers which had demonstrated LOH on chromosome 8p in a previous study of colon cancer. Gene markers used in that study are listed according to approximate chromosomal location from telomere to centromere and heterozygosity status was examined using constitutive and tumour DNA in PCR and Southern blotting studies.25 The approximate region to which NAT2 maps is indicated on the left of the figure. The pattern of allele loss in 13 patients with colorectal cancer is shown. In patients 1-10 NAT2 heterozygosity was retained, and in patients 11-13 NAT2 heterozygosity was lost.
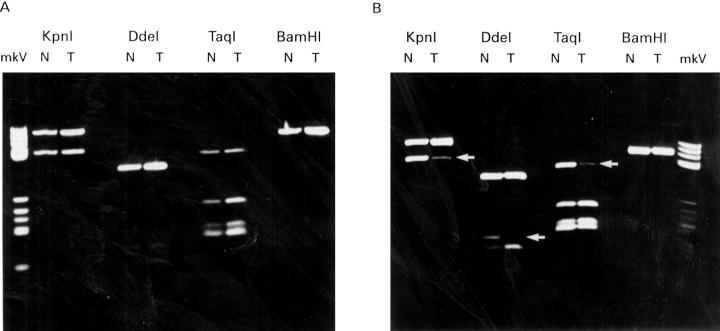
Figure 3 .
: NAT2 alleles of two patients with colorectal cancer which had previously shown LOH on chromosome 8p. Patient A demonstrates allele retention and patient B allele loss. Restriction digests for each enzyme, KpnI, DdeI, TaqI, and BamHI are shown for matched pairs of constitutional DNA (N) and tumour DNA (T). Arrows indicate alleles showing loss.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe M., Deguchi T., Suzuki T. The structure and characteristics of a fourth allele of polymorphic N-acetyltransferase gene found in the Japanese population. Biochem Biophys Res Commun. 1993 Mar 31;191(3):811–816. doi: 10.1006/bbrc.1993.1289. [DOI] [PubMed] [Google Scholar]
- Bell D. A., Taylor J. A., Butler M. A., Stephens E. A., Wiest J., Brubaker L. H., Kadlubar F. F., Lucier G. W. Genotype/phenotype discordance for human arylamine N-acetyltransferase (NAT2) reveals a new slow-acetylator allele common in African-Americans. Carcinogenesis. 1993 Aug;14(8):1689–1692. doi: 10.1093/carcin/14.8.1689. [DOI] [PubMed] [Google Scholar]
- Blum M., Demierre A., Grant D. M., Heim M., Meyer U. A. Molecular mechanism of slow acetylation of drugs and carcinogens in humans. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5237–5241. doi: 10.1073/pnas.88.12.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlay A. M., Lamb D., Gillooly M., Norrman J., Morrison D., Smith C. A., Harrison D. J. Association between the CYP1A1 gene polymorphism and susceptibility to emphysema and lung cancer. Clin Mol Pathol. 1995 Aug;48(4):M210–M214. doi: 10.1136/mp.48.4.m210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantlay A. M., Smith C. A., Wallace W. A., Yap P. L., Lamb D., Harrison D. J. Heterogeneous expression and polymorphic genotype of glutathione S-transferases in human lung. Thorax. 1994 Oct;49(10):1010–1014. doi: 10.1136/thx.49.10.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevix-Trench G., Young J., Coggan M., Board P. Glutathione S-transferase M1 and T1 polymorphisms: susceptibility to colon cancer and age of onset. Carcinogenesis. 1995 Jul;16(7):1655–1657. doi: 10.1093/carcin/16.7.1655. [DOI] [PubMed] [Google Scholar]
- Cunningham C., Dunlop M. G., Wyllie A. H., Bird C. C. Deletion mapping in colorectal cancer of a putative tumour suppressor gene in 8p22-p21.3. Oncogene. 1993 May;8(5):1391–1396. [PubMed] [Google Scholar]
- Eggertsen G., Tegelman R., Ericsson S., Angelin B., Berglund L. Apolipoprotein E polymorphism in a healthy Swedish population: variation of allele frequency with age and relation to serum lipid concentrations. Clin Chem. 1993 Oct;39(10):2125–2129. [PubMed] [Google Scholar]
- Emi M., Fujiwara Y., Nakajima T., Tsuchiya E., Tsuda H., Hirohashi S., Maeda Y., Tsuruta K., Miyaki M., Nakamura Y. Frequent loss of heterozygosity for loci on chromosome 8p in hepatocellular carcinoma, colorectal cancer, and lung cancer. Cancer Res. 1992 Oct 1;52(19):5368–5372. [PubMed] [Google Scholar]
