Abstract
Background and aim—Some of the recently observed functional features characteristic of immunocompetent cells residing in the human intestinal lamina propria could be mediated by interleukin- 10 (IL-10). To investigate the role of IL-10 in the human intestinal mucosa, the regulation of IL-10 production by lamina propria T lymphocytes (LPL-T) was determined and compared with that of peripheral blood T lymphocytes (PBL-T). Methods—Following activation by using different stimuli, IL-10 release by LPL-T and PBL-T into the supernatant was measured by enzyme linked immunosorbent assay (ELISA). In parallel, cell growth was determined by [3H]-thymidine incorporation. Results—Neither LPL-T nor PBL-T release IL-10 constitutively. Triggering through CD2 or the T cell receptor (TCR)/CD3 complex in the presence of autologous monocytes induces significantly greater IL-10 secretion by LPL-T than by PBL-T. Engagement of the CD45 receptor enhances IL-10 release and proliferation of CD2 triggered CD45RO+ PBL-T. In contrast, it reduces CD2 induced IL-10 production by LPL-T without altering cell growth significantly. Conclusions—Activated LPL-T release relatively high amounts of IL-10. Enhanced IL-10 production by activated LPL-T, in comparison with activated PBL-T, is not only related to the presence of a higher proportion of CD45RO+ T cells in the intestinal lamina propria, but is also caused by increased sensitivity of LPL-T to CD2 co-stimulation. The differential responsiveness of LPL-T, compared with PBL-T, to CD45 engagement demonstrates that CD45 could be involved in the altered CD2 reactivity of LPL-T.
Keywords: CD2; CD45; interleukin 10; lamina propria; T cell subsets; T lymphocytes
Full Text
The Full Text of this article is available as a PDF (125.7 KB).
Figure 1 .
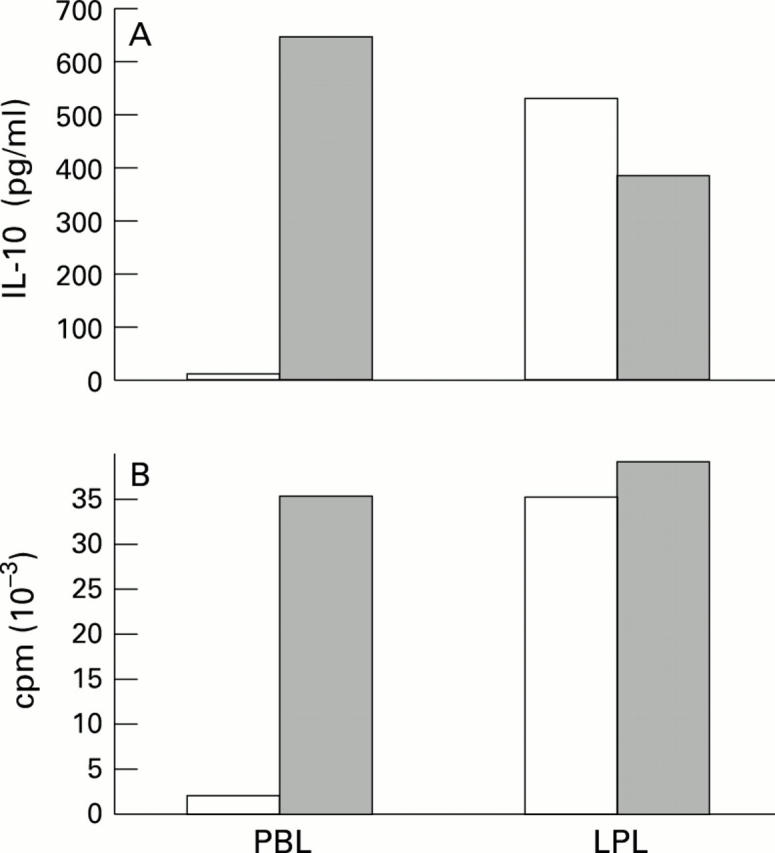
: PBL-T and LPL-T were activated by CD2 monoclonal antibodies T112 and T113 (1/1000 v/v) in the absence (open columns) or presence (shaded columns) of CD45 monoclonal antibody AlCD45.1/B211 (1/400 v/v). (A) Supernatants were harvested after 60 hours and IL-10 content (results expressed in pg/ml) were determined by using ELISA. (B) Cell growth was measured by incorporation of [3H]-thymidine after four days. Results are expressed as the mean cpm of triplicate cultures. SD < 20%. The results shown are representative of four different experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Beckman I., Shepherd K., Dimopoulos K., Ahern M., Firgaira F., Bradley J. Differential expression and regulation of cytokine mRNAs in normal human CD45R T cell subsets. Cytokine. 1994 Mar;6(2):116–123. doi: 10.1016/1043-4666(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Berkman N., John M., Roesems G., Jose P. J., Barnes P. J., Chung K. F. Inhibition of macrophage inflammatory protein-1 alpha expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J Immunol. 1995 Nov 1;155(9):4412–4418. [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Buelens C., Willems F., Delvaux A., Piérard G., Delville J. P., Velu T., Goldman M. Interleukin-10 differentially regulates B7-1 (CD80) and B7-2 (CD86) expression on human peripheral blood dendritic cells. Eur J Immunol. 1995 Sep;25(9):2668–2672. doi: 10.1002/eji.1830250940. [DOI] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Furue M., Tamaki K. B7-1 expression of Langerhans cells is up-regulated by proinflammatory cytokines, and is down-regulated by interferon-gamma or by interleukin-10. Eur J Immunol. 1995 Feb;25(2):394–398. doi: 10.1002/eji.1830250213. [DOI] [PubMed] [Google Scholar]
