Abstract
Background—It is well established that glutamine supplemented elemental diets result in less severe intestinal damage in experimental colitis. However, few studies have examined the mode of action of glutamine in reducing intestinal damage. Aims—To examine the effects of glutamine supplemented elemental diets on the potent inflammatory cytokines interleukin 8 (IL-8) and tumour necrosis factor α (TNF-α) in trinitrobenzene sulphonic acid (TNBS) induced colitis which presents with both acute and chronic features of ulcerative colitis. Methods—Sprague-Dawley rats were randomised into three dietary groups and fed 20% casein (controls), or 20% casein supplemented with either 2% glutamine (2% Gln) or 4% glutamine (4% Gln). After two weeks they received intracolonic TNBS to induce colitis. Results—Both Gln groups of rats gained more weight than the control group (p<0.05) which had progressive weight loss. Colon weight, macroscopic, and microscopic damage scores for the Gln groups were lower than in the control group (p<0.05). IL-8 and TNF-α concentrations in inflamed colonic tissues were lower in the Gln groups than in the control group (p<0.05), and correlated well with disease severity. Bacterial translocation was lower both in incidence (p<0.05) and in the number of colony forming units (p<0.05) for the Gln groups, than in the control group. With respect to all indices studied, the 4% Gln group performed better than did the 2% Gln group. Conclusion—Prophylactic glutamine supplementation modulates the inflammatory activities of IL-8 and TNF-α in TNBS induced colitis.
Keywords: glutamine; trinitrobenzene sulphonic acid; inflammatory bowel disease; rats; interleukin 8; tumour necrosis factor α
Full Text
The Full Text of this article is available as a PDF (185.0 KB).
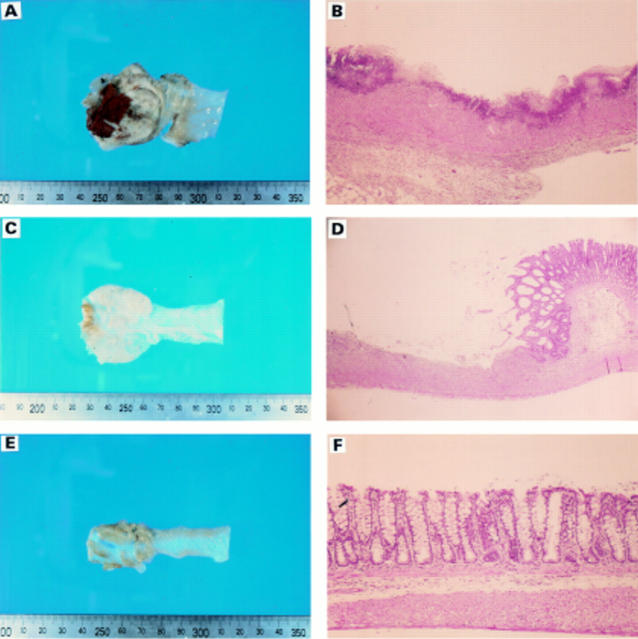
Figure 1 .
: Sample sections of rat colonic mucosa from each dietary group. Control (A,B); 2% Gln (C,D); 4% Gln (E,F). The varying degrees of necrosis of the intestinal mucosa and infiltration by inflammatory cells among the groups are evident.
Figure 2 .

: Colon macroscopic (A) and microscopic (B) scores of rats. Values represent mean (SEM). Bars with different letters indicate significant difference (p<0.05).
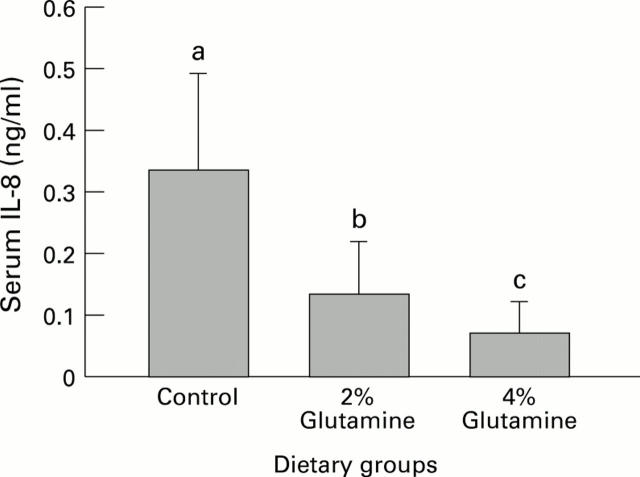
Figure 3 .
