Abstract
Background—Atropine decreases the frequency of transient lower oesophageal sphincter relaxation (TLOSR) through an unknown mechanism. Gastric distension and pharyngeal receptor excitation are two possible sources for the afferent stimulus responsible for TLOSR. Aims—To determine whether atropine affects gastric distension induced TLOSR and pharyngeal receptor mediated lower oesophageal sphincter (LOS) relaxation. Methods—Oesophageal manometry and pH recordings were performed in 10 healthy volunteers on two separate days in the postprandial setting, following either atropine (15 µg/kg intravenous bolus and 4 µg/kg/h as a maintenance dose) or placebo. Pharyngeal receptor mediated LOS relaxation was studied in nine subjects by rapid injection of minute amounts of water (0.05, 0.1, 0.2, 0.3, and 0.4 ml) in the pharynx before and after atropine. Gastric distension mediated TLOSR was studied in eight subjects by insufflating the stomach with 300, 600 and 900 ml of CO2 before and after atropine. Results—Atropine reduced the frequency of spontaneous gastro-oesophageal reflux and TLOSR compared with placebo (p<0.05). Pharyngeal stimulation resulted in bolus volume dependent LOS relaxation. Atropine decreased the frequency and amplitude of pharyngeal receptor mediated LOS relaxation at bolus volumes of 0.05, 0.1, and 0.2 ml. Gastric distension resulted in intermittent episodes of TLOSR. The frequency of gastric distension induced TLOSR was significantly decreased by atropine. Conclusion—(1) Atropine reduces the frequency of spontaneous reflux and TLOSR in normal subjects; and (2) gastric distension induced TLOSR and pharyngeal receptor mediated LOS relaxation is inhibited by atropine.
Keywords: lower oesophageal sphincter relaxation; anticholinergic; pharynx; gastric distension
Full Text
The Full Text of this article is available as a PDF (145.7 KB).
Figure 1 .
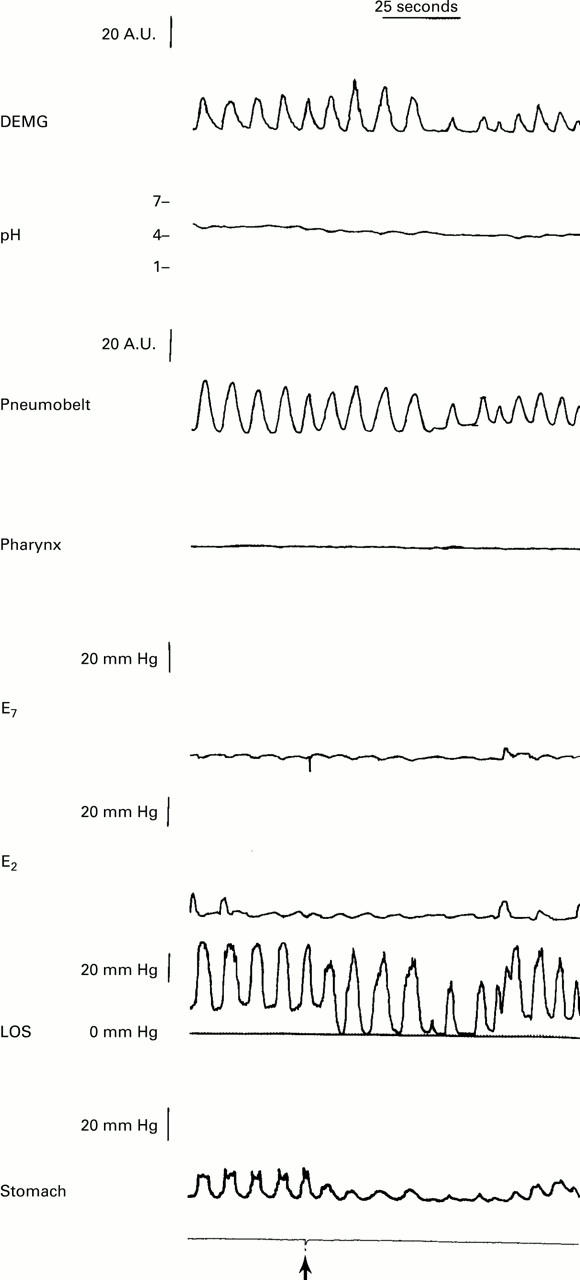
: Effects of pharyngeal stimulus on LOS relaxation. A manometric record of the effect of injection of water in the pharynx (marked by the arrow) on LOS pressure. Note that soon after the injection of water the LOS relaxes for 30 seconds. There is no relaxation of the crural diaphragm as shown by continued inspiratory pressure oscillations in the LOS pressure tracing and crural diaphragmatic electromyographic activity.
