Abstract
Background—The diagnosis and classification of oesophageal motility disorders is currently based on assessment of the phasic contractile activity of the oesophagus. Tonic muscular contraction of the oesophageal body (oesophageal tone) has not been well characterised. Aim—To quantify oesophageal tonic activity in healthy subjects and in patients with achalasia. Patients—Oesophageal tone was measured in 14 patients with untreated achalasia and in 14 healthy subjects. In eight patients with achalasia, oesophageal tone was again measured one month after either endoscopic or surgical treatment. Methods—Tonic wall activity was quantified by means of a flaccid intraoesophageal bag, 5 cm long and of 120 ml maximal capacity, which was placed and maintained 5 cm above the lower oesophageal sphincter and connected to an external electronic barostat. The experimental design included measurement of oesophageal basal tone and compliance as well as the oesophageal tone response to a nitric oxide donor (0.5 ml amyl nitrite inhalation). Results—Oesophageal basal tone, expressed as the intrabag (intraoesophageal) volume at a minimal distending pressure (2 mm Hg), did not differ significantly between patients with achalasia and healthy controls (6.6 (2.5) ml versus 4.1 (0.8) ml, respectively). Oesophageal compliance (volume/pressure relation during intraoesophageal distension) was significantly increased in achalasia (oesophageal extension ratio: 3.2 (0.4) ml/mm Hg versus 1.9 (0.2) ml/mm Hg; p< 0.01). Amyl nitrite inhalation induced oesophageal relaxation both in patients and in controls, but the magnitude of relaxation was greater in the latter (intrabag volume increase: 15.3 (2.4) ml versus 36.2 (7.1) ml; p<0.01). Conclusion—In patients with achalasia, oesophageal tonic activity, and not only phasic activity, is impaired. Although oesophageal compliance is increased, residual oesophageal tone is maintained so that a significant relaxant response may occur after pharmacological stimulation.
Keywords: achalasia; oesophageal tonic activity; oesophageal barostat; amyl nitrite; NO donor
Full Text
The Full Text of this article is available as a PDF (135.9 KB).
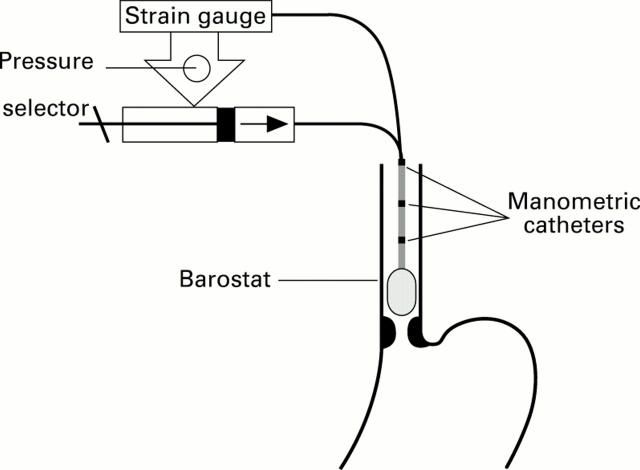
Figure 1 .
: Schematic representation of the oesophageal barostat and three proximal manometric sites which allowed simultaneous recording of tonic and phasic oesophageal motility.
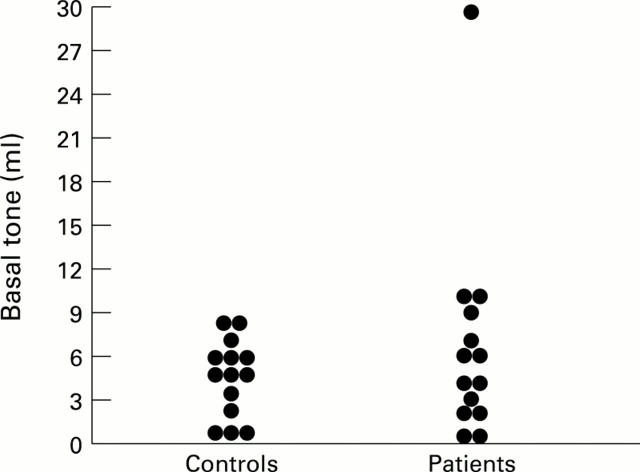
Figure 2 .
