Abstract
Background—Recent reports have described a modulating influence of nitric oxide (NO) on intestinal mucosal permeability and have implicated a role for mast cells in this NO mediated process. Aims—To assess further the contribution of mast cells to the mucosal permeability changes elicited by the NO synthase (NOS) inhibitor NG-nitro-L-arginine methylester (L-NAME), using mast cell deficient (W/WV) and mast cell replete mice (+/+). Methods—Chromium-51 EDTA clearance (from blood to jejunal lumen), jejunal NOS and myeloperoxidase (MPO) activities, and plasma nitrate/nitrite levels were monitored. Results—The increased EDTA clearance elicited by intraluminal L-NAME in W/WV mice (4.4-fold) was significantly greater than the response observed in control (+/+) mice (1.8-fold). The exacerbated response in W/Wv mice was greatly attenuated by pretreatment with either dexamethasone (1.3-fold) or the selective inducible NOS inhibitor, aminoguanidine (1.4-fold), and partially attenuated by the mast cell stabiliser, lodoxamide (2.9-fold). Jejunal inducible NOS activity was significantly higher in W/WV than in +/+ mice, while jejunal MPO was lower in W/WV mice than in +/+ mice, suggesting that the higher inducible NOS in W/WV does not result from the recruitment of inflammatory cells into the gut. The higher inducible NOS activity in the jejunum of W/WV was significantly reduced by dexamethasone treatment. Conclusions—Our results suggest that mast cells normally serve to inhibit inducible NOS activity tonically in the gut and that inhibitors of NOS elicit a larger permeability response when this tonic inhibitory influence is released by mast cell depletion.
Keywords: aminoguanidine; c-kit; dexamethasone; epithelium; neutrophils
Full Text
The Full Text of this article is available as a PDF (130.5 KB).
Figure 1 .
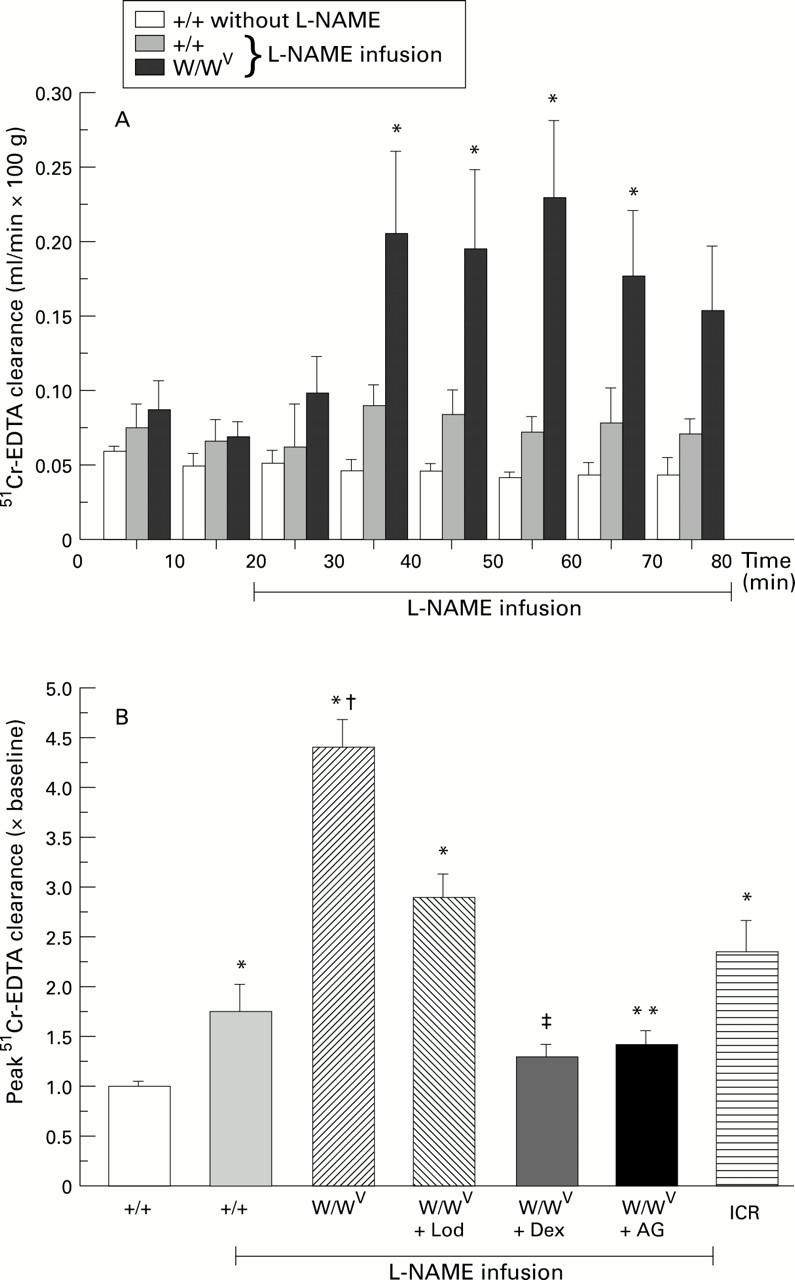
: (A) Time course of mucosal permeability changes in untreated controls (+/+) (n=5), and elicited by L-NAME in +/+ (n=6), and mast cell deficient (W/WV) mice (n=6). (B) L-NAME induced mucosal permeability changes expressed as a ratio of the maximum clearance to baseline value (10-20 minute clearance value) in the following groups: +/+, W/WV, W/WV pretreated with lodoxamide (Lod) (n=5), W/WV pretreated with dexamethasone (Dex) (n=5), and W/WV pretreated with aminoguanidine (AG) (n=6) and ICR mice (n=6). *p<0.05 relative to respective baseline value, †p<0.001 relative to L-NAME-treated +/+, *p<0.05, ‡p<0.001 relative to the peak clearance response of W/WV.
