Abstract
Background—The use of corticosteroids in active Crohn's disease often becomes limited by side effects. Budesonide is a potent corticosteroid with low systemic bioavailability due to an extensive first pass liver metabolism. Aims—To compare the efficacy and safety of two dosage regimens of budesonide and prednisolone in patients with active Crohn's disease affecting the ileum and/or the ascending colon. Patients and methods—One hundred and seventy eight patients were randomised to receive budesonide controlled ileal release (CIR) capsules 9 mg once daily or 4.5 mg twice daily, or prednisolone tablets 40 mg once daily. The treatment period was 12 weeks. The primary efficacy variable was clinical remission, defined as a Crohn's Disease Activity Index (CDAI) of 150 or less. Results—After eight weeks of treatment, remission occurred in 60% of patients receiving budesonide once daily or prednisolone and in 42% of those receiving budesonide twice daily (p=0.062). The presence of glucocorticoid associated side effects was similar in all groups; however, moon face was more common in the prednisolone group (p=0.0005). The highest frequency of impaired adrenal function, as measured by a short ACTH test, was found in the prednisolone group (p=0.0023). Conclusions—Budesonide CIR, administered at 9 mg once daily or 4.5 mg twice daily, is comparable to prednisolone in inducing remission in active Crohn's disease. The single dose administration is as promptly effective as prednisolone and represents a simpler and safer therapeutic approach, with a considerable reduction in side effects.
Keywords: adrenal function; CDAI; glucocorticoid; glucocorticoid associated side effects
Full Text
The Full Text of this article is available as a PDF (138.4 KB).
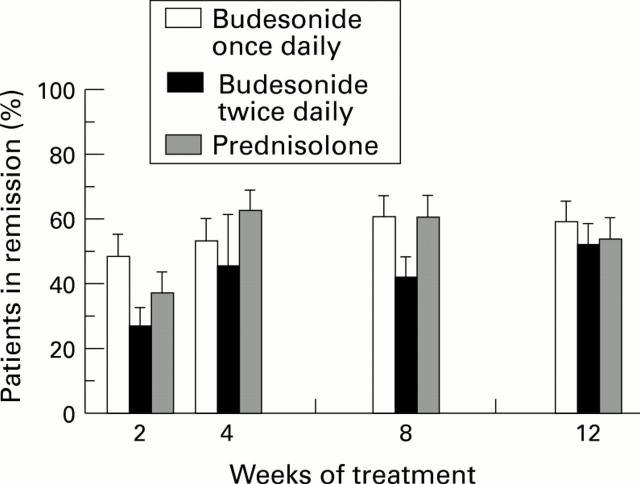
Figure 1 .
: Mean (SE) proportion of patients in remission after two, four, eight, and 12 weeks of treatment with budesonide or prednisolone.
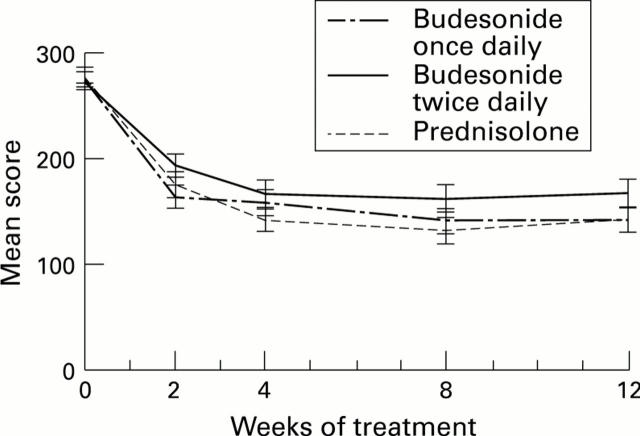
Figure 2 .
: Mean (SE) CDAI score at randomisation and after two, four, eight, and 12 weeks of treatment with budesonide or prednisolone.
