Abstract
Background—It has been reported that infection with vacuolating cytotoxin positive Helicobacter pylori strains is associated with gastroduodenal disease in Western countries. Aims—To evaluate the prevalence of cytotoxin producing strains among patients with H pylori infection in relation to gastrointestinal diseases in Japan. Patients—Ninety seven patients undergoing endoscopy. Methods—A Western blot assay was conducted to detect serum antibodies against the cytotoxin using recombinant cytotoxin (VacA protein) as an antigen. To obtain a purified recombinant cytotoxin, the vacA gene (2233 nucleotides) was cloned into an expression vector to produce the protein (744 amino acids), which was expressed in Escherichia coli. Results—Serum IgG antibodies to the cytotoxin were present in 85%, 95%, 95%, and 100% of infected patients with gastric ulcer (n=26), duodenal ulcer (n=21), chronic gastritis (n=19), and endoscopically normal mucosa (n=14), respectively. Conclusion—The western blot method using recombinant VacA protein is simple and useful for detecting antibody to vacuolating cytotoxin. This method showed antibodies against cytotoxin were highly prevalent, even in subjects with endoscopically normal mucosa in Japan, indicating that the cytotoxin may not be an independent cause of gastrointestinal diseases induced by H pylori infection.
Keywords: stomach; Helicobacter pylori; vacA; ulcer; gastritis
Full Text
The Full Text of this article is available as a PDF (169.2 KB).
Figure 1 .
: Schematic representation of plasmid constructs used for sequence analysis of the vacA gene and expression of cytotoxin protein in E coli. P, promoter; 6× His, histidine hexamer tag; LP, leader peptide; OM exp, outer membrane exporter.
Figure 2 .

: Alignment of the deduced VacA amino acid sequences between Tox+ strain 43526 (sequenced in this study), Tox+ strain 60190, and Tox- strain Tx30a. The 3873 bp vacA ORF encodes 1291 amino acids. Colons denote identity, and asterisks indicate conserved amino acids.
Figure 3 .

: Expression of the vacA gene product in E coli. SDS-PAGE with Coomassie brilliant blue staining shows the recombinant VacA protein with the expected molecular weight of about 80 kDa (arrow). The migration of protein molecular mass standards is shown.
Figure 4 .
: Western blotting analysis of VacA in H pylori. Culture supernatants from H pylori strains were probed with control rabbit serum (lanes 1 and 2), rabbit serum raised against the recombinant VacA protein expressed in E coli (lanes 3-4, 6-8), and rabbit serum raised against VA-1 (lane 5). The size variability of VacA protein in Tox+ strain 43526 (lanes 1, 3, and 5) or Tox- Tx30a (lanes 2 and 4) is shown. The position of protein molecular mass standards is indicated. H pylori clinical isolate culture supernatants were also blotted with antirecombinant VacA rabbit sera (lane 6, Tox+; lane 7, Tox+; lane 8, Tox-).
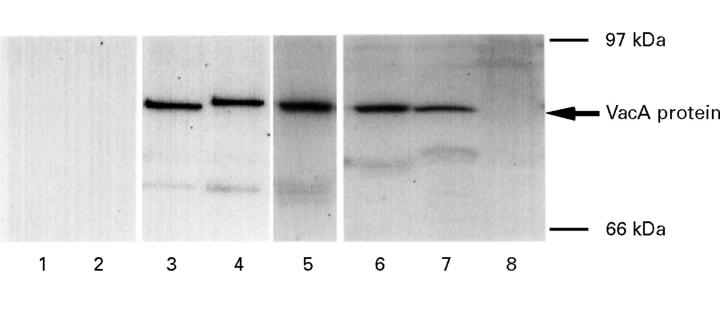
Figure 5 .