: Serum IL-8 concentrations in rats. Values represent mean (SEM). Bars with different letters indicate significant difference (p<0.05).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adjei A. A., Morioka T., Ameho C. K., Yamauchi K., Kulkarni A. D., Al-Mansouri H. M., Kawajiri A., Yamamoto S. Nucleoside-nucleotide free diet protects rat colonic mucosa from damage induced by trinitrobenzene sulphonic acid. Gut. 1996 Sep;39(3):428–433. doi: 10.1136/gut.39.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgayer H., Deschryver K., Stenson W. F. Treatment with 16,16'-dimethyl prostaglandin E2 before and after induction of colitis with trinitrobenzenesulfonic acid in rats decreases inflammation. Gastroenterology. 1989 May;96(5 Pt 1):1290–1300. doi: 10.1016/s0016-5085(89)80016-2. [DOI] [PubMed] [Google Scholar]
- Barber A. E., Jones W. G., 2nd, Minei J. P., Fahey T. J., 3rd, Moldawer L. L., Rayburn J. L., Fischer E., Keogh C. V., Shires G. T., Lowry S. F. Harry M. Vars award. Glutamine or fiber supplementation of a defined formula diet: impact on bacterial translocation, tissue composition, and response to endotoxin. JPEN J Parenter Enteral Nutr. 1990 Jul-Aug;14(4):335–343. doi: 10.1177/0148607190014004335. [DOI] [PubMed] [Google Scholar]
- Bergmann K. C., Waldman R. H. Stimulation of secretory antibody following oral administration of antigen. Rev Infect Dis. 1988 Sep-Oct;10(5):939–950. doi: 10.1093/clinids/10.5.939. [DOI] [PubMed] [Google Scholar]
- Burke D. J., Alverdy J. C., Aoys E., Moss G. S. Glutamine-supplemented total parenteral nutrition improves gut immune function. Arch Surg. 1989 Dec;124(12):1396–1399. doi: 10.1001/archsurg.1989.01410120042009. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Falk W., Leonard E. J. Human monocyte chemotaxis: migrating cells are a subpopulation with multiple chemotaxin specificities on each cell. Infect Immun. 1980 Sep;29(3):953–959. doi: 10.1128/iai.29.3.953-959.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fiocchi C. Cytokines and intestinal inflammation. Transplant Proc. 1996 Oct;28(5):2442–2443. [PubMed] [Google Scholar]
- Ford-Hutchinson A. W., Bray M. A., Doig M. V., Shipley M. E., Smith M. J. Leukotriene B, a potent chemokinetic and aggregating substance released from polymorphonuclear leukocytes. Nature. 1980 Jul 17;286(5770):264–265. doi: 10.1038/286264a0. [DOI] [PubMed] [Google Scholar]
- Fox A. D., Kripke S. A., De Paula J., Berman J. M., Settle R. G., Rombeau J. L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1988 Jul-Aug;12(4):325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Fujita T., Sakurai K. Efficacy of glutamine-enriched enteral nutrition in an experimental model of mucosal ulcerative colitis. Br J Surg. 1995 Jun;82(6):749–751. doi: 10.1002/bjs.1800820611. [DOI] [PubMed] [Google Scholar]
- Higa A., McKnight G. W., Wallace J. L. Attenuation of epithelial injury in acute experimental colitis by immunomodulators. Eur J Pharmacol. 1993 Aug 3;239(1-3):171–176. doi: 10.1016/0014-2999(93)90990-y. [DOI] [PubMed] [Google Scholar]
- Higashiguchi T., Hasselgren P. O., Wagner K., Fischer J. E. Effect of glutamine on protein synthesis in isolated intestinal epithelial cells. JPEN J Parenter Enteral Nutr. 1993 Jul-Aug;17(4):307–314. doi: 10.1177/0148607193017004307. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Dolson D. J., Salloum R. M., Hautamaki R. D., Plumley D. A., Mendenhall W. M., Bova F. J., Khan S. R., Hackett R. L. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62–68. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Levi A. J. Diet in the management of Crohn's disease. Gut. 1985 Oct;26(10):985–988. doi: 10.1136/gut.26.10.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Ceska M., Effenberger F., Kurlak L., Lindley I., Hawkey C. J. Enhanced synthesis of neutrophil-activating peptide-1/interleukin-8 in active ulcerative colitis. Clin Sci (Lond) 1992 Mar;82(3):273–275. doi: 10.1042/cs0820273. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama K., Toyonaga A., Sasaki E., Watanabe K., Tateishi H., Nishiyama T., Saiki T., Ikeda H., Tsuruta O., Tanikawa K. IL-8 as an important chemoattractant for neutrophils in ulcerative colitis and Crohn's disease. Clin Exp Immunol. 