- Cohen S. B., Katsikis P. D., Feldmann M., Londei M. IL-10 enhances expression of the IL-2 receptor alpha chain on T cells. Immunology. 1994 Nov;83(3):329–332. [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Shevach E. M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992 May 15;148(10):3133–3139. [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Autschbach F., Schürmann G., Golling M., Braunstein J., Qiao L. Molecular mechanisms securing "unresponsiveness" in lamina propria T lymphocytes. Ann N Y Acad Sci. 1996 Feb 13;778:174–184. doi: 10.1111/j.1749-6632.1996.tb21126.x. [DOI] [PubMed] [Google Scholar]
- Moebius U., Kober G., Griscelli A. L., Hercend T., Meuer S. C. Expression of different CD8 isoforms on distinct human lymphocyte subpopulations. Eur J Immunol. 1991 Aug;21(8):1793–1800. doi: 10.1002/eji.1830210803. [DOI] [PubMed] [Google Scholar]
- Moore K. W., O'Garra A., de Waal Malefyt R., Vieira P., Mosmann T. R. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- Niessner M., Volk B. A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995 Sep;101(3):428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirzer U. C., Schürmann G., Post S., Betzler M., Meuer S. C. Differential responsiveness to CD3-Ti vs. CD2-dependent activation of human intestinal T lymphocytes. Eur J Immunol. 1990 Oct;20(10):2339–2342. doi: 10.1002/eji.1830201025. [DOI] [PubMed] [Google Scholar]
- Qiao L., Schürmann G., Autschbach F., Wallich R., Meuer S. C. Human intestinal mucosa alters T-cell reactivities. Gastroenterology. 1993 Sep;105(3):814–819. doi: 10.1016/0016-5085(93)90899-n. [DOI] [PubMed] [Google Scholar]
- Qiao L., Schürmann G., Betzler M., Meuer S. C. Activation and signaling status of human lamina propria T lymphocytes. Gastroenterology. 1991 Dec;101(6):1529–1536. doi: 10.1016/0016-5085(91)90388-2. [DOI] [PubMed] [Google Scholar]
- Qiao L., Schürmann G., Betzler M., Meuer S. C. Down-regulation of protein kinase C activation in human lamina propria T lymphocytes: influence of intestinal mucosa on T cell reactivity. Eur J Immunol. 1991 Oct;21(10):2385–2389. doi: 10.1002/eji.1830211014. [DOI] [PubMed] [Google Scholar]
- Reinecker H. C., Steffen M., Witthoeft T., Pflueger I., Schreiber S., MacDermott R. P., Raedler A. Enhanced secretion of tumour necrosis factor-alpha, IL-6, and IL-1 beta by isolated lamina propria mononuclear cells from patients with ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1993 Oct;94(1):174–181. doi: 10.1111/j.1365-2249.1993.tb05997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset F., Garcia E., Defrance T., Péronne C., Vezzio N., Hsu D. H., Kastelein R., Moore K. W., Banchereau J. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1890–1893. doi: 10.1073/pnas.89.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Schieferdecker H. L., Ullrich R., Weiss-Breckwoldt A. N., Schwarting R., Stein H., Riecken E. O., Zeitz M. The HML-1 antigen of intestinal lymphocytes is an activation antigen. J Immunol. 1990 Apr 1;144(7):2541–2549. [PubMed] [Google Scholar]