: Oesophageal basal tone (intrabag volume at the minimal distending pressure: 2 mm Hg) in healthy controls and untreated patients with achalasia.
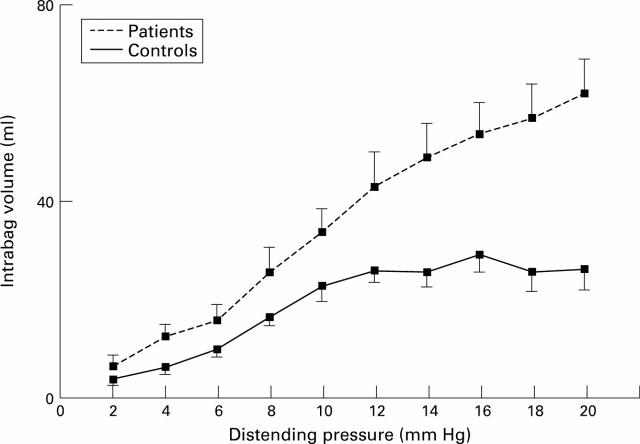
Figure 3 .
: Increase in oesophageal compliance in patients with achalasia: higher volumes were obtained at similar distending pressures (p<0.05 versus controls for cumulative values and slope) (means (SE)).
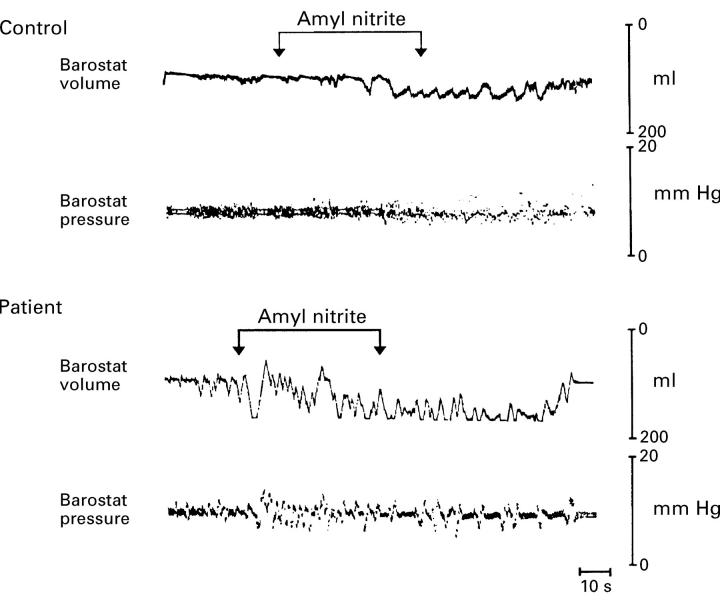
Figure 4 .
: Actual tracing illustrating the relaxant effect of amyl nitrite inhalation on the oesophageal body (increase in intra-oesophageal volume) both in controls and patients. The magnitude of the relaxant response was larger in the patients.
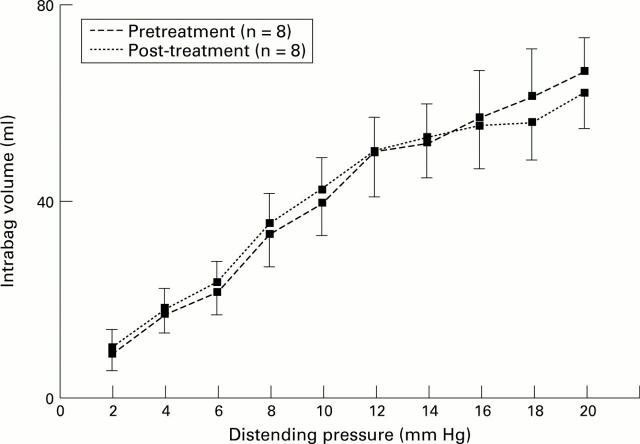
Figure 5 .
