Abstract
Background—The aetiology and pathology of both idiopathic megarectum and idiopathic megacolon are unknown. In particular, it is unknown whether there are abnormalities involving enteric nerves or smooth muscle. Methods—Resected tissue was examined from 24 patients who underwent surgery for idiopathic megarectum, from six patients who had tissue resected for idiopathic megacolon, and 17 control patients who had surgery for non-obstructing large bowel cancer. Qualitative and quantitative histological examination was performed after staining with haematoxylin and eosin, periodic acid Schiff (PAS), Martius scarlet blue (MSB), and phosphotungstic acid haematoxylin (PTAH). Neural and glial tissue were examined after immunostaining with S100 and PGP9.5. Results—Compared with controls, patients with idiopathic megarectum had significant thickening of their muscularis mucosae (median 78 v 33 µm, p<0.005), circular muscle (1000 v 633 µm, p<0.005), and longitudinal muscle (1083 v 303 µm, p<0.005), despite rectal dilatation. This thickening was relatively greater in the longitudinal than in the circular muscle. Fibrosis of the longitudinal muscle was seen, using MSB staining, in 58%, of circular muscle in 38%, and of muscularis mucosae in 29% of patients. The relation between muscle thickening and fibrosis was variable. The density of neural tissue in the longitudinal muscle seemed to be reduced in patients with idiopathic megarectum. There was no thickening of enteric muscle or alteration in the density of innervation in patients with idiopathic megacolon. Conclusion—There is notable thickening of the enteric smooth muscle in patients with idiopathic megarectum, but the architecture of the enteric innervation seems to be intact. Functional abnormalities of the latter remain a possible cause of the smooth muscle hypertrophy.
Keywords: idiopathic megarectum; idiopathic megacolon
Full Text
The Full Text of this article is available as a PDF (141.7 KB).
Figure 1 .
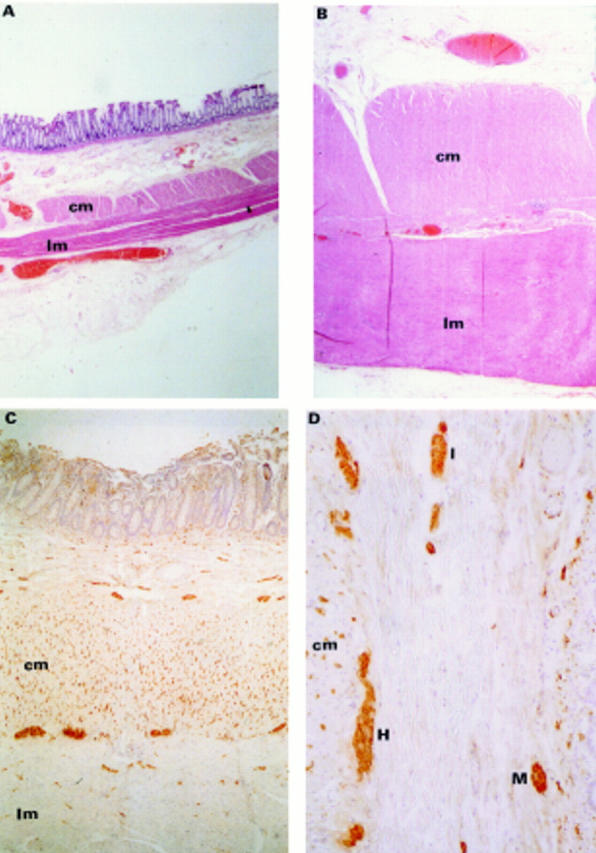
: Haematoxylin and eosin stained sections of normal control tissue (A) and tissue from a patient with idiopathic megarectum (B), taken at the same magnification (originally ×15). Thickened muscularis externa can be seen in the patient with idiopathic megarectum. (C) and (D) PGP 9.5 immunoreactivity in rectal tissue of a patient with idiopathic megarectum. The dense staining in the circular muscle is in contrast to the sparse staining within the longitudinal muscle (C). The ganglia shown in (D) are from Henle's plexus (H), the intermediate plexus (I), and Meissner's plexus (M). cm = circular muscle; lm = longitudinal muscle.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. R., Lennard-Jones J. E., Hawley P. R., Todd I. P. Hirschsprung's disease and idiopathic megacolon in adults and adolescents. Gut. 1986 May;27(5):534–541. doi: 10.1136/gut.27.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckxstaens G. E., Pelckmans P. A., Herman A. G., Van Maercke Y. M. Involvement of nitric oxide in the inhibitory innervation of the human isolated colon. Gastroenterology. 1993 Mar;104(3):690–697. doi: 10.1016/0016-5085(93)91003-z. [DOI] [PubMed] [Google Scholar]
- Burleigh D. E. Ng-nitro-L-arginine reduces nonadrenergic, noncholinergic relaxations of human gut. Gastroenterology. 1992 Feb;102(2):679–683. doi: 10.1016/0016-5085(92)90120-n. [DOI] [PubMed] [Google Scholar]
- Crowe R., Kamm M. A., Burnstock G., Lennard-Jones J. E. Peptide-containing neurons in different regions of the submucous plexus of human sigmoid colon. Gastroenterology. 1992 Feb;102(2):461–467. doi: 10.1016/0016-5085(92)90091-c. [DOI] [PubMed] [Google Scholar]
- Howard E. R., Garrett J. R., Kidd A. Constipation and congenital disorders of the myenteric plexus. J R Soc Med. 1984;77 (Suppl 3):13–19. [PMC free article] [PubMed] [Google Scholar]
- Hoyle C. H., Burnstock G. Neuronal populations in the submucous plexus of the human colon. J Anat. 1989 Oct;166:7–22. [PMC free article] [PubMed] [Google Scholar]