- Farrington S. M., Cunningham C., Boyle S. M., Wyllie A. H., Dunlop M. G. Detailed physical and deletion mapping of 8p with isolation of YAC clones from tumour suppressor loci involved in colorectal cancer. Oncogene. 1996 Apr 18;12(8):1803–1808. [PubMed] [Google Scholar]
- Fettman M. J., Butler R. N., McMichael A. J., Roberts-Thomson I. C. Metabolic phenotypes and colorectal neoplasia. J Gastroenterol Hepatol. 1991 Jan-Feb;6(1):81–89. doi: 10.1111/j.1440-1746.1991.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Franke S., Klawitz I., Schnakenberg E., Rommel B., Van de Ven W., Bullerdiek J., Schloot W. Isolation and mapping of a cosmid clone containing the human NAT2 gene. Biochem Biophys Res Commun. 1994 Feb 28;199(1):52–55. doi: 10.1006/bbrc.1994.1192. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Emi M., Ohata H., Kato Y., Nakajima T., Mori T., Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993 Mar 1;53(5):1172–1174. [PubMed] [Google Scholar]
- Grant D. M., Blum M., Meyer U. A. Polymorphisms of N-acetyltransferase genes. Xenobiotica. 1992 Sep-Oct;22(9-10):1073–1081. doi: 10.3109/00498259209051861. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Lottspeich F., Meyer U. A. Evidence for two closely related isozymes of arylamine N-acetyltransferase in human liver. FEBS Lett. 1989 Feb 13;244(1):203–207. doi: 10.1016/0014-5793(89)81193-7. [DOI] [PubMed] [Google Scholar]
- Grant D. M. Molecular genetics of the N-acetyltransferases. Pharmacogenetics. 1993 Feb;3(1):45–50. doi: 10.1097/00008571-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Mörike K., Eichelbaum M., Meyer U. A. Acetylation pharmacogenetics. The slow acetylator phenotype is caused by decreased or absent arylamine N-acetyltransferase in human liver. J Clin Invest. 1990 Mar;85(3):968–972. doi: 10.1172/JCI114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein D. W. Acetylator genotype and arylamine-induced carcinogenesis. Biochim Biophys Acta. 1988 Aug 3;948(1):37–66. doi: 10.1016/0304-419x(88)90004-2. [DOI] [PubMed] [Google Scholar]
- Hickman D., Sim E. N-acetyltransferase polymorphism. Comparison of phenotype and genotype in humans. Biochem Pharmacol. 1991 Aug 8;42(5):1007–1014. doi: 10.1016/0006-2952(91)90282-a. [DOI] [PubMed] [Google Scholar]
- Ilett K. F., David B. M., Detchon P., Castleden W. M., Kwa R. Acetylation phenotype in colorectal carcinoma. Cancer Res. 1987 Mar 1;47(5):1466–1469. [PubMed] [Google Scholar]
- Ilett K. F., Ingram D. M., Carpenter D. S., Teitel C. H., Lang N. P., Kadlubar F. F., Minchin R. F. Expression of monomorphic and polymorphic N-acetyltransferases in human colon. Biochem Pharmacol. 1994 Mar 2;47(5):914–917. doi: 10.1016/0006-2952(94)90493-6. [DOI] [PubMed] [Google Scholar]
- Jacobs R. Role of dietary factors in cell replication and colon cancer. Am J Clin Nutr. 1988 Sep;48(3 Suppl):775–779. doi: 10.1093/ajcn/48.3.775. [DOI] [PubMed] [Google Scholar]
- Ladero J. M., González J. F., Benítez J., Vargas E., Fernández M. J., Baki W., Diaz-Rubio M. Acetylator polymorphism in human colorectal carcinoma. Cancer Res. 1991 Apr 15;51(8):2098–2100. [PubMed] [Google Scholar]
- Lang N. P., Chu D. Z., Hunter C. F., Kendall D. C., Flammang T. J., Kadlubar F. F. Role of aromatic amine acetyltransferase in human colorectal cancer. Arch Surg. 1986 Nov;121(11):1259–1261. doi: 10.1001/archsurg.121.11.1259. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., Kadlubar F. F., Ilett K. F. Role of acetylation in colorectal cancer. Mutat Res. 1993 Nov;290(1):35–42. doi: 10.1016/0027-5107(93)90030-j. [DOI] [PubMed] [Google Scholar]
- Mommsen S., Barfod N. M., Aagaard J. N-Acetyltransferase phenotypes in the urinary bladder carcinogenesis of a low-risk population. Carcinogenesis. 1985 Feb;6(2):199–201. doi: 10.1093/carcin/6.2.199. [DOI] [PubMed] [Google Scholar]
