Abstract
Background—Cancer of the oesophagus has so far eluded every attempt at pharmacological treatment. The recent advent of somatic gene therapy offers a new therapeutic approach to malignant tumours. Aim—To investigate whether and how gene transfer into the oesophagus can be achieved. Methods—A LacZ reporter gene was used as marker and transferred into the oesophagus of rats using cationic liposomes. Gene transfer was achieved by either luminal instillation into a closed segment using a double balloon catheter, or by intramural injection through a needle. Expression of β-galactosidase was monitored in the oesophagus and various control tissues by histochemistry, polymerase chain reaction (PCR), reverse transcriptase PCR, and Southern blotting. Results—Up to 100 days after in vivo gene transfer β-galactosidase activity could be demonstrated in the oesophagus. Following luminal application, the transgene was expressed in epithelial cells whereas intramural injection induced preferential expression in fibroblasts. Conclusion—In vivo gene transfer into the oesophagus is feasible and safe, and the route of administration largely determines cell type specificity. This novel approach will enable in vivo studies of growth, differentiation, and malignant transformation in the oesophagus, and may open new avenues to the confinement of oesophageal malignancies.
Keywords: oesophagus; gene transfer; gene therapy; liposomes; balloon catheter
Full Text
The Full Text of this article is available as a PDF (239.9 KB).
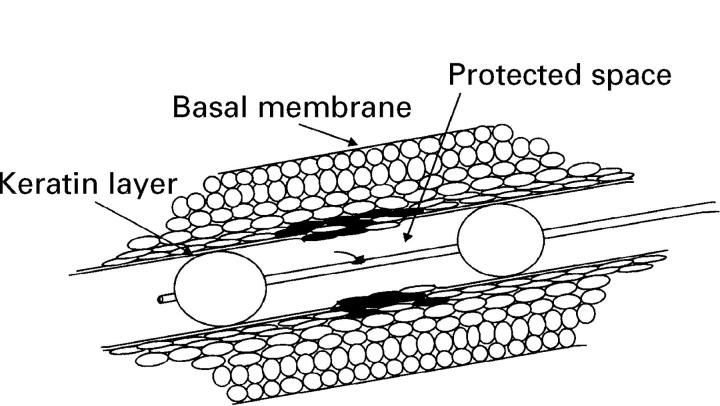
Figure 1 .
: Method of introducing DNA-liposome complexes with a double balloon catheter into the oesophageal epithelium.
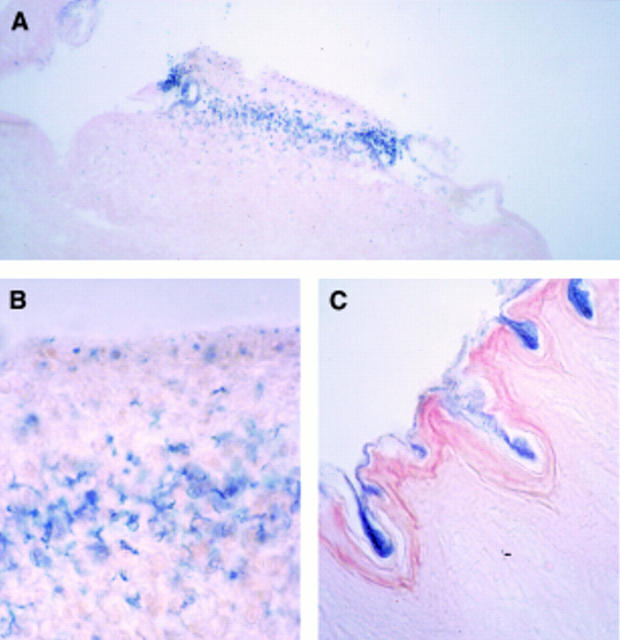
Figure 2 .
: Microscopic view of sections from transduced oesophageal epithelium using the double balloon catheter system. (A) Representative area positively stained for β-galactosidase activity (original magnification ×50); (B) an oesophageal section three days after luminal gene transfer, indicating transfected epithelial layers (original magnification ×250); (C) an oesophageal section with an intact keratin layer which acts as a barrier for gene transfer (original magnification ×100). Sections were counterstained with eosin.
