Abstract
Background—The "topical" effect of non-steroidal anti-inflammatory drugs (NSAIDs) seems to be an important cause of NSAID induced gastrointestinal damage. Aim—To examine the possible mechanism of the "topical" phase of damage in the small intestine. Methods—Electron microscopy and subcellular organelle marker enzyme studies were done in rat small intestine after oral administration of indomethacin (doses varied between 5 and 30 mg/kg). The effect of conventional and non-acidic NSAIDs on rat liver mitochondrial respiration was measured in vitro in a Clarke-type oxygen electrode. Results—The subcellular organelle marker enzymes showed mitochondrial and brush border involvement within an hour of indomethacin administration. Electron microscopy showed dose dependent mitochondrial changes following indomethacin administration consistent with uncoupling of oxidative phosphorylation (or inhibition of electron transport) which were indistinguishable from those seen with the uncoupler dinitrophenol. Parenteral indomethacin caused similar changes, but not in rats with ligated bile ducts. A range of NSAIDs, but not paracetamol or non-acidic NSAIDs which have a favourable gastrointestinal tolerability profile, uncoupled oxidative phosphorylation in vitro at micromolar concentrations and inhibited respiration at higher concentrations. In vivo studies with nabumetone and aspirin further suggested that uncoupling or inhibition of electron transport underlies the "topical" phase of NSAID induced damage. Conclusion—Collectively, these studies suggest that NSAID induced changes in mitochondrial energy production may be an important component of the "topical" phase of damage induction.
Keywords: intestinal inflammation; intestinal toxicity; mitochondrial function; drug induced toxicity
Full Text
The Full Text of this article is available as a PDF (253.1 KB).
Figure 1 .
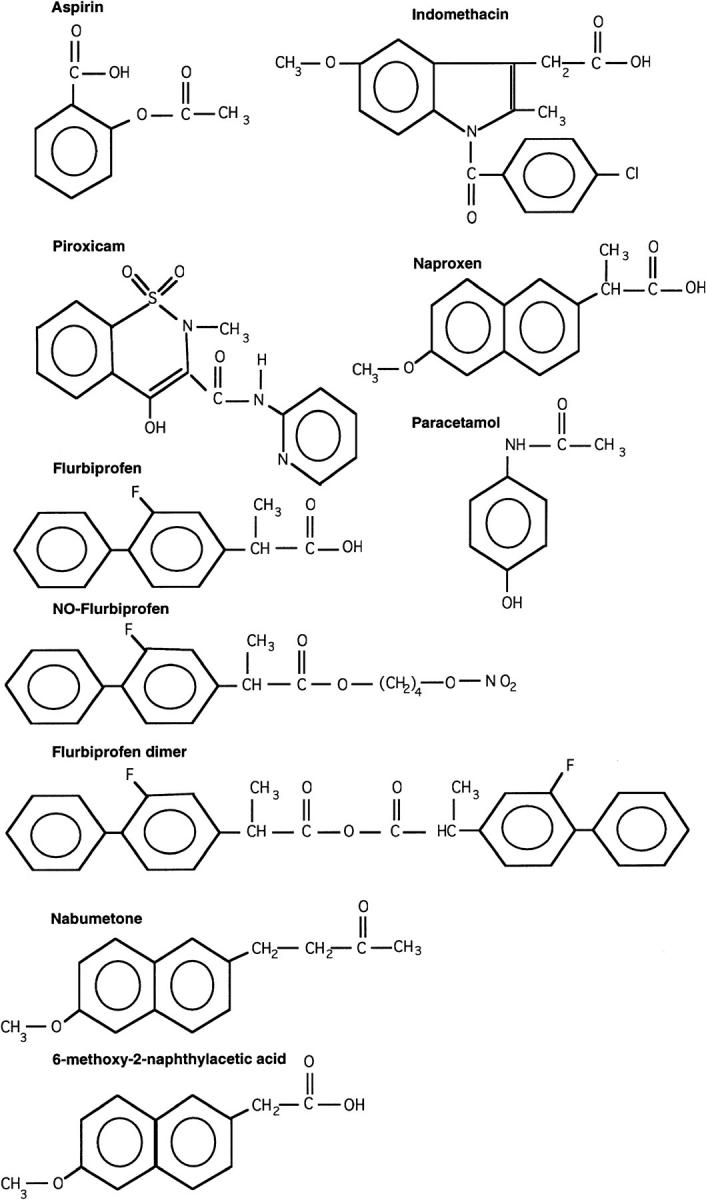
: Chemical structure of the NSAIDs and paracetamol used in the study.