: Western blotting with human sera or rabbit anti-VA-1 antiserum of the purified recombinant VacA protein. The recombinant VacA proteins were electrophoresed on 10% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted with human sera (lane 2, negative; lane 3, positive) or rabbit antiserum raised against VA-1 (lane 4, control; lane 5, immunised by VA-1). Gold staining of the cellulose membrane to which purified recombinant VacA protein was electroblotted is also shown (lane 1). The migration of protein molecular mass standards is indicated.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anda R. F., Williamson D. F., Escobedo L. G., Remington P. L. Smoking and the risk of peptic ulcer disease among women in the United States. Arch Intern Med. 1990 Jul;150(7):1437–1441. [PubMed] [Google Scholar]
- Asaka M., Kimura T., Kudo M., Takeda H., Mitani S., Miyazaki T., Miki K., Graham D. Y. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992 Mar;102(3):760–766. doi: 10.1016/0016-5085(92)90156-s. [DOI] [PubMed] [Google Scholar]
- Atherton J. C., Cao P., Peek R. M., Jr, Tummuru M. K., Blaser M. J., Cover T. L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995 Jul 28;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- Breuer-Katschinski B. D., Armstrong D., Goebell H., Arnold R., Classen M., Fischer M., Blum A. L. Smoking as a risk factor for duodenal ulcer relapse. RUDER Study Group. Z Gastroenterol. 1995 Sep;33(9):509–512. [PubMed] [Google Scholar]
- Chuong J. J., Fisher R. L., Chuong R. L., Spiro H. M. Duodenal ulcer. Incidence, risk factors, and predictive value of plasma pepsinogen. Dig Dis Sci. 1986 Nov;31(11):1178–1184. doi: 10.1007/BF01296515. [DOI] [PubMed] [Google Scholar]
- Cover T. L., Blaser M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992 May 25;267(15):10570–10575. [PubMed] [Google Scholar]
- Cover T. L., Cao P., Murthy U. K., Sipple M. S., Blaser M. J. Serum neutralizing antibody response to the vacuolating cytotoxin of Helicobacter pylori. J Clin Invest. 1992 Sep;90(3):913–918. doi: 10.1172/JCI115967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Tummuru M. K., Cao P., Thompson S. A., Blaser M. J. Divergence of genetic sequences for the vacuolating cytotoxin among Helicobacter pylori strains. J Biol Chem. 1994 Apr 8;269(14):10566–10573. [PubMed] [Google Scholar]
- Figura N., Guglielmetti P., Rossolini A., Barberi A., Cusi G., Musmanno R. A., Russi M., Quaranta S. Cytotoxin production by Campylobacter pylori strains isolated from patients with peptic ulcers and from patients with chronic gastritis only. J Clin Microbiol. 1989 Jan;27(1):225–226. doi: 10.1128/jcm.27.1.225-226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann R. D., Adams M. D., White O., Clayton R. A., Kirkness E. F., Kerlavage A. R., Bult C. J., Tomb J. F., Dougherty B. A., Merrick J. M. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995 Jul 28;269(5223):496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- Fox J. G., Correa P., Taylor N. S., Thompson N., Fontham E., Janney F., Sobhan M., Ruiz B., Hunter F. High prevalence and persistence of cytotoxin-positive Helicobacter pylori strains in a population with high prevalence of atrophic gastritis. Am J Gastroenterol. 1992 Nov;87(11):1554–1560. [PubMed] [Google Scholar]
- Ghiara P., Marchetti M., Blaser M. J., Tummuru M. K., Cover T. L., Segal E. D., Tompkins L. S., Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995 Oct;63(10):4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Azuma T., Ito S., Kato T., Kohli Y., Fujiki N. High prevalence of neutralizing activity to Helicobacter pylori cytotoxin in serum of gastric-carcinoma patients. Int J Cancer. 1994 Jan 2;56(1):56–60. doi: 10.1002/ijc.2910560111. [DOI] [PubMed] [Google Scholar]
- Hochuli E., Döbeli H., Schacher A. New metal chelate adsorbent selective for proteins and peptides containing neighbouring histidine residues. J Chromatogr. 1987 Dec 18;411:177–184. doi: 10.1016/s0021-9673(00)93969-4. [DOI] [PubMed] [Google Scholar]