Figure 3 .
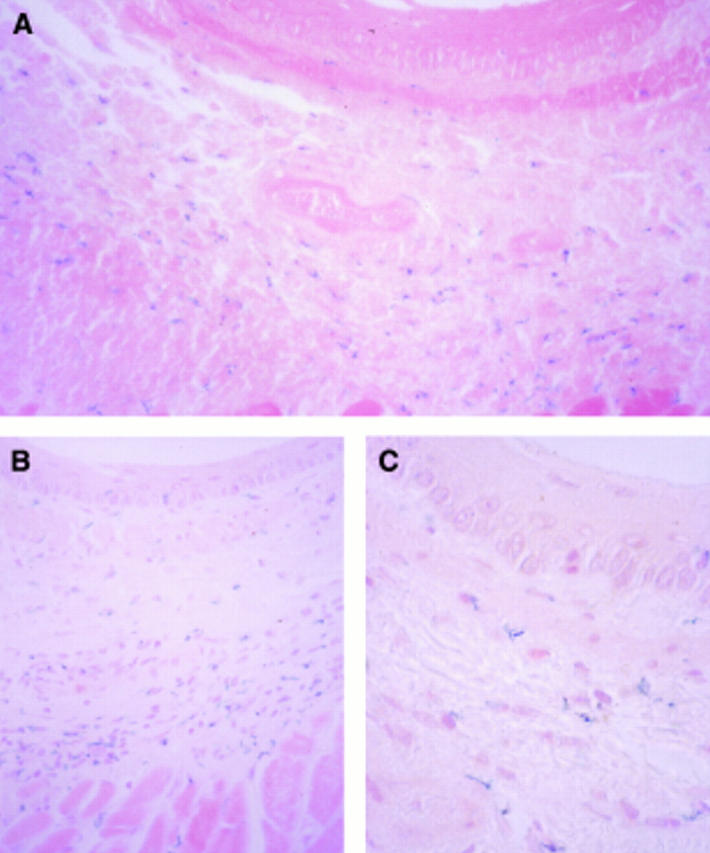
: Microscopic view of sections from oesophagus transduced by injection. β-Galactosidase activity was detectable in the submucosa (A and B) and in the connective tissue of the muscular layer (B). Occasionally epithelial cells were positive (C). Original magnification ×100 (A), ×100 (B), ×250 (C). All sections were counterstained with eosin (A) or eosin and haematoxylin (B and C).
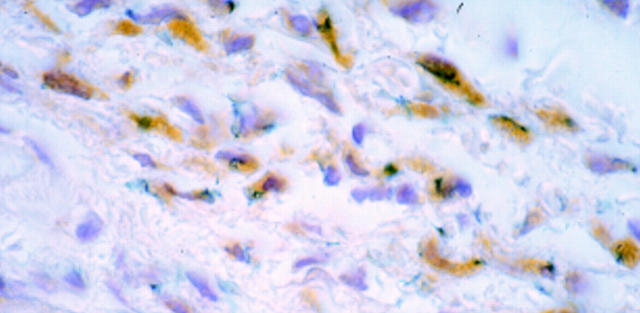
Figure 4 .
: Microscopic view of sections from oesophagus transduced by injection. Sections were incubated with a monoclonal anti-β-galactosidase antibody followed by a secondary biotin conjugated rabbit antimouse antibody and streptavidin-peroxidase complexes with DAB. β-Galactosidase activity is presented as a blue precipitate, whereas detection by the anti-β-galactosidase antibody is displayed as brown precipitate throughout the cytoplasm. The section was counterstained with haematoxylin. Original magnification ×250.
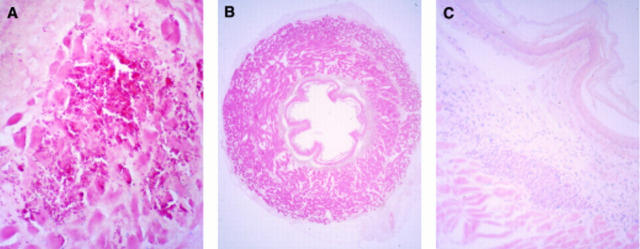
Figure 5 .