- Probst-Hensch N. M., Haile R. W., Ingles S. A., Longnecker M. P., Han C. Y., Lin B. K., Lee D. B., Sakamoto G. T., Frankl H. D., Lee E. R. Acetylation polymorphism and prevalence of colorectal adenomas. Cancer Res. 1995 May 15;55(10):2017–2020. [PubMed] [Google Scholar]
- Risch A., Smelt V., Lane D., Stanley L., van der Slot W., Ward A., Sim E. Arylamine N-acetyltransferase in erythrocytes of cystic fibrosis patients. Pharmacol Toxicol. 1996 Apr;78(4):235–240. doi: 10.1111/j.1600-0773.1996.tb00211.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez J. W., Kirlin W. G., Ferguson R. J., Doll M. A., Gray K., Rustan T. D., Lee M. E., Kemp K., Urso P., Hein D. W. Human acetylator genotype: relationship to colorectal cancer incidence and arylamine N-acetyltransferase expression in colon cytosol. Arch Toxicol. 1993;67(7):445–452. doi: 10.1007/BF01969914. [DOI] [PubMed] [Google Scholar]
- Smith G., Harrison D. J., East N., Rae F., Wolf H., Wolf C. R. Regulation of cytochrome P450 gene expression in human colon and breast tumour xenografts. Br J Cancer. 1993 Jul;68(1):57–63. doi: 10.1038/bjc.1993.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr N. K., Blanton S., Bookstein R., Clarke R., Cottingham R., Daiger S., Drayna D., Faber P., Horrigan S., Kas K. Report and abstracts of the second international workshop on human chromosome 8 mapping 1994. Oxford, United Kingdom, September 16-18, 1994. Cytogenet Cell Genet. 1995;68(3-4):147–164. doi: 10.1159/000133908. [DOI] [PubMed] [Google Scholar]
- Stern C. THE HARDY-WEINBERG LAW. Science. 1943 Feb 5;97(2510):137–138. doi: 10.1126/science.97.2510.137. [DOI] [PubMed] [Google Scholar]
- Sugimura T. Carcinogenicity of mutagenic heterocyclic amines formed during the cooking process. Mutat Res. 1985 Jun-Jul;150(1-2):33–41. doi: 10.1016/0027-5107(85)90098-3. [DOI] [PubMed] [Google Scholar]
- Sugimura T., Nagao M., Wakabayashi K. Heterocyclic amines in cooked foods: candidates for causation of common cancers. J Natl Cancer Inst. 1994 Jan 5;86(1):2–4. doi: 10.1093/jnci/86.1.2. [DOI] [PubMed] [Google Scholar]
- Vatsis K. P., Martell K. J., Weber W. W. Diverse point mutations in the human gene for polymorphic N-acetyltransferase. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6333–6337. doi: 10.1073/pnas.88.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatsis K. P., Weber W. W., Bell D. A., Dupret J. M., Evans D. A., Grant D. M., Hein D. W., Lin H. J., Meyer U. A., Relling M. V. Nomenclature for N-acetyltransferases. Pharmacogenetics. 1995 Feb;5(1):1–17. doi: 10.1097/00008571-199502000-00001. [DOI] [PubMed] [Google Scholar]
- Vatsis K. P., Weber W. W. Structural heterogeneity of Caucasian N-acetyltransferase at the NAT1 gene locus. Arch Biochem Biophys. 1993 Feb 15;301(1):71–76. doi: 10.1006/abbi.1993.1116. [DOI] [PubMed] [Google Scholar]
- Wohlleb J. C., Hunter C. F., Blass B., Kadlubar F. F., Chu D. Z., Lang N. P. Aromatic amine acetyltransferase as a marker for colorectal cancer: environmental and demographic associations. Int J Cancer. 1990 Jul 15;46(1):22–30. doi: 10.1002/ijc.2910460107. [DOI] [PubMed] [Google Scholar]
- Yaremko M. L., Wasylyshyn M. L., Paulus K. L., Michelassi F., Westbrook C. A. Deletion mapping reveals two regions of chromosome 8 allele loss in colorectal carcinomas. Genes Chromosomes Cancer. 1994 May;10(1):1–6. doi: 10.1002/gcc.2870100102. [DOI] [PubMed] [Google Scholar]
- Zhong S., Wyllie A. H., Barnes D., Wolf C. R., Spurr N. K. Relationship between the GSTM1 genetic polymorphism and susceptibility to bladder, breast and colon cancer. Carcinogenesis. 1993 Sep;14(9):1821–1824. doi: 10.1093/carcin/14.9.1821. [DOI] [PubMed] [Google Scholar]