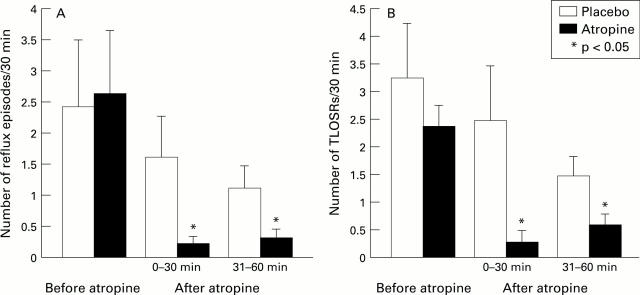
Figure 2 .
: Effects of atropine on the frequency of postprandial GOR and TLOSR. Mean (SEM) data on the frequency of TLOSR and GOR in 10 subjects. Data were collected in the postprandial periods.
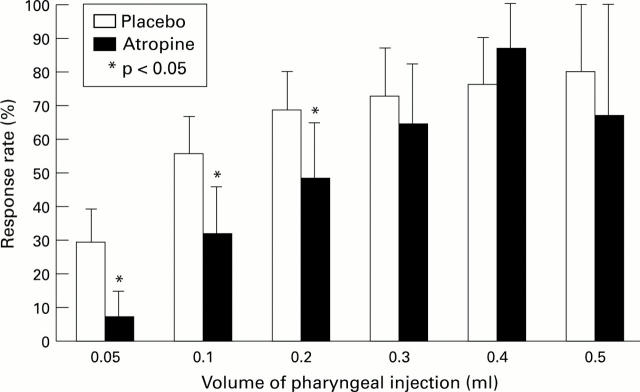
Figure 3 .
: Effects of atropine on the incidence of pharyngeal stimulus induced LOS relaxation. Mean (SEM) data in nine normal subjects. The LOS response to pharyngeal stimulus was defined as a decrease in LOS pressure of more than 4 mm Hg.
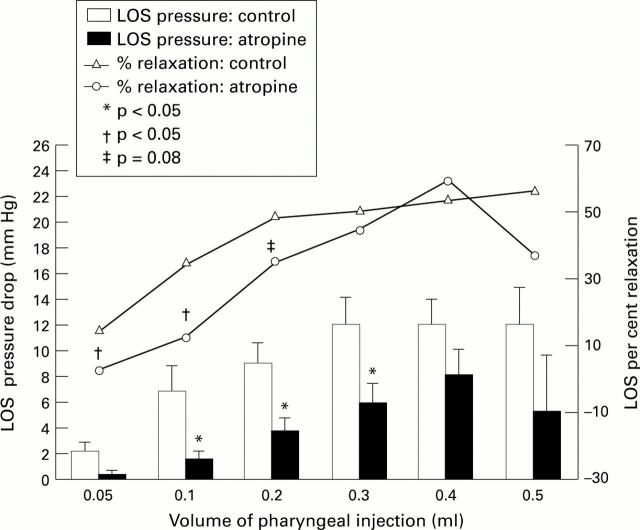
Figure 4 .