- Schraven B., Roux M., Hutmacher B., Meuer S. C. Triggering of the alternative pathway of human T cell activation involves members of the T 200 family of glycoproteins. Eur J Immunol. 1989 Feb;19(2):397–403. doi: 10.1002/eji.1830190226. [DOI] [PubMed] [Google Scholar]
- Schraven B., Samstag Y., Altevogt P., Meuer S. C. Association of CD2 and CD45 on human T lymphocytes. Nature. 1990 May 3;345(6270):71–74. doi: 10.1038/345071a0. [DOI] [PubMed] [Google Scholar]
- Schreiber S., Heinig T., Thiele H. G., Raedler A. Immunoregulatory role of interleukin 10 in patients with inflammatory bowel disease. Gastroenterology. 1995 May;108(5):1434–1444. doi: 10.1016/0016-5085(95)90692-4. [DOI] [PubMed] [Google Scholar]
- Schwarz M., Majdic O., Knapp W., Holter W. High-level IL-10 production by monoclonal antibody-stimulated human T cells. Immunology. 1995 Nov;86(3):364–371. [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Stordeur P., Schandené L., Durez P., Gérard C., Goldman M., Velu T. Spontaneous and cycloheximide-induced interleukin-10 mRNA expression in human mononuclear cells. Mol Immunol. 1995 Mar;32(4):233–239. doi: 10.1016/0161-5890(94)00158-w. [DOI] [PubMed] [Google Scholar]
- Taga K., Mostowski H., Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993 Jun 1;81(11):2964–2971. [PubMed] [Google Scholar]
- Taga K., Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992 Feb 15;148(4):1143–1148. [PubMed] [Google Scholar]
- Targan S. R., Deem R. L., Liu M., Wang S., Nel A. Definition of a lamina propria T cell responsive state. Enhanced cytokine responsiveness of T cells stimulated through the CD2 pathway. J Immunol. 1995 Jan 15;154(2):664–675. [PubMed] [Google Scholar]
- Wang P., Wu P., Siegel M. I., Egan R. W., Billah M. M. IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol. 1994 Jul 15;153(2):811–816. [PubMed] [Google Scholar]
- Wang P., Wu P., Siegel M. I., Egan R. W., Billah M. M. Interleukin (IL)-10 inhibits nuclear factor kappa B (NF kappa B) activation in human monocytes. IL-10 and IL-4 suppress cytokine synthesis by different mechanisms. J Biol Chem. 1995 Apr 21;270(16):9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- Willems F., Marchant A., Delville J. P., Gérard C., Delvaux A., Velu T., de Boer M., Goldman M. Interleukin-10 inhibits B7 and intercellular adhesion molecule-1 expression on human monocytes. Eur J Immunol. 1994 Apr;24(4):1007–1009. doi: 10.1002/eji.1830240435. [DOI] [PubMed] [Google Scholar]
- Yssel H., De Waal Malefyt R., Roncarolo M. G., Abrams J. S., Lahesmaa R., Spits H., de Vries J. E. IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol. 1992 Oct 1;149(7):2378–2384. [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., de Vries J. E. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993 Jun 1;150(11):4754–4765. [PubMed] [Google Scholar]