- Huizinga J. D., Tomlinson J., Pintin-Quezada J. Involvement of nitric oxide in nerve-mediated inhibition and action of vasoactive intestinal peptide in colonic smooth muscle. J Pharmacol Exp Ther. 1992 Feb;260(2):803–808. [PubMed] [Google Scholar]
- Kamm M. A., Stabile G. Management of idiopathic megarectum and megacolon. Br J Surg. 1991 Aug;78(8):899–900. doi: 10.1002/bjs.1800780803. [DOI] [PubMed] [Google Scholar]
- Keef K. D., Du C., Ward S. M., McGregor B., Sanders K. M. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology. 1993 Oct;105(4):1009–1016. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S., Schuffler M. D. Pathology of neuromuscular disorders of the small intestine and colon. Gastroenterology. 1987 Sep;93(3):610–639. doi: 10.1016/0016-5085(87)90926-7. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy S., Schuffler M. D., Rohrmann C. A., Pope C. E., 2nd Severe idiopathic constipation is associated with a distinctive abnormality of the colonic myenteric plexus. Gastroenterology. 1985 Jan;88(1 Pt 1):26–34. doi: 10.1016/s0016-5085(85)80128-1. [DOI] [PubMed] [Google Scholar]
- Kune G. A. Megacolon in adults. Br J Surg. 1966 Mar;53(3):199–205. doi: 10.1002/bjs.1800530312. [DOI] [PubMed] [Google Scholar]
- Nissan S., Bar-Maor J. A. Further experience in the diagnosis and surgical treatment of short-segment Hirschsprung's disease and idiopathic megacolon. J Pediatr Surg. 1971 Dec;6(6):738–741. doi: 10.1016/0022-3468(71)90854-2. [DOI] [PubMed] [Google Scholar]
- O'Kelly T., Brading A., Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut. 1993 May;34(5):689–693. doi: 10.1136/gut.34.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. A., McBirnie J. E. Atonic megacolon. Can J Surg. 1967 Jan;10(1):15–20. [PubMed] [Google Scholar]
- Preston D. M., Lennard-Jones J. E., Thomas B. M. Towards a radiologic definition of idiopathic megacolon. Gastrointest Radiol. 1985;10(2):167–169. doi: 10.1007/BF01893094. [DOI] [PubMed] [Google Scholar]
- Roy A. D. Resection of hindgut for idiopathic megacolon in adolescence. Br J Surg. 1968 Feb;55(2):106–109. doi: 10.1002/bjs.1800550209. [DOI] [PubMed] [Google Scholar]
- Scott M. J. Idiopathic megacolon presenting with fatal inferior vena caval obstruction and colonic perforation. Case report. Acta Chir Scand. 1988 Oct;154(10):605–607. [PubMed] [Google Scholar]
- Smith B., Grace R. H., Todd I. P. Organic constipation in adults. Br J Surg. 1977 May;64(5):313–314. doi: 10.1002/bjs.1800640504. [DOI] [PubMed] [Google Scholar]
- Stabile G., Kamm M. A., Hawley P. R., Lennard-Jones J. E. Colectomy for idiopathic megarectum and megacolon. Gut. 1991 Dec;32(12):1538–1540. doi: 10.1136/gut.32.12.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile G., Kamm M. A., Hawley P. R., Lennard-Jones J. E. Results of the Duhamel operation in the treatment of idiopathic megarectum and megacolon. Br J Surg. 1991 Jun;78(6):661–663. doi: 10.1002/bjs.1800780609. [DOI] [PubMed] [Google Scholar]
- Stabile G., Kamm M. A., Phillips R. K., Hawley P. R., Lennard-Jones J. E. Partial colectomy and coloanal anastomosis for idiopathic megarectum and megacolon. Dis Colon Rectum. 1992 Feb;35(2):158–162. doi: 10.1007/BF02050671. [DOI] [PubMed] [Google Scholar]
- Stark M. E., Szurszewski J. H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992 Dec;103(6):1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- Watkins G. L. Operative treatment of acquired megacolon in adults. Arch Surg. 1966 Oct;93(4):620–624. doi: 10.1001/archsurg.1966.01330040084015. [DOI] [PubMed] [Google Scholar]
- Wilson P. O., Barber P. C., Hamid Q. A., Power B. F., Dhillon A. P., Rode J., Day I. N., Thompson R. J., Polak J. M. The immunolocalization of protein gene product 9.5 using rabbit polyclonal and mouse monoclonal antibodies. Br J Exp Pathol. 1988 Feb;69(1):91–104. [PMC free article] [PubMed] [Google Scholar]