Figure 2 .
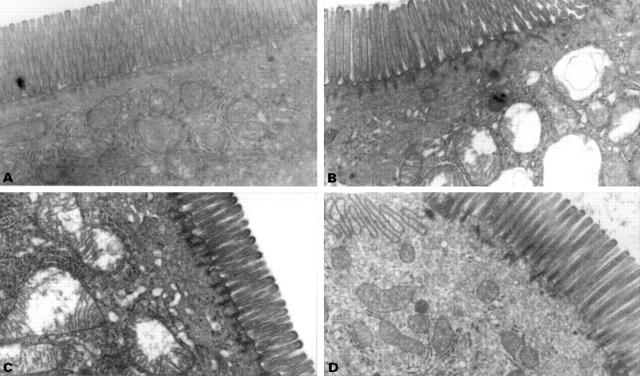
: Representative electron transmission micrographs of rat small intestine (jejunum, 30 cm distal to the ligament of Treitz) demonstrating (A) normal enterocyte mitochondria, (B) vacuolisation of mitochondria one hour after indomethacin administration, (C) vacuolisation of mitochondria one hour after the administration of the uncoupler dinitrophenol, and (D) normal enterocyte mitochondria one hour after nabumetone administration. Note normal brush border appearances. Original magnification, ×10 000.
Figure 3 .
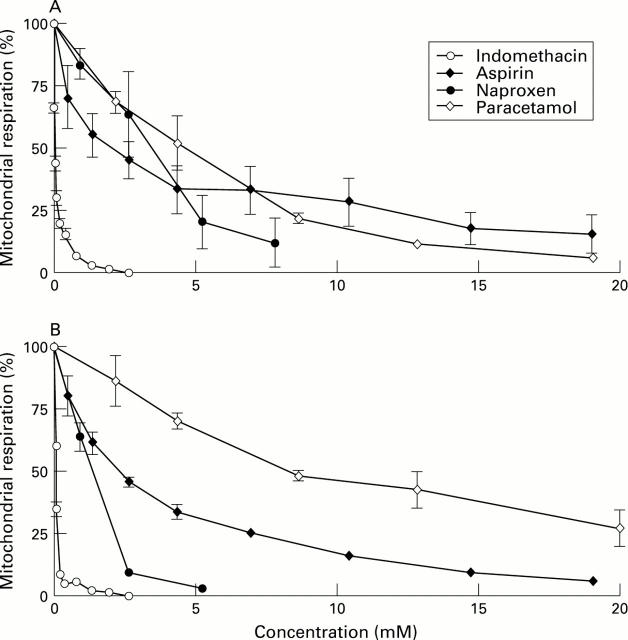
: Indomethacin, aspirin, naproxen, and piroxicam stimulate mitochondrial respiration (uncouple oxidative phosphorylation) at concentrations between 30 and 1500 µM, depending on the particular NSAID, and inhibit respiration at higher concentrations. Paracetamol, a non-NSAID analgesic control, reduces respiration, without an uncoupling effect. Each data point represents the mean (SE) of 3-5 experiments, performed on different days.
Figure 4 .