: Microscopic view of a section from the oesophagus injected with 200 µl DNA-liposomes. Three days following gene transfer, signs of inflammation, necrosis, and bleeding were visible at the area where the injection took place. Sections were fixed in 4% paraformaldehyde and stained with eosin (A) (original magnification ×50). Twenty eight days after gene transfer no signs of trauma, necrosis, or major inflammation were visible (B) (original magnification ×6.25). In these areas a local increase in small lymphoid aggregates remained in the submucosa (C) (original magnification ×50). Sections were counterstained with eosin (A) or with eosin in combination with haematoxylin (B and C).
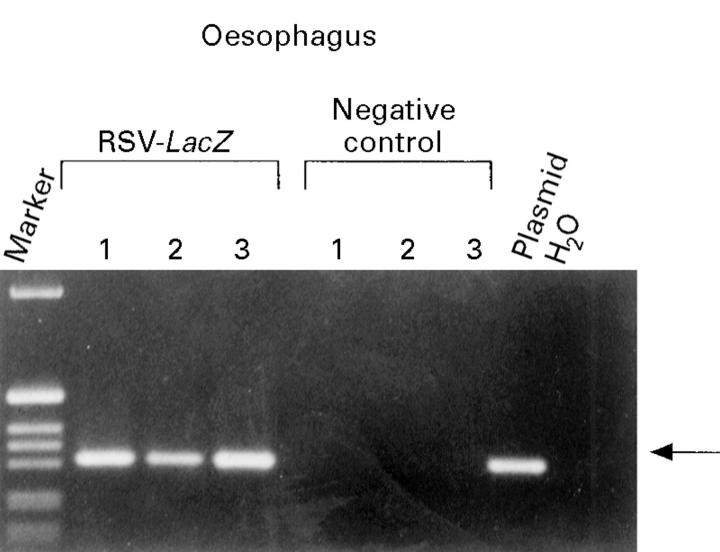
Figure 6 .
: Detection of plasmid DNA in oesophageal tissue three days after direct gene transfer with DNA- liposome complexes by PCR. A specific band of 318 bp was detected after transduction with RSV-LacZ using direct injection (RSV-LacZ lanes 1-3), but not in controls, where Bluescript SK with liposomes was injected (control lanes 1-3). The 1 kB ladder (Gibco) was used as a marker.
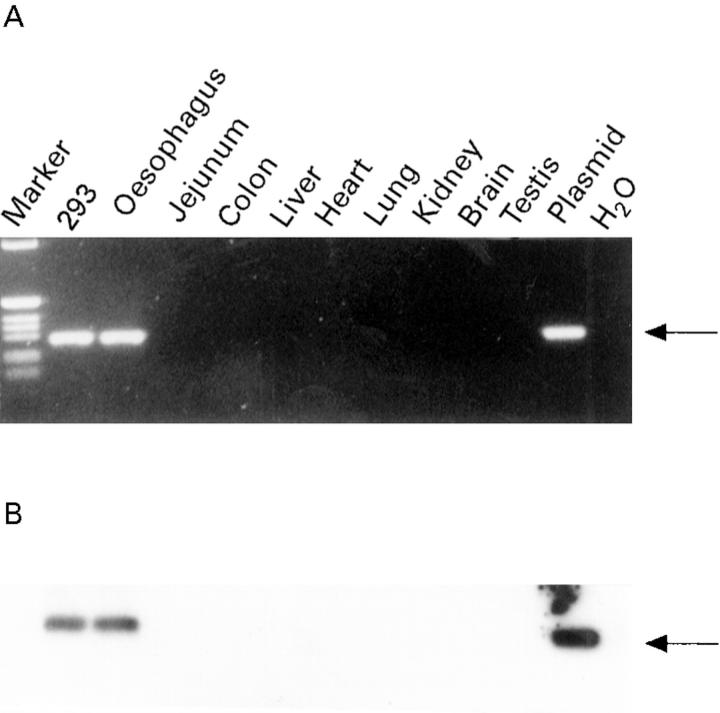
Figure 7 .