: Effects of atropine on the amplitude of LOS pressure drop and percentage LOS relaxation induced by pharyngeal stimulus. The data summarise the absolute LOS pressure fall and percentage LOS relaxation by pharyngeal stimulus before and after injection of atropine in nine subjects (control and atropine, respectively).
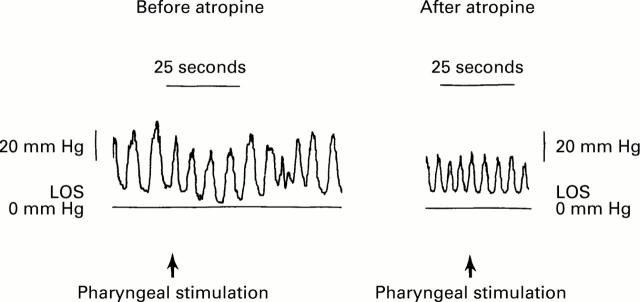
Figure 5 .
: Effects of atropine on pharyngeal stimulus induced LOS relaxation: LOS pressure tracing before and after injection of atropine in the same individual. The same bolus volume (0.3 ml) was used for both the pharyngeal stimulations. Note the absence of LOS relaxation following atropine injection. Crural diaphragmatic activity (inspiratory pressure oscillations) was not affected by pharyngeal stimulus and atropine.
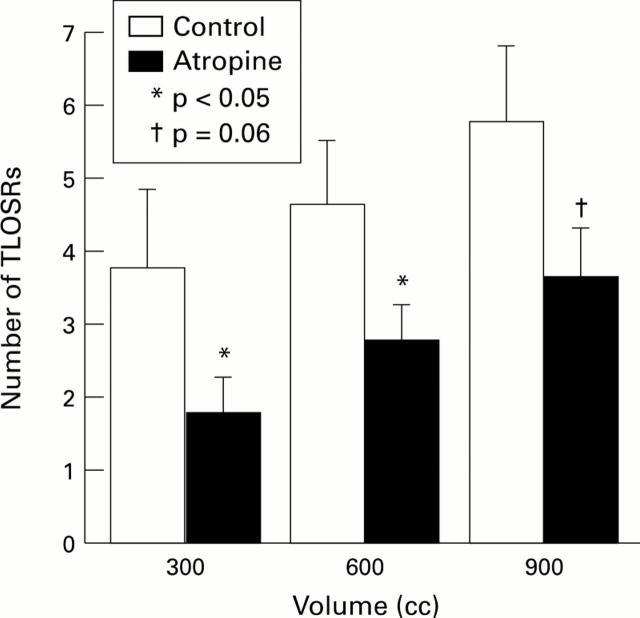
Figure 6 .
