Abstract
Background—Lectins are proteins capable of specific binding to carbohydrates without altering their covalent structure. As an essential part of plants they are ingested in our daily diet. By binding to glycosyl side chains of receptors lectins can mimic or inhibit the action of the ligand. Oral administration of phytohaemagglutinin (PHA) in rats dose dependently induces growth of the small intestine and the pancreas by an unknown mechanism. Aims—To investigate the mechanism of PHA induced intestinal and pancreatic growth. Methods—Thirty day old male rats were pairfed for 10 days with lactalbumin as a control diet or lactalbumin plus PHA or purified soybean trypsin inhibitor (STI) as a positive control (42 mg/rat/day) with or without 20 µg of the cholecystokinin A (CCK-A) antagonist MK 329. To investigate further the effect of PHA on CCK release intestinal mucosal cells were isolated from rats which were continuously perfused in a perfusion apparatus. CCK release into the medium was assayed. Results—PHA and STI significantly stimulated growth of the pancreas and the small intestine. MK 329 blocked this growth effect in the pancreas but not in the small intestine. In vivo, PHA significantly increased CCK plasma levels from 0.75 to 6.67 (SEM 2.23) compared with 2.3 (0.35) pM in the control group. In addition, in vitro PHA dose dependently stimulated CCK release with a maximal effect at 100 ng/ml. Conclusion—In vivo and in vitro PHA is a potent stimulus for CCK release in the rat, thereby inducing pancreatic growth, whereas intestinal growth is stimulated by a CCK independent mechanism.
Keywords: phytohaemagglutinin; cholecystokinin; cholecystokinin A receptor antagonist; pancreatic growth; intestinal growth
Full Text
The Full Text of this article is available as a PDF (137.4 KB).
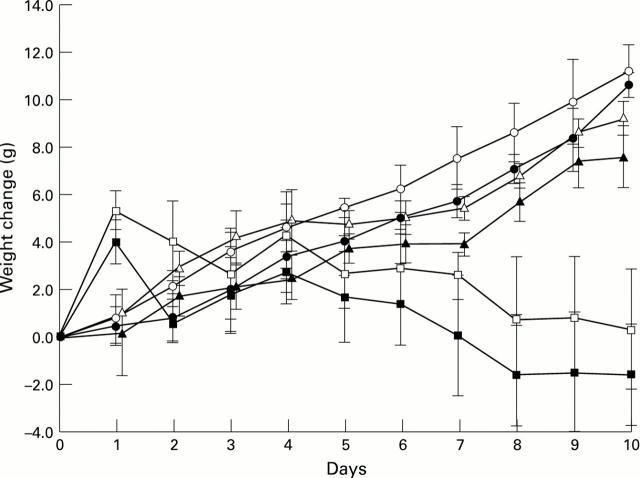
Figure 1 .
: Growth of rats fed control (lactalbumin) diet (open circles), control diet + MK 329 (closed circles) or diets containing kidney bean lectin (PHA) (open squares), PHA + MK 329 (closed squares), STI (open triangles), or STI + MK 329 (closed triangles) for 10 days (initial weight 82 (1) g; daily food intake 6 g/rat).
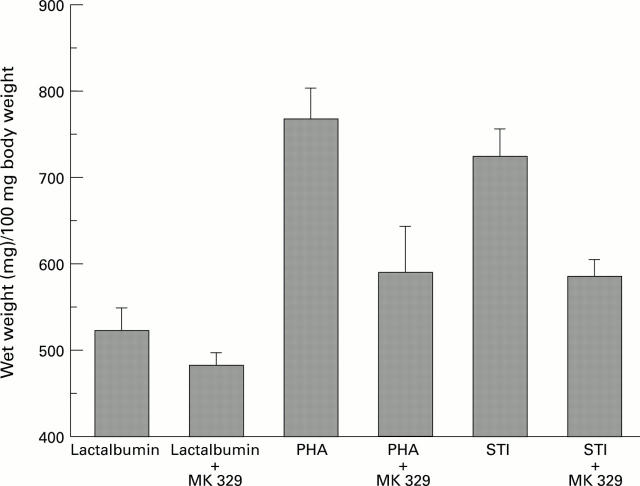
Figure 2 .
: Effects of lactalbumin, PHA, and STI with or without MK 329 on growth of the small bowel (n=5; p<0.05).