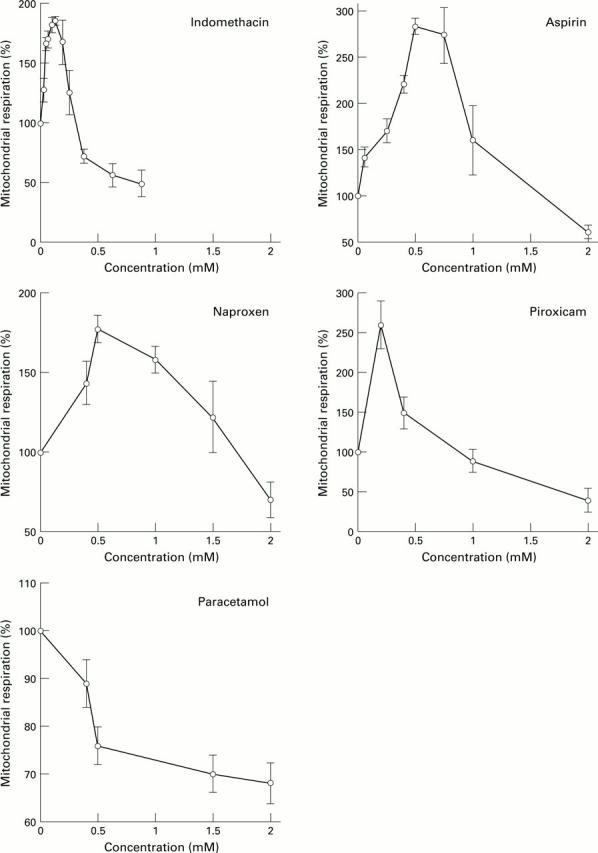
: Mitochondrial preparation was as in fig 3, but with the addition of 0.1 µM FCCP, a potent uncoupler, prior to the addition of the drugs. (A) Glutamate/malate and (B) succinate (in the presence of rotenone) were used as electron donors for complexes I and II, respectively, at different concentrations of NSAIDs as shown in the figure. Indomethacin, aspirin, naproxen, and paracetamol all inhibit complexes I and II at different concentrations in a dose dependent manner. Each point represents the mean (SE) of 3-5 separate experiments, performed on different days.
Figure 5 .
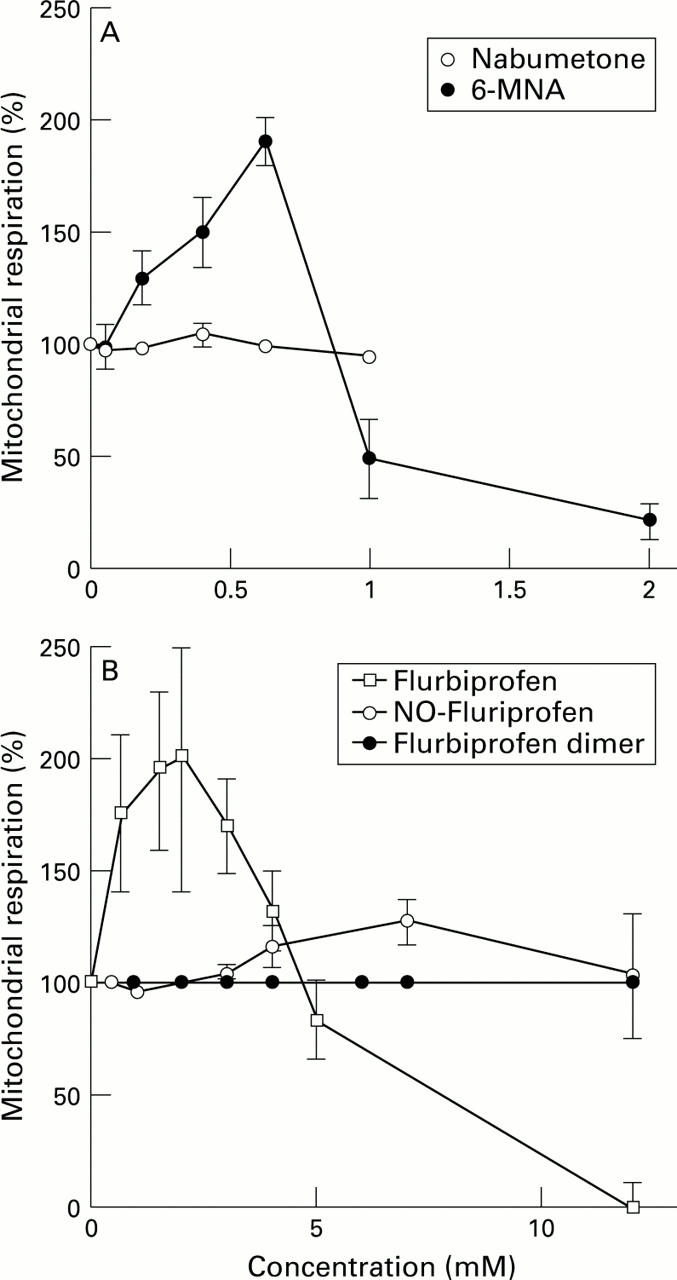
: (A) The non-acidic pro-NSAID, nabumetone, had no significant effects on mitochondrial respiration whereas its active component, 6-MNA, stimulated respiration in a similar fashion to the other NSAIDs. (B) Similarly, flurbiprofen stimulated mitochondrial respiration whereas its chemical modification to a nitroxybutyl ester or a flurbiprofen dimer, renders the drugs incapable of uncoupling oxidative phosphorylation.