: Detection of plasmid DNA in tissue samples after gene transfer. Three days after injection of oesophageal segments with RSV-LacZ DNA-liposomes genomic DNA was isolated. Genomic DNA (1 µg) was subjected to PCR analysis; 1 ng RSV-LacZ or 293 cells transfected with 5 µg RSV-LacZ were used as positive controls. One tenth of the PCR reaction was electrophoresed on a 1.6% agarose gel, stained with ethidium bromide, and photographed. Recombinant RSV-LacZ DNA was not detected as a specific 318 bp band in all tissues except in the oesophagus (A). Positive and negative controls are shown along with DNA markers. To enhance the signal Southern blot analysis was performed (B). The gel was blotted onto nitrocellulose and hybridised to a LacZ specific probe. No signals were detected in the negative control or in any tissue except the oesophagus.
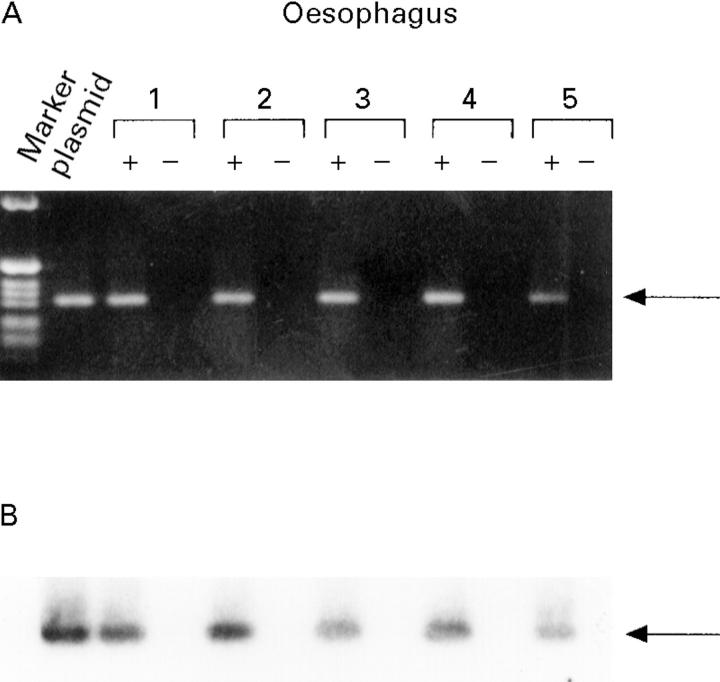
Figure 8 .
: Expression of β-galactosidase mRNA in the oesophagus in vivo. A specific band of 318 bp was detected in RNA from oesophageal segments when reverse transcriptase was used (+) (A). The 1 kB ladder (Gibco) was used as a marker and 1 ng RSV-LacZ as a positive control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Jiao S. S., Jani A., Duke D., Williams P., Chong W., Wolff J. A. Direct gene transfer and expression into rat heart in vivo. New Biol. 1991 Jan;3(1):71–81. [PubMed] [Google Scholar]
- Alton E. W., Middleton P. G., Caplen N. J., Smith S. N., Steel D. M., Munkonge F. M., Jeffery P. K., Geddes D. M., Hart S. L., Williamson R. Non-invasive liposome-mediated gene delivery can correct the ion transport defect in cystic fibrosis mutant mice. Nat Genet. 1993 Oct;5(2):135–142. doi: 10.1038/ng1093-135. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Meyrick B., Christman B., Magnuson M., King G., Berry L. C., Jr In vivo transfection of murine lungs with a functioning prokaryotic gene using a liposome vehicle. Am J Med Sci. 1989 Oct;298(4):278–281. doi: 10.1097/00000441-198910000-00013. [DOI] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Chang A. G., Wu G. Y. Gene therapy: applications to the treatment of gastrointestinal and liver diseases. Gastroenterology. 1994 Apr;106(4):1076–1084. doi: 10.1016/0016-5085(94)90771-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner P. L., Holm M., Chan H. Cationic liposome mediated transfection. Proc West Pharmacol Soc. 1989;32:115–121. [PubMed] [Google Scholar]
- Felgner P. L., Rhodes G. Gene therapeutics. Nature. 1991 Jan 24;349(6307):351–352. doi: 10.1038/349351a0. [DOI] [PubMed] [Google Scholar]
- Ferry N., Duplessis O., Houssin D., Danos O., Heard J. M. Retroviral-mediated gene transfer into hepatocytes in vivo. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8377–8381. doi: 10.1073/pnas.88.19.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Garlick N., Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990 Feb;4(2):203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Immunotherapy of malignancy by in vivo gene transfer into tumors. Hum Gene Ther. 1992 Aug;3(4):399–410. doi: 10.1089/hum.1992.3.4-399. [DOI] [PubMed] [Google Scholar]
