Abstract
Background—Low fat and wheat bran interventions significantly reduced the growth of small to large adenomas and modestly suppressed rectal epithelial cell proliferation in the Australian Polyp Prevention Project. Aim—To study the effect of unprocessed wheat bran, unprocessed oat bran and processed wheat bran (Kellogg's All Bran) on rectal epithelial cell proliferation. Patients—Twenty subjects with recent adenomas and a high fat background diet were recruited. Methods—Rectal biopsy specimens were taken at entry and at the end of three six-week periods of oat bran (64 g/day), wheat bran (25 g/day) and All Bran (38 g/day), all in association with a diet <25% energy as fat, in a randomised cross-over trial. Each of the bran supplements had a total of 11 g dietary fibre. The biopsy specimens were fixed in methacarn and stained immunohistochemically for presence of the proliferating cell nuclear antigen (PCNA). The kinetics used to measure proliferation were labelling index, whole distribution of labelled cells, and labelled cells in the top two-fifths of crypts using analysis of variance. Results—There were no significant differences in mean labelling indexes between the four diets or in the percentage of labelled cells in the top two-fifths (p=0.59), but activity in the top two-fifths of crypts was lowest with wheat bran. The mean (SD) labelling indexes were 2.23 (0.11)% for control, 2.13 (0.08)% for wheat bran, 2.19 (0.09)% for oat bran, and 2.12 (0.08)% for All Bran. The proportion in the top two-fifths of the crypts was 2.6 (0.6)% for control, 2.15 (0.5)% for wheat bran, 3.3 (0.9)% for oat bran, and 3.1 (0.9)% for All Bran. On analysis of whole distribution, there was no significant overall effect of diets but there was a difference between subjects. Analysis including total fibre intake also did not identify effects on proliferation. Conclusion—In this study of high risk subjects with initial high fat diets, dietary fibre in association with a low fat diet had no effect on rectal epithelial cell proliferation, although wheat bran had the greatest effect on percentage of labelled cells in the top two-fifths of crypts.
Keywords: cereal fibre; rectal epithelial proliferation; PCNA; fibre solubility
Full Text
The Full Text of this article is available as a PDF (149.0 KB).
Figure 1 .
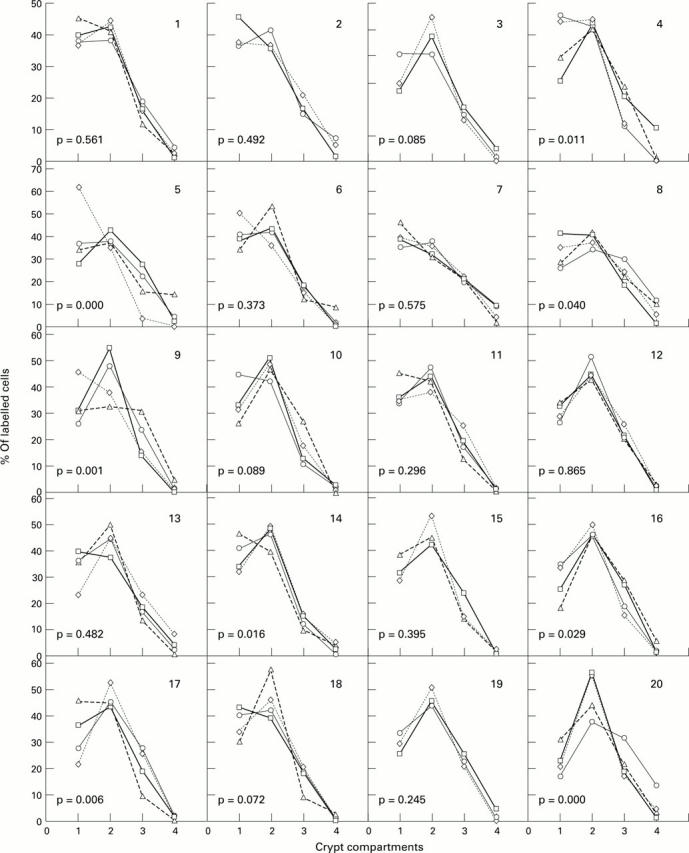
: Individual distribution patterns for each subject and for each dietary group. Each of the graphs represents the number of labelled cells in each of the four crypt compartments, comprising the bottom three-fifths and the top two-fifths combined. Differences in the distribution patterns between the four groups were found in eight of the 20 subjects (p<0.05). Control, open squares; wheat bran, diamonds; oat bran, open circles; All Bran, open triangles.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alabaster O., Tang Z. C., Frost A., Shivapurkar N. Potential synergism between wheat bran and psyllium: enhanced inhibition of colon cancer. Cancer Lett. 1993 Nov 30;75(1):53–58. doi: 10.1016/0304-3835(93)90207-p. [DOI] [PubMed] [Google Scholar]
- Alberts D. S., Einspahr J., Rees-McGee S., Ramanujam P., Buller M. K., Clark L., Ritenbaugh C., Atwood J., Pethigal P., Earnest D. Effects of dietary wheat bran fiber on rectal epithelial cell proliferation in patients with resection for colorectal cancers. J Natl Cancer Inst. 1990 Aug 1;82(15):1280–1285. doi: 10.1093/jnci/82.15.1280. [DOI] [PubMed] [Google Scholar]