1994 Jun;96(3):432–436. doi: 10.1111/j.1365-2249.1994.tb06047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- O'Morain C. Diet and Crohn's disease. Mol Aspects Med. 1987;9(1):113–118. doi: 10.1016/0098-2997(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Ohkusa T., Yamada M., Takenaga T., Kitazume C., Yamamoto N., Sasabe M., Takashimizu I., Tamura Y., Sakamoto E., Kurosawa H. [Protective effect of metronidazole in experimental ulcerative colitis induced by dextran sulfate sodium]. Nihon Shokakibyo Gakkai Zasshi. 1987 Oct;84(10):2337–2346. [PubMed] [Google Scholar]
- Onderdonk A. B., Bartlett J. G. Bacteriological studies of experimental ulcerative colitis. Am J Clin Nutr. 1979 Jan;32(1):258–265. doi: 10.1093/ajcn/32.1.258. [DOI] [PubMed] [Google Scholar]
- Onderdonk A. B., Hermos J. A., Dzink J. L., Bartlett J. G. Protective effect of metronidazole in experimental ulcerative colitis. Gastroenterology. 1978 Mar;74(3):521–526. [PubMed] [Google Scholar]
- Pons L., Droy-Lefaix M. T., Bueno L. Leukotriene D4 participates in colonic transit disturbances induced by intracolonic administration of trinitrobenzene sulfonic acid in rats. Gastroenterology. 1992 Jan;102(1):149–156. doi: 10.1016/0016-5085(92)91794-5. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Simon P. L., Schwartz L. W., Griswold D. E., Fondacaro J. D., Wasserman M. A. Inflammatory mediators of experimental colitis in rats. Gastroenterology. 1989 Aug;97(2):326–337. doi: 10.1016/0016-5085(89)90068-1. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Rolandelli R. H., Saul S. H., Settle R. G., Jacobs D. O., Trerotola S. O., Rombeau J. L. Comparison of parenteral nutrition and enteral feeding with pectin in experimental colitis in the rat. Am J Clin Nutr. 1988 Apr;47(4):715–721. doi: 10.1093/ajcn/47.4.715. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Seidman E. G., Roy C. C., Weber A. M., Morin C. L. Nutritional therapy of Crohn's disease in childhood. Dig Dis Sci. 1987 Dec;32(12 Suppl):82S–88S. doi: 10.1007/BF01312470. [DOI] [PubMed] [Google Scholar]
- Seidman E., LeLeiko N., Ament M., Berman W., Caplan D., Evans J., Kocoshis S., Lake A., Motil K., Sutphen J. Nutritional issues in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1991 May;12(4):424–438. doi: 10.1097/00005176-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Klimberg V. S., Plumley D. A., Salloum R. M., Flynn T. C., Bland K. I., Copeland E. M., 3rd The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res. 1990 Apr;48(4):383–391. doi: 10.1016/0022-4804(90)90080-l. [DOI] [PubMed] [Google Scholar]
- Souba W. W., Smith R. J., Wilmore D. W. Glutamine metabolism by the intestinal tract. JPEN J Parenter Enteral Nutr. 1985 Sep-Oct;9(5):608–617. doi: 10.1177/0148607185009005608. [DOI] [PubMed] [Google Scholar]
- Suzuki I., Matsumoto Y., Adjei A. A., Asato L., Shinjo S., Yamamoto S. Effect of a glutamine-supplemented diet on response to methicillin-resistant Staphylococcus aureus infection in mice. J Nutr Sci Vitaminol (Tokyo) 1993 Aug;39(4):405–410. doi: 10.3177/jnsv.39.405. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Thomsen M. K., Larsen C. G., Thomsen H. K., Kirstein D., Skak-Nielsen T., Ahnfelt-Rønne I., Thestrup-Pedersen K. Recombinant human interleukin-8 is a potent activator of canine neutrophil aggregation, migration, and leukotriene B4 biosynthesis. J Invest Dermatol. 1991 Feb;96(2):260–266. doi: 10.1111/1523-1747.ep12464458. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Gale D., Shoupe T. S. Exacerbation of experimental colitis by nonsteroidal anti-inflammatory drugs is not related to elevated leukotriene B4 synthesis. Gastroenterology. 1992 Jan;102(1):18–27. doi: 10.1016/0016-5085(92)91779-4. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., MacNaughton W. K., Morris G. P., Beck P. L. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989 Jan;96(1):29–36. doi: 10.1016/0016-5085(89)90760-9. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]