Figure 6 .
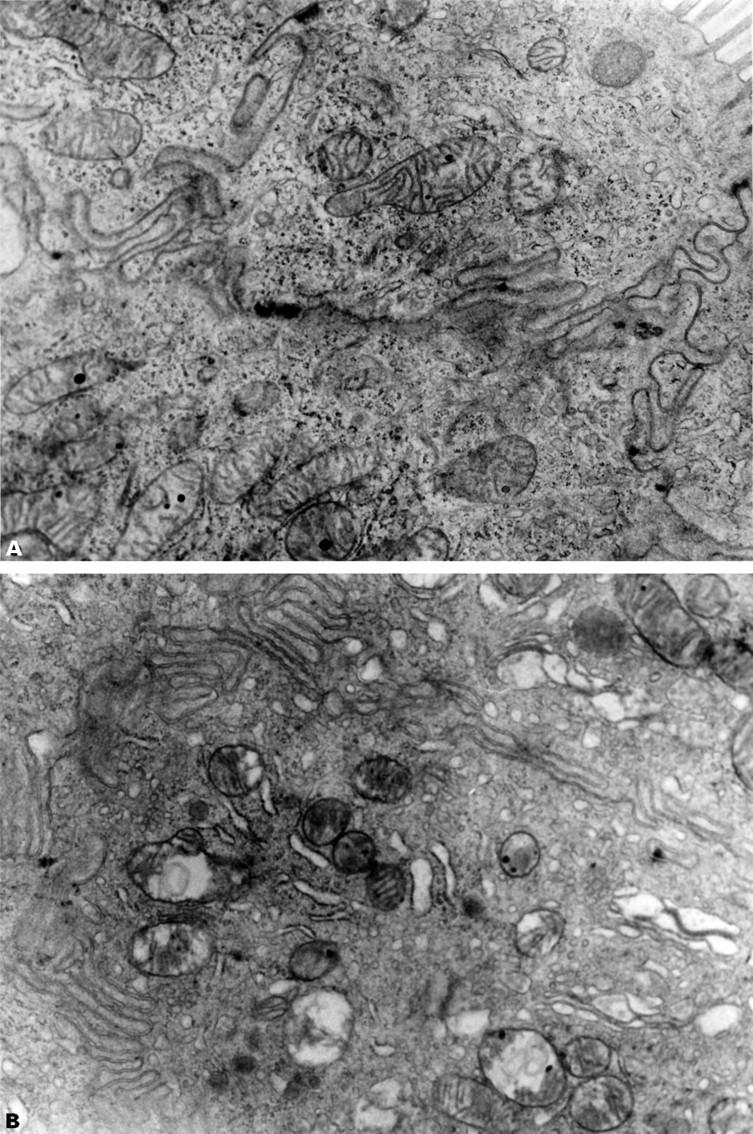
: Representative electron transmission micrographs of rat small intestine following intraperitoneal indomethacin (20 mg/kg) in (A) a rat with a ligated bile duct showing normal intestinal mitochondria with discrete cristae and (B) a sham operated animal showing disruption of mitochondrial cristae, vacuolisation and occasional disruption of both the outer and inner mitochondrial membranes. Endoplasmic reticulum is also dilated. Original magnification, × 30 000.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS S. S., COBB R. A possible basis for the anti-inflammatory activity of salicylates and other non-hormonal anti-rheumatic drugs. Nature. 1958 Mar 15;181(4611):773–774. doi: 10.1038/181773b0. [DOI] [PubMed] [Google Scholar]
- Anthony A., Dhillon A. P., Nygard G., Hudson M., Piasecki C., Strong P., Trevethick M. A., Clayton N. M., Jordan C. C., Pounder R. E. Early histological features of small intestinal injury induced by indomethacin. Aliment Pharmacol Ther. 1993 Feb;7(1):29–39. doi: 10.1111/j.1365-2036.1993.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Barrier C. H., Hirschowitz B. I. Controversies in the detection and management of nonsteroidal antiinflammatory drug-induced side effects of the upper gastrointestinal tract. Arthritis Rheum. 1989 Jul;32(7):926–932. [PubMed] [Google Scholar]
- Baskin W. N., Ivey K. J., Krause W. J., Jeffrey G. E., Gemmell R. T. Aspirin-induced ultrastructural changes in human gastric mucosa: correlation with potential difference. Ann Intern Med. 1976 Sep;85(3):299–303. doi: 10.7326/0003-4819-85-3-299. [DOI] [PubMed] [Google Scholar]
- Bennett A., Tavares I. A. NSAIDs, Cox-2 inhibitors, and the gut. Lancet. 1995 Oct 21;346(8982):1105–1105. [PubMed] [Google Scholar]
- Bjarnason I., Fehilly B., Smethurst P., Menzies I. S., Levi A. J. Importance of local versus systemic effects of non-steroidal anti-inflammatory drugs in increasing small intestinal permeability in man. Gut. 1991 Mar;32(3):275–277. doi: 10.1136/gut.32.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., Hayllar J., MacPherson A. J., Russell A. S. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology. 1993 Jun;104(6):1832–1847. doi: 10.1016/0016-5085(93)90667-2. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Smethurst P., Fenn C. G., Lee C. E., Menzies I. S., Levi A. J. Misoprostol reduces indomethacin-induced changes in human small intestinal permeability. Dig Dis Sci. 1989 Mar;34(3):407–411. doi: 10.1007/BF01536263. [DOI] [PubMed] [Google Scholar]
- Bjarnason I., Williams P., Smethurst P., Peters T. J., Levi A. J. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut. 1986 Nov;27(11):1292–1297. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie D. A., Cook P. G., Bauer B. J., Dagle G. E. Indomethacin-induced intestinal lesions in the rat. Toxicol Appl Pharmacol. 1970 Nov;17(3):615–624. doi: 10.1016/0041-008x(70)90036-0. [DOI] [PubMed] [Google Scholar]