- Anti M., Marra G., Armelao F., Bartoli G. M., Ficarelli R., Percesepe A., De Vitis I., Maria G., Sofo L., Rapaccini G. L. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992 Sep;103(3):883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- Anti M., Marra G., Armelao F., Percesepe A., Ficarelli R., Ricciuto G. M., Valenti A., Rapaccini G. L., De Vitis I., D'Agostino G. Rectal epithelial cell proliferation patterns as predictors of adenomatous colorectal polyp recurrence. Gut. 1993 Apr;34(4):525–530. doi: 10.1136/gut.34.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkitt D. P. Epidemiology of cancer of the colon and rectum. Cancer. 1971 Jul;28(1):3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Cahill R. J., O'Sullivan K. R., Mathias P. M., Beattie S., Hamilton H., O'Morain C. Effects of vitamin antioxidant supplementation on cell kinetics of patients with adenomatous polyps. Gut. 1993 Jul;34(7):963–967. doi: 10.1136/gut.34.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron I. L., Ord V. A., Hunter K. E., Padilla G. M., Heitman D. W. Suppression of a a carcinogen (1,2-dimethylhydrazine dihydrochloride)-induced increase in mitotic activity in the colonic crypts of rats by addition of dietary cellulose. Cancer Res. 1989 Feb 15;49(4):991–995. [PubMed] [Google Scholar]
- Cummings J. H. Constipation, dietary fibre and the control of large bowel function. Postgrad Med J. 1984 Nov;60(709):811–819. doi: 10.1136/pgmj.60.709.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. A., Wilson R. G., Hanlon L., Eastwood M. A. Effect of the dietary fibre content of lifelong diet on colonic cellular proliferation in the rat. Gut. 1992 Aug;33(8):1076–1079. doi: 10.1136/gut.33.8.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenheim J. L., Graham S., Horvath P. J., Marshall J. R., Haughey B. P., Wilkinson G. Risks associated with source of fiber and fiber components in cancer of the colon and rectum. Cancer Res. 1990 Jun 1;50(11):3295–3300. [PubMed] [Google Scholar]
- Gibson P. R., Folino M., Rosella O., Finch C. F., Moeller I., Alexeyeff M., Lindley J., Young G. P. Neoplasia and hyperplasia of large bowel: focal lesions in an abnormal epithelium. Gastroenterology. 1992 Nov;103(5):1452–1459. doi: 10.1016/0016-5085(92)91164-y. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Stampfer M. J., Colditz G. A., Ascherio A., Willett W. C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994 May 1;54(9):2390–2397. [PubMed] [Google Scholar]
- Jacobs L. R., Lupton J. R. Relationship between colonic luminal pH, cell proliferation, and colon carcinogenesis in 1,2-dimethylhydrazine treated rats fed high fiber diets. Cancer Res. 1986 Apr;46(4 Pt 1):1727–1734. [PubMed] [Google Scholar]
- Jacobs L. R. Modification of experimental colon carcinogenesis by dietary fibers. Adv Exp Med Biol. 1986;206:105–118. doi: 10.1007/978-1-4613-1835-4_11. [DOI] [PubMed] [Google Scholar]
- Kashtan H., Stern H. S., Jenkins D. J., Jenkins A. L., Thompson L. U., Hay K., Marcon N., Minkin S., Bruce W. R. Colonic fermentation and markers of colorectal-cancer risk. Am J Clin Nutr. 1992 Mar;55(3):723–728. doi: 10.1093/ajcn/55.3.723. [DOI] [PubMed] [Google Scholar]
- Kune S., Kune G. A., Watson L. F. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr Cancer. 1987;9(1):21–42. doi: 10.1080/01635588709513908. [DOI] [PubMed] [Google Scholar]