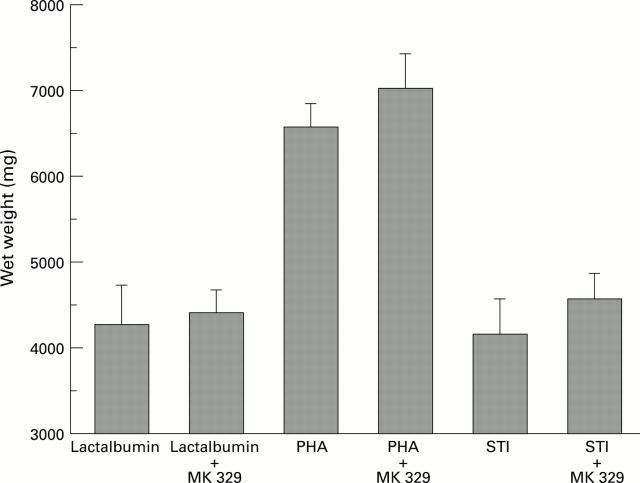
Figure 3 .
: Effects of lactalbumin, PHA, and STI with or without MK 329 on growth of the pancreas (n=5; p<0.05).
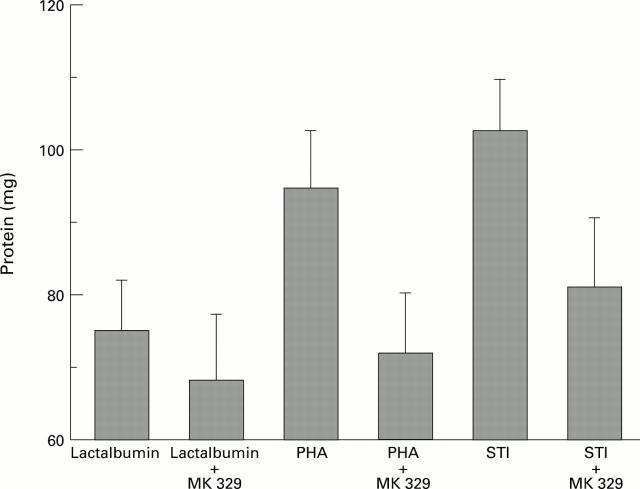
Figure 4 .
: Effects of lactalbumin, PHA, and STI with or without MK 329 on protein content of the pancreas (n=5; p<0.05).
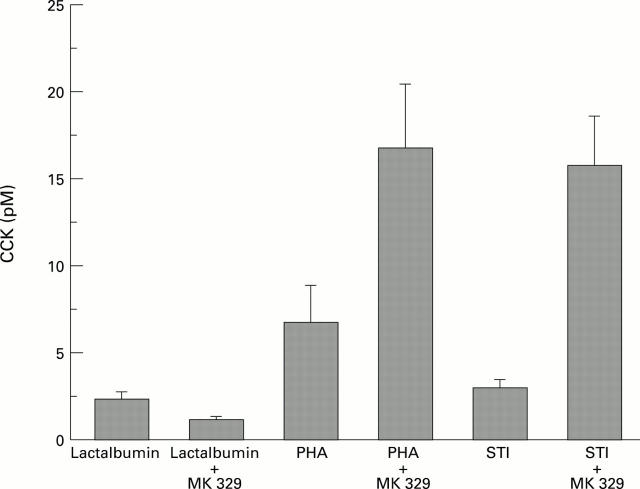
Figure 5 .
: Effects of lactalbumin, PHA, and STI with or without MK 329 on CCK plasma levels (n=5; p<0.05).
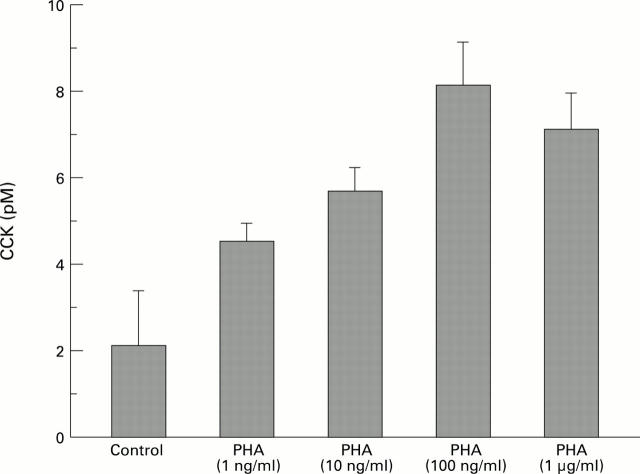
Figure 6 .