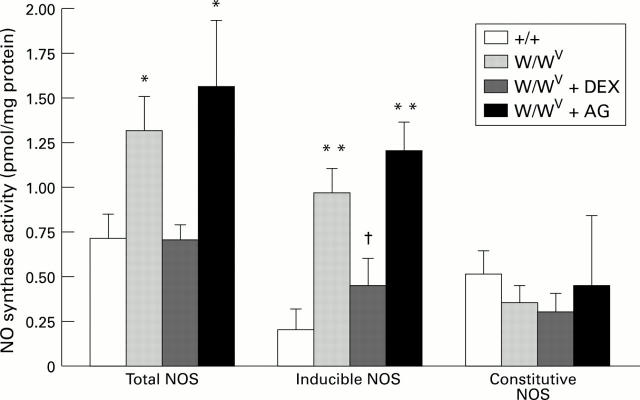
Figure 2 .
: Nitric oxide synthase (NOS) activity in control (+/+) mice (n=7), mast cell deficient (W/WV) mice (n=8), W/WV mice pretreated with dexamethasone (DEX) (n=8), and W/WV mice pretreated with aminoguanidine (AG) (n=6). *p<0.05, **p<0.001 relative to +/+, †p<0.05 relative to W/WV.
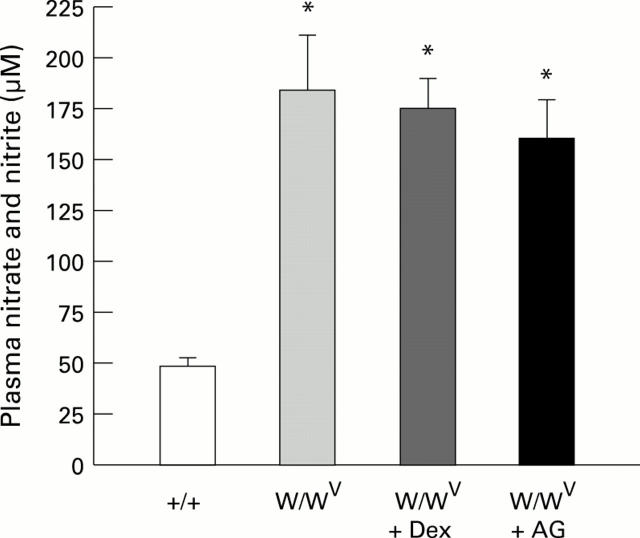
Figure 3 .
: Plasma levels of nitrite and nitrate in control (+/+) (n=6), mast cell deficient (W/WV) (n=6), and W/WV mice pretreated with either dexamethasone (Dex) (n=6) or aminoguanidine (AG) (n=6). *p<0.01 relative to +/+.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alican I., Kubes P. A critical role for nitric oxide in intestinal barrier function and dysfunction. Am J Physiol. 1996 Feb;270(2 Pt 1):G225–G237. doi: 10.1152/ajpgi.1996.270.2.G225. [DOI] [PubMed] [Google Scholar]
- Brown J. F., Tepperman B. L., Hanson P. J., Whittle B. J., Moncada S. Differential distribution of nitric oxide synthase between cell fractions isolated from the rat gastric mucosa. Biochem Biophys Res Commun. 1992 Apr 30;184(2):680–685. doi: 10.1016/0006-291x(92)90643-y. [DOI] [PubMed] [Google Scholar]
- Burd P. R., Thompson W. C., Max E. E., Mills F. C. Activated mast cells produce interleukin 13. J Exp Med. 1995 Apr 1;181(4):1373–1380. doi: 10.1084/jem.181.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Crissinger K. D., Kvietys P. R., Granger D. N. Pathophysiology of gastrointestinal mucosal permeability. J Intern Med Suppl. 1990;732:145–154. doi: 10.1111/j.1365-2796.1990.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Dileepan K. N., Lorsbach R. B., Stechschulte D. J. Mast cell granules inhibit macrophage-mediated lysis of mastocytoma cells (P815) and nitric oxide production. J Leukoc Biol. 1993 Apr;53(4):446–453. doi: 10.1002/jlb.53.4.446. [DOI] [PubMed] [Google Scholar]
- Fujiskai J., Fujimoto K., Oohara A., Sakata T., Hirano M., Ohyama T., Iwakiri R., Yamaguchi M. Roles of histamine and diamine oxidase in mucosa of rat small intestine after ischemia-reperfusion. Dig Dis Sci. 1993 Jul;38(7):1195–1200. doi: 10.1007/BF01296067. [DOI] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Geng Y., Maier R., Lotz M. Tyrosine kinases are involved with the expression of inducible nitric oxide synthase in human articular chondrocytes. J Cell Physiol. 1995 Jun;163(3):545–554. doi: 10.1002/jcp.1041630315. [DOI] [PubMed] [Google Scholar]
- Griffiths M. J., Messent M., MacAllister R. J., Evans T. W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br J Pharmacol. 1993 Nov;110(3):963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscavage J. M., Rogers N. E., Sherman M. P., Ignarro L. J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993 Dec 1;151(11):6329–6337. [PubMed] [Google Scholar]
- Grisham M. B., Specian R. D., Zimmerman T. E. Effects of nitric oxide synthase inhibition on the pathophysiology observed in a model of chronic granulomatous colitis. J Pharmacol Exp Ther. 1994 Nov;271(2):1114–1121. [PubMed] [Google Scholar]