- Lipkin M., Enker W. E., Winawer S. J. Tritiated-thymidine labeling of rectal epithelial cells in 'non-prep' biopsies of individuals at increased risk for colonic neoplasia. Cancer Lett. 1987 Oct 30;37(2):153–161. doi: 10.1016/0304-3835(87)90158-3. [DOI] [PubMed] [Google Scholar]
- MacLennan R., Macrae F., Bain C., Battistutta D., Chapuis P., Gratten H., Lambert J., Newland R. C., Ngu M., Russell A. Randomized trial of intake of fat, fiber, and beta carotene to prevent colorectal adenomas. J Natl Cancer Inst. 1995 Dec 6;87(23):1760–1766. doi: 10.1093/jnci/87.23.1760. [DOI] [PubMed] [Google Scholar]
- Macrae F. A., Kilias D., Sharpe K., Hughes N., Young G. P., MacLennan R. Rectal epithelial cell proliferation: comparison of errors of measurement with inter-subject variance. Australian Polyp Prevention Project Investigators. J Cell Biochem Suppl. 1994;19:84–90. [PubMed] [Google Scholar]
- McIntyre A., Gibson P. R., Young G. P. Butyrate production from dietary fibre and protection against large bowel cancer in a rat model. Gut. 1993 Mar;34(3):386–391. doi: 10.1136/gut.34.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre A., Young G. P., Taranto T., Gibson P. R., Ward P. B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology. 1991 Nov;101(5):1274–1281. doi: 10.1016/0016-5085(91)90077-x. [DOI] [PubMed] [Google Scholar]
- Nugent K. P., Farmer K. C., Spigelman A. D., Williams C. B., Phillips R. K. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993 Dec;80(12):1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- Ponz de Leon M., Roncucci L., Di Donato P., Tassi L., Smerieri O., Amorico M. G., Malagoli G., De Maria D., Antonioli A., Chahin N. J. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res. 1988 Jul 15;48(14):4121–4126. [PubMed] [Google Scholar]
- Rozen P., Fireman Z., Fine N., Wax Y., Ron E. Oral calcium suppresses increased rectal epithelial proliferation of persons at risk of colorectal cancer. Gut. 1989 May;30(5):650–655. doi: 10.1136/gut.30.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg A. S., Andersson H., Carlsson N. G., Sandström B. Degradation products of bran phytate formed during digestion in the human small intestine: effect of extrusion cooking on digestibility. J Nutr. 1987 Dec;117(12):2061–2065. doi: 10.1093/jn/117.12.2061. [DOI] [PubMed] [Google Scholar]
- Slattery M. L., Sorenson A. W., Mahoney A. W., French T. K., Kritchevsky D., Street J. C. Diet and colon cancer: assessment of risk by fiber type and food source. J Natl Cancer Inst. 1988 Nov 16;80(18):1474–1480. doi: 10.1093/jnci/80.18.1474. [DOI] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Trock B., Lanza E., Greenwald P. Dietary fiber, vegetables, and colon cancer: critical review and meta-analyses of the epidemiologic evidence. J Natl Cancer Inst. 1990 Apr 18;82(8):650–661. doi: 10.1093/jnci/82.8.650. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Isbell G., Shabot M., Winn R., Lanza F., Hochman L., Larson E., Lynch P., Roubein L., Levin B. Calcium supplementation decreases rectal epithelial cell proliferation in subjects with sporadic adenoma. Gastroenterology. 1992 Jul;103(1):92–97. doi: 10.1016/0016-5085(92)91100-i. [DOI] [PubMed] [Google Scholar]
- Willett W. C., Stampfer M. J., Colditz G. A., Rosner B. A., Speizer F. E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990 Dec 13;323(24):1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]