- Hosking S. W., Ling T. K., Chung S. C., Yung M. Y., Cheng A. F., Sung J. J., Li A. K. Duodenal ulcer healing by eradication of Helicobacter pylori without anti-acid treatment: randomised controlled trial. Lancet. 1994 Feb 26;343(8896):508–510. doi: 10.1016/s0140-6736(94)91460-5. [DOI] [PubMed] [Google Scholar]
- Kawamata O., Yoshida H., Hirota K., Yoshida A., Kawaguchi R., Shiratori Y., Omata M. Nested-polymerase chain reaction for the detection of Helicobacter pylori infection with novel primers designed by sequence analysis of urease A gene in clinically isolated bacterial strains. Biochem Biophys Res Commun. 1996 Feb 6;219(1):266–272. doi: 10.1006/bbrc.1996.0216. [DOI] [PubMed] [Google Scholar]
- Leoci C., Ierardi E., Chiloiro M., Piccioli E., Di Matteo G., Misciagna G., Giorgio I. Incidence and risk factors of duodenal ulcer. A retrospective cohort study. J Clin Gastroenterol. 1995 Mar;20(2):104–109. doi: 10.1097/00004836-199503000-00006. [DOI] [PubMed] [Google Scholar]
- Leunk R. D., Ferguson M. A., Morgan D. R., Low D. E., Simor A. E. Antibody to cytotoxin in infection by Helicobacter pylori. J Clin Microbiol. 1990 Jun;28(6):1181–1184. doi: 10.1128/jcm.28.6.1181-1184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Leunk R. D. Production of a cytotoxin by Helicobacter pylori. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 8):S686–S689. doi: 10.1093/clinids/13.supplement_8.s686. [DOI] [PubMed] [Google Scholar]
- Levenstein S., Prantera C., Varvo V., Scribano M. L., Berto E., Spinella S., Lanari G. Patterns of biologic and psychologic risk factors in duodenal ulcer patients. J Clin Gastroenterol. 1995 Sep;21(2):110–117. doi: 10.1097/00004836-199509000-00010. [DOI] [PubMed] [Google Scholar]
- Manetti R., Massari P., Burroni D., de Bernard M., Marchini A., Olivieri R., Papini E., Montecucco C., Rappuoli R., Telford J. L. Helicobacter pylori cytotoxin: importance of native conformation for induction of neutralizing antibodies. Infect Immun. 1995 Nov;63(11):4476–4480. doi: 10.1128/iai.63.11.4476-4480.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. J., Goodwin C. S., Warren J. R., Murray R., Blincow E. D., Blackbourn S. J., Phillips M., Waters T. E., Sanderson C. R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988 Dec 24;2(8626-8627):1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Pereira Lage A., Glupczynski Y., Goossens H., Burette A., Butzler J. P. Neutralising antibodies to the vacuolating toxin of Helicobacter pylori in gastritis only and peptic ulcer patients. Zentralbl Bakteriol. 1993 Sep;280(1-2):197–202. doi: 10.1016/s0934-8840(11)80956-4. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Ilver D., Janzon L., Normark S., Westblom T. U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994 May;62(5):1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt W., Haas R. Genetic analysis of the Helicobacter pylori vacuolating cytotoxin: structural similarities with the IgA protease type of exported protein. Mol Microbiol. 1994 Apr;12(2):307–319. doi: 10.1111/j.1365-2958.1994.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Sipponen P., Aärynen M., Käriäinen I., Kettunen P., Helske T., Seppälä K. Chronic antral gastritis, Lewis(a+) phenotype, and male sex as factors in predicting coexisting duodenal ulcer. Scand J Gastroenterol. 1989 Jun;24(5):581–588. doi: 10.3109/00365528909093093. [DOI] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Lambert J. R., Dwyer B. Cytotoxin production by Helicobacter pylori from patients with upper gastrointestinal tract diseases. J Clin Microbiol. 1995 May;33(5):1203–1205. doi: 10.1128/jcm.33.5.1203-1205.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J. L., Ghiara P., Dell'Orco M., Comanducci M., Burroni D., Bugnoli M., Tecce M. F., Censini S., Covacci A., Xiang Z. Gene structure of the Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994 May 1;179(5):1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]