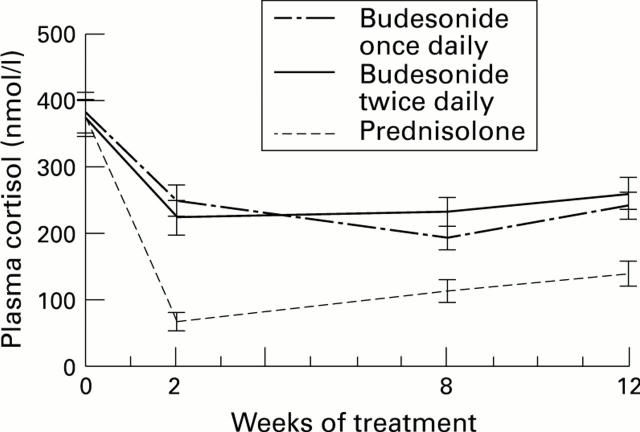
Figure 3 .
: Mean (SE) morning plasma cortisol at randomisation and after two, four, eight, and 12 weeks of treatment with budesonide or prednisolone.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Brogden R. N., McTavish D. Budesonide. An updated review of its pharmacological properties, and therapeutic efficacy in asthma and rhinitis. Drugs. 1992 Sep;44(3):375–407. doi: 10.2165/00003495-199244030-00007. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C., Balow J. E. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976 Mar;84(3):304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Feagan B. G., Martin F., Sutherland L. R., Thomson A. B., Williams C. N., Nilsson L. G., Persson T. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994 Sep 29;331(13):836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- Hanauer S. B., Stathopoulos G. Risk-benefit assessment of drugs used in the treatment of inflammatory bowel disease. Drug Saf. 1991 May-Jun;6(3):192–219. doi: 10.2165/00002018-199106030-00005. [DOI] [PubMed] [Google Scholar]
- Löfberg R., Danielsson A., Salde L. Oral budesonide in active Crohn's disease. Aliment Pharmacol Ther. 1993 Dec;7(6):611–616. doi: 10.1111/j.1365-2036.1993.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Löfberg R., Ostergaard Thomsen O., Langholz E., Schiöler R., Danielsson A., Suhr O., Graffner H., Påhlman L., Matzen P., Møller-Petersen J. F. Budesonide versus prednisolone retention enemas in active distal ulcerative colitis. Aliment Pharmacol Ther. 1994 Dec;8(6):623–629. doi: 10.1111/j.1365-2036.1994.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J. W., Goebell H., Ehms H., Sommer H., Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984 Feb;86(2):249–266. [PubMed] [Google Scholar]
- Meyers S., Sachar D. B. Medical management of Crohn's disease. Hepatogastroenterology. 1990 Feb;37(1):42–55. [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (2) N Engl J Med. 1991 Oct 3;325(14):1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- Reinberg A., Smolensky M. H., D'Alonzo G. E., McGovern J. P. Chronobiology and asthma. III. Timing corticotherapy to biological rhythms to optimize treatment goals. J Asthma. 1988;25(4):219–248. doi: 10.3109/02770908809071368. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Löfberg R., Malchow H., Lamers C., Olaison G., Jewell D., Danielsson A., Goebell H., Thomsen O. O., Lorenz-Meyer H. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994 Sep 29;331(13):842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- Sachar D. B. Budesonide for inflammatory bowel disease. It is a magic bullet? N Engl J Med. 1994 Sep 29;331(13):873–874. doi: 10.1056/NEJM199409293311311. [DOI] [PubMed] [Google Scholar]
- Summers R. W., Switz D. M., Sessions J. T., Jr, Becktel J. M., Best W. R., Kern F., Jr, Singleton J. W. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979 Oct;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- Tarpila S., Turunen U., Seppälä K., Aukee S., Pikkarainen P., Elomaa I., Karvonen A. L., Käriäinen I., Sipponen P., Toivanen E. Budesonide enema in active haemorrhagic proctitis--a controlled trial against hydrocortisone foam enema. Aliment Pharmacol Ther. 1994 Dec;8(6):591–595. doi: 10.1111/j.1365-2036.1994.tb00335.x. [DOI] [PubMed] [Google Scholar]