- Brooks P. M., Day R. O. Nonsteroidal antiinflammatory drugs--differences and similarities. N Engl J Med. 1991 Jun 13;324(24):1716–1725. doi: 10.1056/NEJM199106133242407. [DOI] [PubMed] [Google Scholar]
- Brune K., Dietzel K., Nürnberg B., Schneider H. T. Recent insight into the mechanism of gastrointestinal tract ulceration. Scand J Rheumatol Suppl. 1987;65:135–140. doi: 10.3109/03009748709102192. [DOI] [PubMed] [Google Scholar]
- Brune K., Schweitzer A., Eckert H. Parietal cells of the stomach trap salicylates during absorption. Biochem Pharmacol. 1977 Sep 15;26(18):1735–1740. doi: 10.1016/0006-2952(77)90155-1. [DOI] [PubMed] [Google Scholar]
- Bugat R., Thompson M. R., Aures D., Grossman M. I. Gastric mucosal lesions produced by intravenous infusion of aspirin in cats. Gastroenterology. 1976 Nov;71(5):754–759. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Carter E. A., Hatz R. A., Yarmush M. L., Tompkins R. G. Injury-induced inhibition of small intestinal protein and nucleic acid synthesis. Gastroenterology. 1990 Jun;98(6):1445–1451. doi: 10.1016/0016-5085(90)91074-g. [DOI] [PubMed] [Google Scholar]
- Daneshmend T. K., Stein A. G., Bhaskar N. K., Hawkey C. J. Abolition by omeprazole of aspirin induced gastric mucosal injury in man. Gut. 1990 May;31(5):514–517. doi: 10.1136/gut.31.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeja P. K., Brasitus T. A. Inactivation of rat small intestinal brush-border membrane alkaline phosphatase by oxygen free radicals. Gastroenterology. 1993 Aug;105(2):357–366. doi: 10.1016/0016-5085(93)90708-k. [DOI] [PubMed] [Google Scholar]
- Gullikson G. W., Cline W. S., Lorenzsonn V., Benz L., Olsen W. A., Bass P. Effects of anionic surfactants on hamster small intestinal membrane structure and function: relationship to surface activity. Gastroenterology. 1977 Sep;73(3):501–511. [PubMed] [Google Scholar]
- Gullikson G. W., Sender M., Bass P. Laxative-like effects of nonsteroidal anti-inflammatory drugs on intestinal fluid movement and membrane integrity. J Pharmacol Exp Ther. 1982 Feb;220(2):236–242. [PubMed] [Google Scholar]
- Ivey K. J., Paone D. B., Krause W. J. Acute effect of systemic aspirin on gastric mucosa in man. Dig Dis Sci. 1980 Feb;25(2):97–99. doi: 10.1007/BF01308304. [DOI] [PubMed] [Google Scholar]
- Kauffman G. L., Jr, Grossman M. I. Prostaglandin and cimetidine inhibit the formation of ulcers produced by parenteral salicylates. Gastroenterology. 1978 Dec;75(6):1099–1102. [PubMed] [Google Scholar]
- Kent T. H., Cardelli R. M., Stamler F. W. Small intestinal ulcers and intestinal flora in rats given indomethacin. Am J Pathol. 1969 Feb;54(2):237–249. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langenbach R., Morham S. G., Tiano H. F., Loftin C. D., Ghanayem B. I., Chulada P. C., Mahler J. F., Lee C. A., Goulding E. H., Kluckman K. D. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995 Nov 3;83(3):483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- Levine R. A., Petokas S., Nandi J., Enthoven D. Effects of nonsteroidal, antiinflammatory drugs on gastrointestinal injury and prostanoid generation in healthy volunteers. Dig Dis Sci. 1988 Jun;33(6):660–666. doi: 10.1007/BF01540427. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M., Wang Z. M., Romero J. J., Ulloa C., Perez J. C., Giraud M. N., Barreto J. C. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nat Med. 1995 Feb;1(2):154–158. doi: 10.1038/nm0295-154. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Golanska E. M., Hansen D. G., Kauffman G. L., Jr Aspirin can inhibit gastric mucosal cyclo-oxygenase without causing lesions in rat. Gastroenterology. 1983 Apr;84(4):756–761. [PubMed] [Google Scholar]
- Ligumsky M., Grossman M. I., Kauffman G. L., Jr Endogenous gastric mucosal prostaglandins: their role in mucosal integrity. Am J Physiol. 1982 Apr;242(4):G337–G341. doi: 10.1152/ajpgi.1982.242.4.G337. [DOI] [PubMed] [Google Scholar]
- Matthews J. B., Smith J. A., Tally K. J., Menconi M. J., Nguyen H., Fink M. P. Chemical hypoxia increases junctional permeability and activates electrogenic ion transport in human intestinal epithelial monolayers. Surgery. 1994 Aug;116(2):150–158. [PubMed] [Google Scholar]
- Mehlman M. A., Tobin R. B., Sporn E. M. Oxidative phosphorylation and respiration by rat liver mitochondria from aspirin-treated rats. Biochem Pharmacol. 1972 Dec 15;21(24):3279–3279. doi: 10.1016/0006-2952(72)90092-5. [DOI] [PubMed] [Google Scholar]
- Melarange R., Gentry C., O'Connell C., Blower P. R., Neil C., Kelvin A. S., Toseland C. D. Antiinflammatory and gastrointestinal effects of nabumetone or its active metabolite, 6-methoxy-2-naphthylacetic acid (6MNA). Comparative studies with indomethacin. Dig Dis Sci. 1992 Dec;37(12):1847–1852. doi: 10.1007/BF01308078. [DOI] [PubMed] [Google Scholar]
- Ohe K., Hayashi K., Shirakawa T., Yamada K., Kawasaki T., Miyoshi A. Aspirin- and taurocholate-induced metabolic damage in mammalian gastric mucosa in vitro. Am J Physiol. 1980 Dec;239(6):G457–G462. doi: 10.1152/ajpgi.1980.239.6.G457. [DOI] [PubMed] [Google Scholar]
- Peters T. J. Investigation of tissue organelles by a combination of analytical subcellular fractionation and enzymic microanalysis: a new approach to pathology. J Clin Pathol. 1981 Jan;34(1):1–12. doi: 10.1136/jcp.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters T. J., Jones P. E., Jenkins W. J., Wells G. Analytical subcellular fractionation of jejunal biopsy specimens: enzyme activities, organelle pathology and response to corticosteroids in patients with non-responsive coeliac disease. Clin Sci Mol Med. 1978 Sep;55(3):293–300. doi: 10.1042/cs0550293. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D. Mechanisms of gastrointestinal toxicity of non-steroidal anti-inflammatory drugs. Scand J Gastroenterol Suppl. 1989;163:9–16. [PubMed] [Google Scholar]
- Rainsford K. D., Whitehouse M. W. Anti-inflammatory/anti-pyretic salicylic acid esters with low gastric ulcerogenic activity. Agents Actions. 1980 Nov;10(5):451–456. doi: 10.1007/BF01968046. [DOI] [PubMed] [Google Scholar]