- Kanwar S., Wallace J. L., Befus D., Kubes P. Nitric oxide synthesis inhibition increases epithelial permeability via mast cells. Am J Physiol. 1994 Feb;266(2 Pt 1):G222–G229. doi: 10.1152/ajpgi.1994.266.2.G222. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go S., Hatanaka K. Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood. 1978 Aug;52(2):447–452. [PubMed] [Google Scholar]
- Knowles R. G., Salter M., Brooks S. L., Moncada S. Anti-inflammatory glucocorticoids inhibit the induction by endotoxin of nitric oxide synthase in the lung, liver and aorta of the rat. Biochem Biophys Res Commun. 1990 Nov 15;172(3):1042–1048. doi: 10.1016/0006-291x(90)91551-3. [DOI] [PubMed] [Google Scholar]
- Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol. 1993 Jan;264(1 Pt 1):G143–G149. doi: 10.1152/ajpgi.1993.264.1.G143. [DOI] [PubMed] [Google Scholar]
- Kubes P. Nitric oxide modulates epithelial permeability in the feline small intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G1138–G1142. doi: 10.1152/ajpgi.1992.262.6.G1138. [DOI] [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose I., Kubes P., Wolf R., Anderson D. C., Paulson J., Miyasaka M., Granger D. N. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ Res. 1993 Jul;73(1):164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- Kurose I., Wolf R., Grisham M. B., Granger D. N. Effects of an endogenous inhibitor of nitric oxide synthesis on postcapillary venules. Am J Physiol. 1995 Jun;268(6 Pt 2):H2224–H2231. doi: 10.1152/ajpheart.1995.268.6.H2224. [DOI] [PubMed] [Google Scholar]
- Kurose I., Wolf R., Grisham M. B., Granger D. N. Modulation of ischemia/reperfusion-induced microvascular dysfunction by nitric oxide. Circ Res. 1994 Mar;74(3):376–382. doi: 10.1161/01.res.74.3.376. [DOI] [PubMed] [Google Scholar]
- Misko T. P., Moore W. M., Kasten T. P., Nickols G. A., Corbett J. A., Tilton R. G., McDaniel M. L., Williamson J. R., Currie M. G. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993 Mar 16;233(1):119–125. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- Mitchell J. A., Kohlhaas K. L., Sorrentino R., Warner T. D., Murad F., Vane J. R. Induction by endotoxin of nitric oxide synthase in the rat mesentery: lack of effect on action of vasoconstrictors. Br J Pharmacol. 1993 May;109(1):265–270. doi: 10.1111/j.1476-5381.1993.tb13563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990 Jun;9(6):1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Karmeli F., Kelly C. P., Eliakim R., Joshi M. A., O'Keane C. J., Castagliuolo I., LaMont J. T., Rachmilewitz D. Ketotifen inhibits Clostridium difficile toxin A-induced enteritis in rat ileum. Gastroenterology. 1993 Sep;105(3):701–707. doi: 10.1016/0016-5085(93)90886-h. [DOI] [PubMed] [Google Scholar]
- Taipale J., Lohi J., Saarinen J., Kovanen P. T., Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995 Mar 3;270(9):4689–4696. doi: 10.1074/jbc.270.9.4689. [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Bogdan C., Paik J., Xie Q. W., Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993 Aug 1;178(2):605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. M., Burns A. J., Torihashi S., Sanders K. M. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994 Oct 1;480(Pt 1):91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]