- Jaffe H. A., Danel C., Longenecker G., Metzger M., Setoguchi Y., Rosenfeld M. A., Gant T. W., Thorgeirsson S. S., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated in vivo gene transfer and expression in normal rat liver. Nat Genet. 1992 Aug;1(5):372–378. doi: 10.1038/ng0892-372. [DOI] [PubMed] [Google Scholar]
- Lin H., Parmacek M. S., Morle G., Bolling S., Leiden J. M. Expression of recombinant genes in myocardium in vivo after direct injection of DNA. Circulation. 1990 Dec;82(6):2217–2221. doi: 10.1161/01.cir.82.6.2217. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Gordon D., Yang Z. Y., Xu L., San H., Plautz G. E., Wu B. Y., Gao X., Huang L., Nabel G. J. Gene transfer in vivo with DNA-liposome complexes: lack of autoimmunity and gonadal localization. Hum Gene Ther. 1992 Dec;3(6):649–656. doi: 10.1089/hum.1992.3.6-649. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Boyce F. M., Stanley J. C., Nabel G. J. Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science. 1989 Jun 16;244(4910):1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Nicolau C., Le Pape A., Soriano P., Fargette F., Juhel M. F. In vivo expression of rat insulin after intravenous administration of the liposome-entrapped gene for rat insulin I. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1068–1072. doi: 10.1073/pnas.80.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Legrand A., Grosse E. Liposomes as carriers for in vivo gene transfer and expression. Methods Enzymol. 1987;149:157–176. doi: 10.1016/0076-6879(87)49054-x. [DOI] [PubMed] [Google Scholar]
- Plautz G. E., Yang Z. Y., Wu B. Y., Gao X., Huang L., Nabel G. J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. A., Siegfried W., Yoshimura K., Yoneyama K., Fukayama M., Stier L. E., Päkkö P. K., Gilardi P., Stratford-Perricaudet L. D., Perricaudet M. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science. 1991 Apr 19;252(5004):431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Stewart M. J., Plautz G. E., Del Buono L., Yang Z. Y., Xu L., Gao X., Huang L., Nabel E. G., Nabel G. J. Gene transfer in vivo with DNA-liposome complexes: safety and acute toxicity in mice. Hum Gene Ther. 1992 Jun;3(3):267–275. doi: 10.1089/hum.1992.3.3-267. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Hart I. R. In vitro and in vivo targeting of gene expression to melanoma cells. Cancer Res. 1993 Mar 1;53(5):962–967. [PubMed] [Google Scholar]
- Westbrook C. A., Chmura S. J., Arenas R. B., Kim S. Y., Otto G. Human APC gene expression in rodent colonic epithelium in vivo using liposomal gene delivery. Hum Mol Genet. 1994 Nov;3(11):2005–2010. doi: 10.1093/hmg/3.11.2005. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Jefferson D. M., Chowdhury J. R., Novikoff P. M., Johnston D. E., Mulligan R. C. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Zhu N., Liggitt D., Liu Y., Debs R. Systemic gene expression after intravenous DNA delivery into adult mice. Science. 1993 Jul 9;261(5118):209–211. doi: 10.1126/science.7687073. [DOI] [PubMed] [Google Scholar]