: In patients with achalasia, oesophageal compliance remained unchanged one month after treatment (means (SE)).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggestrup S., Uddman R., Sundler F., Fahrenkrug J., Håkanson R., Sørensen H. R., Hambraeus G. Lack of vasoactive intestinal polypeptide nerves in esophageal achalasia. Gastroenterology. 1983 May;84(5 Pt 1):924–927. [PubMed] [Google Scholar]
- Azpiroz F., Malagelada J. R. Physiological variations in canine gastric tone measured by an electronic barostat. Am J Physiol. 1985 Feb;248(2 Pt 1):G229–G237. doi: 10.1152/ajpgi.1985.248.2.G229. [DOI] [PubMed] [Google Scholar]
- Cohen S. Motor disorders of the esophagus. N Engl J Med. 1979 Jul 26;301(4):184–192. doi: 10.1056/NEJM197907263010404. [DOI] [PubMed] [Google Scholar]
- Frieling T., Berges W., Borchard F., Lübke H. J., Enck P., Wienbeck M. Family occurrence of achalasia and diffuse spasm of the oesophagus. Gut. 1988 Nov;29(11):1595–1602. doi: 10.1136/gut.29.11.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway R. H., Dodds W. J., Helm J. F., Hogan W. J., Dent J., Arndorfer R. C. Integrity of cholinergic innervation to the lower esophageal sphincter in achalasia. Gastroenterology. 1986 Apr;90(4):924–929. doi: 10.1016/0016-5085(86)90869-3. [DOI] [PubMed] [Google Scholar]
- Kahrilas P. J., Kishk S. M., Helm J. F., Dodds W. J., Harig J. M., Hogan W. J. Comparison of pseudoachalasia and achalasia. Am J Med. 1987 Mar;82(3):439–446. doi: 10.1016/0002-9343(87)90443-8. [DOI] [PubMed] [Google Scholar]
- Mayrand S., Diamant N. E. Measurement of human esophageal tone in vivo. Gastroenterology. 1993 Nov;105(5):1411–1420. doi: 10.1016/0016-5085(93)90146-4. [DOI] [PubMed] [Google Scholar]
- Mearin F., Cucala M., Azpiroz F., Malagelada J. R. The origin of symptoms on the brain-gut axis in functional dyspepsia. Gastroenterology. 1991 Oct;101(4):999–1006. doi: 10.1016/0016-5085(91)90726-2. [DOI] [PubMed] [Google Scholar]
- Mearin F., Mourelle M., Guarner F., Salas A., Riveros-Moreno V., Moncada S., Malagelada J. R. Patients with achalasia lack nitric oxide synthase in the gastro-oesophageal junction. Eur J Clin Invest. 1993 Nov;23(11):724–728. doi: 10.1111/j.1365-2362.1993.tb01292.x. [DOI] [PubMed] [Google Scholar]
- Mellow M. H. Return of esophageal peristalsis in idiopathic achalasia. Gastroenterology. 1976 Jun;70(6):1148–1151. [PubMed] [Google Scholar]
- Mertz H., Naliboff B., Munakata J., Niazi N., Mayer E. A. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterology. 1995 Jul;109(1):40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Parrilla P., Aguayo J. L., Martinez de Haro L., Ortiz A., Martinez D. A., Morales G. Reversible achalasia-like motor pattern of esophageal body secondary to postoperative stricture of gastroesophageal junction. Dig Dis Sci. 1992 Nov;37(11):1781–1784. doi: 10.1007/BF01299876. [DOI] [PubMed] [Google Scholar]
- Ponce J., Miralbés M., Garrigues V., Berenguer J. Return of esophageal peristalsis after Heller's myotomy for idiopathic achalasia. Dig Dis Sci. 1986 May;31(5):545–547. doi: 10.1007/BF01320323. [DOI] [PubMed] [Google Scholar]
- Robertson C. S., Hardy J. G., Atkinson M. Quantitative assessment of the response to therapy in achalasia of the cardia. Gut. 1989 Jun;30(6):768–773. doi: 10.1136/gut.30.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M. A., Hasler W. L., Chey W. D., Kim M. S., Owyang C. Hyperglycemia inhibits mechanoreceptor-mediated gastrocolonic responses and colonic peristaltic reflexes in healthy humans. Gastroenterology. 1995 Feb;108(2):350–359. doi: 10.1016/0016-5085(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Tøttrup A., Svane D., Forman A. Nitric oxide mediating NANC inhibition in opossum lower esophageal sphincter. Am J Physiol. 1991 Mar;260(3 Pt 1):G385–G389. doi: 10.1152/ajpgi.1991.260.3.G385. [DOI] [PubMed] [Google Scholar]