- Rainsford K. D., Whitehouse M. W. Biochemical gastroprotection from acute ulceration induced by aspirin and related drugs. Biochem Pharmacol. 1980 May 1;29(9):1281–1289. doi: 10.1016/0006-2952(80)90286-5. [DOI] [PubMed] [Google Scholar]
- Robins P. G. Ultrastructural observations on the pathogenesis of aspirin-induced gastric erosions. Br J Exp Pathol. 1980 Oct;61(5):497–504. [PMC free article] [PubMed] [Google Scholar]
- Roth S. H. New understandings of NSAID gastropathy. Scand J Rheumatol Suppl. 1989;78:24–32. doi: 10.3109/03009748909101460. [DOI] [PubMed] [Google Scholar]
- Somasundaram S., Hayllar H., Rafi S., Wrigglesworth J. M., Macpherson A. J., Bjarnason I. The biochemical basis of non-steroidal anti-inflammatory drug-induced damage to the gastrointestinal tract: a review and a hypothesis. Scand J Gastroenterol. 1995 Apr;30(4):289–299. doi: 10.3109/00365529509093280. [DOI] [PubMed] [Google Scholar]
- Spenney J. G., Bhown M. Effect of acetylsalicylic acid on gastric mucosa. II. Mucosal ATP and phosphocreatine content, and salicylate effects on mitochondrial metabolism. Gastroenterology. 1977 Nov;73(5):995–999. [PubMed] [Google Scholar]
- Szabo S., Spill W. F., Rainsford K. D. Non-steroidal anti-inflammatory drug-induced gastropathy. Mechanisms and management. Med Toxicol Adverse Drug Exp. 1989 Mar-Apr;4(2):77–94. doi: 10.1007/BF03259905. [DOI] [PubMed] [Google Scholar]
- Tokumitsu Y., Lee S., Ui M. In vitro effects of nonsteroidal anti-inflammatory drugs on oxidative phosphorylation in rats liver mitochondria. Biochem Pharmacol. 1977 Nov 15;26(22):2101–2106. doi: 10.1016/0006-2952(77)90258-1. [DOI] [PubMed] [Google Scholar]
- WHITEHOUSE M. W. BIOCHEMICAL PROPERTIES OF ANTI-INFLAMMATORY DRUGS--III. UNCOUPLING OF OXIDATIVE PHOSPHORYLATION IN A CONNECTIVE TISSUE (CARTILAGE) AND LIVER MITOCHONDRIA BY SALICYLATE ANALOGUES: RELATIONSHIP OF STRUCTURE TO ACTIVITY. Biochem Pharmacol. 1964 Mar;13:319–336. doi: 10.1016/0006-2952(64)90148-0. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Reuter B., Cicala C., McKnight W., Grisham M. B., Cirino G. Novel nonsteroidal anti-inflammatory drug derivatives with markedly reduced ulcerogenic properties in the rat. Gastroenterology. 1994 Jul;107(1):173–179. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Wallace J. L. The 1994 Merck Frosst Award. Mechanisms of nonsteroidal anti-inflammatory drug (NSAID) induced gastrointestinal damage--potential for development of gastrointestinal tract safe NSAIDs. Can J Physiol Pharmacol. 1994 Dec;72(12):1493–1498. doi: 10.1139/y94-215. [DOI] [PubMed] [Google Scholar]
- Wax J., Clinger W. A., Varner P., Bass P., Winder C. V. Relationship of the enterohepatic cycle to ulcerogenesis in the rat small bowel with flufenamic acid. Gastroenterology. 1970 Jun;58(6):772–780. [PubMed] [Google Scholar]
- Whittle B. J. Mechanisms underlying gastric mucosal damage induced by indomethacin and bile-salts, and the actions of prostaglandins. Br J Pharmacol. 1977 Jul;60(3):455–460. doi: 10.1111/j.1476-5381.1977.tb07522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]