: Dose dependent effect of PHA on CCK release from rat isolated intestinal mucosal cells (n=6-12; p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelson J., Håkanson R., Ihse I., Lilja I., Rehfeld J. F., Sundler F. Effects of endogenous and exogenous cholecystokinin and of infusion with the cholecystokinin antagonist L-364,718 on pancreatic and gastrointestinal growth. Scand J Gastroenterol. 1990 May;25(5):471–480. doi: 10.3109/00365529009095518. [DOI] [PubMed] [Google Scholar]
- Balas D., Senegas-Balas F., Pradayrol L., Vayssette J., Bertrand C., Ribet A. Long-term comparative effect of cholecystokinin and gastrin on mouse stomach, antrum, intestine, and exocrine pancreas. Am J Anat. 1985 Sep;174(1):27–43. doi: 10.1002/aja.1001740104. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Howard R., Kabir I., Adrian T. E., Diamond R. H., Abramowsky C. Small intestinal growth caused by feeding red kidney bean phytohemagglutinin lectin to rats. Gastroenterology. 1993 Jun;104(6):1669–1677. doi: 10.1016/0016-5085(93)90644-r. [DOI] [PubMed] [Google Scholar]
- Banwell J. G., Howard R., Kabir I., Costerton J. W. Bacterial overgrowth by indigenous microflora in the phytohemagglutinin-fed rat. Can J Microbiol. 1988 Aug;34(8):1009–1013. doi: 10.1139/m88-177. [DOI] [PubMed] [Google Scholar]
- Bardocz S., Grant G., Ewen S. W., Duguid T. J., Brown D. S., Englyst K., Pusztai A. Reversible effect of phytohaemagglutinin on the growth and metabolism of rat gastrointestinal tract. Gut. 1995 Sep;37(3):353–360. doi: 10.1136/gut.37.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardocz S., Grant G., Pusztai A. The effect of phytohaemagglutinin at different dietary concentrations on the growth, body composition and plasma insulin of the rat. Br J Nutr. 1996 Oct;76(4):613–626. doi: 10.1079/bjn19960067. [DOI] [PubMed] [Google Scholar]
- Bouras E. P., Misukonis M. A., Liddle R. A. Role of calcium in monitor peptide-stimulated cholecystokinin release from perifused intestinal cells. Am J Physiol. 1992 May;262(5 Pt 1):G791–G796. doi: 10.1152/ajpgi.1992.262.5.G791. [DOI] [PubMed] [Google Scholar]
- Felsted R. L., Egorin M. J., Leavitt R. D., Bachur N. R. Recombinations of subunits of Phaseolus vulgaris isolectins. J Biol Chem. 1977 May 10;252(9):2967–2971. [PubMed] [Google Scholar]
- Green G. M., Levan V. H., Liddle R. A. Interaction of dietary protein and trypsin inhibitor on plasma cholecystokinin and pancreatic growth in rats. Adv Exp Med Biol. 1986;199:123–132. doi: 10.1007/978-1-4757-0022-0_7. [DOI] [PubMed] [Google Scholar]
- Herzig K. H., Brunke G., Schön I., Schäffer M., Fölsch U. R. Mechanism of galanin's inhibitory action on pancreatic enzyme secretion: modulation of cholinergic transmission--studies in vivo and in vitro. Gut. 1993 Nov;34(11):1616–1621. doi: 10.1136/gut.34.11.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Bates T., Dowling R. H. Cholecystokinin and secretin prevent the intestinal mucosal hypoplasia of total parenteral nutrition in the dog. Gastroenterology. 1978 Jul;75(1):34–41. [PubMed] [Google Scholar]
- Johnson L. R., Guthrie P. Effect of cholecystokinin and 16,16-dimethyl prostaglandin E2 on RNA and DNA of gastric and duodenal mucosa. Gastroenterology. 1976 Jan;70(1):59–65. [PubMed] [Google Scholar]
- King T. P., Pusztai A., Clarke E. M. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in rat small intestine. 3. Ultrastructural studies. J Comp Pathol. 1982 Jul;92(3):357–373. doi: 10.1016/0021-9975(82)90021-4. [DOI] [PubMed] [Google Scholar]