: Effects of atropine on gastric distension induced TLOSR. Data are mean (SEM) in seven normal subjects. The number of TLOSRs was measured in the 15 minute periods after the ingestion of each volume of CO2.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azpiroz F., Malagelada J. R. Importance of vagal input in maintaining gastric tone in the dog. J Physiol. 1987 Mar;384:511–524. doi: 10.1113/jphysiol.1987.sp016467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger D. Muscarinic activation of rhombencephalic neurones controlling oesophageal peristalsis in the rat. Neuropharmacology. 1984 Dec;23(12A):1451–1464. doi: 10.1016/0028-3908(84)90088-1. [DOI] [PubMed] [Google Scholar]
- Boulant J., Fioramonti J., Dapoigny M., Bommelaer G., Bueno L. Cholecystokinin and nitric oxide in transient lower esophageal sphincter relaxation to gastric distention in dogs. Gastroenterology. 1994 Oct;107(4):1059–1066. doi: 10.1016/0016-5085(94)90230-5. [DOI] [PubMed] [Google Scholar]
- Dodds W. J., Dent J., Hogan W. J., Helm J. F., Hauser R., Patel G. K., Egide M. S. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982 Dec 16;307(25):1547–1552. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- Franzi S. J., Martin C. J., Cox M. R., Dent J. Response of canine lower esophageal sphincter to gastric distension. Am J Physiol. 1990 Sep;259(3 Pt 1):G380–G385. doi: 10.1152/ajpgi.1990.259.3.G380. [DOI] [PubMed] [Google Scholar]
- Gidda J. S., Buyniski J. P. Swallow-evoked peristalsis in opossum esophagus: role of cholinergic mechanisms. Am J Physiol. 1986 Dec;251(6 Pt 1):G779–G785. doi: 10.1152/ajpgi.1986.251.6.G779. [DOI] [PubMed] [Google Scholar]
- Gidda J. S., Goyal R. K. Swallow-evoked action potentials in vagal preganglionic efferents. J Neurophysiol. 1984 Dec;52(6):1169–1180. doi: 10.1152/jn.1984.52.6.1169. [DOI] [PubMed] [Google Scholar]
- Helm J. F., Dodds W. J., Pelc L. R., Palmer D. W., Hogan W. J., Teeter B. C. Effect of esophageal emptying and saliva on clearance of acid from the esophagus. N Engl J Med. 1984 Feb 2;310(5):284–288. doi: 10.1056/NEJM198402023100503. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Patrikios J., Dent J. Abolition of gas reflux and transient lower esophageal sphincter relaxation by vagal blockade in the dog. Gastroenterology. 1986 Oct;91(4):890–896. doi: 10.1016/0016-5085(86)90691-8. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., Chiareli C., Liu J., Shaker R. Characteristics of lower esophageal sphincter relaxation induced by pharyngeal stimulation with minute amounts of water. Gastroenterology. 1996 Aug;111(2):378–384. doi: 10.1053/gast.1996.v111.pm8690202. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., Fisher M. J. Electrical and mechanical inhibition of the crural diaphragm during transient relaxation of the lower esophageal sphincter. Gastroenterology. 1990 Nov;99(5):1265–1268. doi: 10.1016/0016-5085(90)91148-y. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., Holloway R. H., Penagini R., Blackshaw L. A., Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995 Aug;109(2):601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., Holloway R., Dent J. Effect of atropine on the frequency of reflux and transient lower esophageal sphincter relaxation in normal subjects. Gastroenterology. 1995 Nov;109(5):1547–1554. doi: 10.1016/0016-5085(95)90643-6. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., McCallum R. W. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988 Sep;95(3):593–599. doi: 10.1016/s0016-5085(88)80003-9. [DOI] [PubMed] [Google Scholar]
- Paterson W. G., Rattan S., Goyal R. K. Experimental induction of isolated lower esophageal sphincter relaxation in anesthetized opossums. J Clin Invest. 1986 Apr;77(4):1187–1193. doi: 10.1172/JCI112420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivri B., Mittal R. K. Reverse-perfused sleeve: an improved device for measurement of sphincteric function of the crural diaphragm. Gastroenterology. 1991 Oct;101(4):962–969. doi: 10.1016/0016-5085(91)90722-w. [DOI] [PubMed] [Google Scholar]
- Trifan A., Ren J., Arndorfer R., Hofmann C., Bardan E., Shaker R. Inhibition of progressing primary esophageal peristalsis by pharyngeal water stimulation in humans. Gastroenterology. 1996 Feb;110(2):419–423. doi: 10.1053/gast.1996.v110.pm8566588. [DOI] [PubMed] [Google Scholar]
- Trifan A., Shaker R., Ren J., Mittal R. K., Saeian K., Dua K., Kusano M. Inhibition of resting lower esophageal sphincter pressure by pharyngeal water stimulation in humans. Gastroenterology. 1995 Feb;108(2):441–446. doi: 10.1016/0016-5085(95)90072-1. [DOI] [PubMed] [Google Scholar]