- King T. P., Pusztai A., Clarke E. M. Kidney bean (Phaseolus vulgaris) lectin-induced lesions in the small intestine: 1. Light microscope studies. J Comp Pathol. 1980 Oct;90(4):585–595. doi: 10.1016/0021-9975(80)90107-3. [DOI] [PubMed] [Google Scholar]
- King T. P., Pusztai A., Grant G., Slater D. Immunogold localization of ingested kidney bean (Phaseolus vulgaris) lectins in epithelial cells of the rat small intestine. Histochem J. 1986 Aug;18(8):413–420. doi: 10.1007/BF01675333. [DOI] [PubMed] [Google Scholar]
- Kruse-Jarres J. D., Kaiser C., Hafkenscheid J. C., Hohenwallner W., Stein W., Bohner J., Klein G., Poppe W., Rauscher E. Evaluation of a new alpha-amylase assay using 4.6-ethylidene-(G7)-1-4-nitrophenyl-(G1)-alpha-D-maltoheptaoside as substrate. J Clin Chem Clin Biochem. 1989 Feb;27(2):103–113. [PubMed] [Google Scholar]
- Liddle R. A., Gertz B. J., Kanayama S., Beccaria L., Coker L. D., Turnbull T. A., Morita E. T. Effects of a novel cholecystokinin (CCK) receptor antagonist, MK-329, on gallbladder contraction and gastric emptying in humans. Implications for the physiology of CCK. J Clin Invest. 1989 Oct;84(4):1220–1225. doi: 10.1172/JCI114288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Williams J. A. Bioassay of plasma cholecystokinin in rats: effects of food, trypsin inhibitor, and alcohol. Gastroenterology. 1984 Sep;87(3):542–549. [PubMed] [Google Scholar]
- Logsdon C. D. Stimulation of pancreatic acinar cell growth by CCK, epidermal growth factor, and insulin in vitro. Am J Physiol. 1986 Oct;251(4 Pt 1):G487–G494. doi: 10.1152/ajpgi.1986.251.4.G487. [DOI] [PubMed] [Google Scholar]
- Löser C., Fölsch U. R., Sahelijo-Krohn P., Creutzfeldt W. Ornithine decarboxylase and polyamines in cholecystokinin-induced pancreatic growth in rats: effects of alpha-difluoromethylornithine and the CCK receptor antagonist L-364,718. Eur J Clin Invest. 1989 Oct;19(5):448–458. doi: 10.1111/j.1365-2362.1989.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Mainz D. L., Black O., Webster P. D. Hormonal control of pancreatic growth. J Clin Invest. 1973 Sep;52(9):2300–2304. doi: 10.1172/JCI107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack S. A., Johnson L. R. Role of polyamines in gastrointestinal mucosal growth. Am J Physiol. 1991 Jun;260(6 Pt 1):G795–G806. doi: 10.1152/ajpgi.1991.260.6.G795. [DOI] [PubMed] [Google Scholar]
- Morisset J., Benrezzak O. Polyamines and pancreatic growth induced by caerulein. Life Sci. 1984 Dec 10;35(24):2471–2480. doi: 10.1016/0024-3205(84)90456-9. [DOI] [PubMed] [Google Scholar]
- Nachbar M. S., Oppenheim J. D. Lectins in the United States diet: a survey of lectins in commonly consumed foods and a review of the literature. Am J Clin Nutr. 1980 Nov;33(11):2338–2345. doi: 10.1093/ajcn/33.11.2338. [DOI] [PubMed] [Google Scholar]
- Niederau C., Heintges T., Rovati L., Strohmeyer G. Effects of loxiglumide on gallbladder emptying in healthy volunteers. Gastroenterology. 1989 Nov;97(5):1331–1336. doi: 10.1016/0016-5085(89)91709-5. [DOI] [PubMed] [Google Scholar]
- Noah N. D., Bender A. E., Reaidi G. B., Gilbert R. J. Food poisoning from raw red kidney beans. Br Med J. 1980 Jul 19;281(6234):236–237. [PMC free article] [PubMed] [Google Scholar]
- Pusztai A., Watt W. B. Isolectins of Phaseolus vulgaris. A comprehensive study of fractionation. Biochim Biophys Acta. 1974 Sep 13;365(1):57–71. doi: 10.1016/0005-2795(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Rattray E. A., Palmer R., Pusztai A. Toxicity of kidney beans (Phaseolus vulgaris L). to conventional and gnotobiotic rats. J Sci Food Agric. 1974 Aug;25(8):1035–1040. doi: 10.1002/jsfa.2740250819. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Parker N., Ecclestone D., Haqqani M. T., Rhodes J. M. Peanut lectin stimulates proliferation in colonic explants from patients with inflammatory bowel disease and colon polyps. Gastroenterology. 1994 Jan;106(1):117–124. doi: 10.1016/s0016-5085(94)94775-9. [DOI] [PubMed] [Google Scholar]
- Ryder S. D., Smith J. A., Rhodes E. G., Parker N., Rhodes J. M. Proliferative responses of HT29 and Caco2 human colorectal cancer cells to a panel of lectins. Gastroenterology. 1994 Jan;106(1):85–93. doi: 10.1016/s0016-5085(94)94527-6. [DOI] [PubMed] [Google Scholar]
- Santer R., Leung Y. K., Alliet P., Lebenthal E., Lee P. C. The role of carbohydrate moieties of cholecystokinin receptors in cholecystokinin octapeptide binding: alteration of binding data by specific lectins. Biochim Biophys Acta. 1990 Jan 23;1051(1):78–83. doi: 10.1016/0167-4889(90)90176-e. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Schafmayer A., Nustede R., Pompino A., Köhler H. Vagal influence on cholecystokinin and neurotensin release in conscious dogs. Scand J Gastroenterol. 1988 Apr;23(3):315–320. doi: 10.3109/00365528809093872. [DOI] [PubMed] [Google Scholar]
- Schafmayer A., Werner M., Becker H. D. Radioimmunological determination of cholecystokinin in tissue extracts. Digestion. 1982;24(3):146–154. doi: 10.1159/000198790. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Choudhury A. R., Siegel E. G., Löser C., Conlon J. M., Fölsch U. R., Creutzfeldt W. CCK-antagonist L-364,718: influence on rat pancreatic growth induced by caerulein and bombesin-like peptides. Regul Pept. 1989 Jan;24(1):67–79. doi: 10.1016/0167-0115(89)90212-7. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Creutzfeldt W., Höcker M., Nustede R., Choudhury A. R., Schleser A., Rovati L. C., Fölsch U. R. Cholecystokinin receptor antagonist loxiglumide modulates plasma levels of gastro-entero-pancreatic hormones in man. Feedback control of cholecystokinin and gastrin secretion. Eur J Clin Invest. 1991 Oct;21(5):501–511. doi: 10.1111/j.1365-2362.1991.tb01402.x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. E., Creutzfeldt W., Schleser A., Choudhury A. R., Nustede R., Höcker M., Nitsche R., Sostmann H., Rovati L. C., Fölsch U. R. Role of CCK in regulation of pancreaticobiliary functions and GI motility in humans: effects of loxiglumide. Am J Physiol. 1991 Feb;260(2 Pt 1):G197–G206. doi: 10.1152/ajpgi.1991.260.2.G197. [DOI] [PubMed] [Google Scholar]
- Seiler N. Polyamine metabolism. Digestion. 1990;46 (Suppl 2):319–330. doi: 10.1159/000200405. [DOI] [PubMed] [Google Scholar]
- Shulman R. J. Oral insulin increases small intestinal mass and disaccharidase activity in the newborn miniature pig. Pediatr Res. 1990 Aug;28(2):171–175. doi: 10.1203/00006450-199008000-00018. [DOI] [PubMed] [Google Scholar]
- Weinman M. D., Allan C. H., Trier J. S., Hagen S. J. Repair of microvilli in the rat small intestine after damage with lectins contained in the red kidney bean. Gastroenterology. 1989 Nov;97(5):1193–1204. doi: 10.1016/0016-5085(89)91690-9. [DOI] [PubMed] [Google Scholar]
- Weser E., Bell D., Tawil T. Effects of octapeptide-cholecystokinin, secretin, and glucagon on intestinal mucosal growth in parenterally nourished rats. Dig Dis Sci. 1981 May;26(5):409–416. doi: 10.1007/BF01313582. [DOI] [PubMed] [Google Scholar]
- Ziegler T. R., Almahfouz A., Pedrini M. T., Smith R. J. A comparison of rat small intestinal insulin and insulin-like growth factor I receptors during fasting and refeeding. Endocrinology. 1995 Nov;136(11):5148–5154. doi: 10.1210/endo.136.11.7588253. [DOI] [PubMed] [Google Scholar]