Abstract
Background—It has recently been shown that humoral antigastric autoreactivities occur in a substantial number of Helicobacter pylori infected patients. Aims—To analyse the relevance of such antigastric autoantibodies for histological and serological parameters of the infection as well as for the clinical course. Methods—Gastric biopsy samples and sera from 126 patients with upper abdominal complaints were investigated for evidence of H pylori infection using histology and serology. Autoantibodies against epitopes in human gastric mucosa were detected by immunohistochemical techniques. Histological and clinical findings of all patients were then correlated with the detection of antigastric autoantibodies. Results—H pylori infection was significantly associated with antigastric autoantibodies reactive with the luminal membrane of the foveolar epithelium and with canalicular structures within parietal cells. The presence of the latter autoantibodies was significantly correlated with the severity of body gastritis, gastric mucosa atrophy, elevated fasting gastrin concentrations, and a decreased ratio of serum pepsinogen I:II. Furthermore the presence of anticanalicular autoantibodies was associated with a greater than twofold reduced prevalence for duodenal ulcer. Conclusion—The data indicate that antigastric autoantibodies play a role in the pathogenesis and outcome of H pylori gastritis, in particular in the development of gastric mucosal atrophy.
Keywords: gastritis; Helicobacter pylori; autoimmunity; gastric atrophy
Full Text
The Full Text of this article is available as a PDF (157.7 KB).
Figure 1 .
: Antiluminal antigastric reactivity against the apical membrane of foveolar epithelium cells in the human gastric antrum and body (original magnification ×40).
Figure 2 .
: Anticanalicular antigastric autoreactivity against canaliculi within parietal cells of the human gastric body (original magnification ×40).
Figure 3 .
: Fasting serum gastrin concentrations in healthy controls, patients with H pylori gastritis with and without anticanalicular autoantibodies, and patients with type A gastritis.
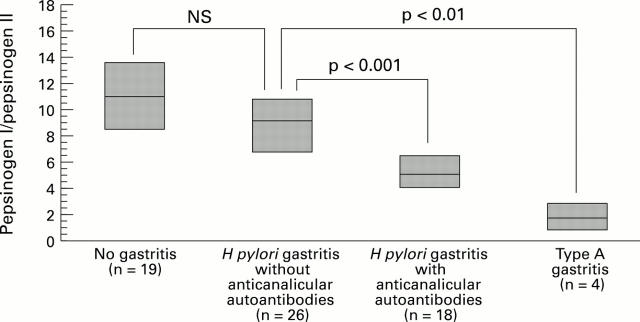
Figure 4 .
: Fasting pepsinogen I:II ratio in healthy controls, patients with H pylori gastritis with and without anticanalicular autoantibodies, and patients with type A gastritis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelmelk B. J., Simoons-Smit I., Negrini R., Moran A. P., Aspinall G. O., Forte J. G., De Vries T., Quan H., Verboom T., Maaskant J. J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996 Jun;64(6):2031–2040. doi: 10.1128/iai.64.6.2031-2040.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstad K., Berstad A. Helicobacter pylori infection in peptic ulcer disease. Scand J Gastroenterol. 1993 Jul;28(7):561–567. doi: 10.3109/00365529309096088. [DOI] [PubMed] [Google Scholar]
- Coghlan J. G., Gilligan D., Humphries H., McKenna D., Dooley C., Sweeney E., Keane C., O'Morain C. Campylobacter pylori and recurrence of duodenal ulcers--a 12-month follow-up study. Lancet. 1987 Nov 14;2(8568):1109–1111. doi: 10.1016/s0140-6736(87)91545-5. [DOI] [PubMed] [Google Scholar]
- Engstrand L., Scheynius A., Påhlson C., Grimelius L., Schwan A., Gustavsson S. Association of Campylobacter pylori with induced expression of class II transplantation antigens on gastric epithelial cells. Infect Immun. 1989 Mar;57(3):827–832. doi: 10.1128/iai.57.3.827-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enno A., O'Rourke J. L., Howlett C. R., Jack A., Dixon M. F., Lee A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am J Pathol. 1995 Jul;147(1):217–222. [PMC free article] [PubMed] [Google Scholar]
- Faller G., Steininger H., Eck M., Hensen J., Hann E. G., Kirchner T. Antigastric autoantibodies in Helicobacter pylori gastritis: prevalence, in-situ binding sites and clues for clinical relevance. Virchows Arch. 1996 Feb;427(5):483–486. doi: 10.1007/BF00199508. [DOI] [PubMed] [Google Scholar]
- Fujioka T., Kubota T., Shuto R., Kodama R., Murakami K., Perparim K., Nasu M. Establishment of an animal model for chronic gastritis with Helicobacter pylori: potential model for long-term observations. Eur J Gastroenterol Hepatol. 1994 Dec;6 (Suppl 1):S73–S78. [PubMed] [Google Scholar]
- Goldschmiedt M., Barnett C. C., Schwarz B. E., Karnes W. E., Redfern J. S., Feldman M. Effect of age on gastric acid secretion and serum gastrin concentrations in healthy men and women. Gastroenterology. 1991 Oct;101(4):977–990. doi: 10.1016/0016-5085(91)90724-y. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Haruma K., Kawaguchi H., Kohmoto K., Okamoto S., Yoshihara M., Sumii K., Kajiyama G. Helicobacter pylori infection, serum gastrin, and gastric acid secretion in teen-age subjects with duodenal ulcer, gastritis, or normal mucosa. Scand J Gastroenterol. 1995 Apr;30(4):322–326. doi: 10.3109/00365529509093284. [DOI] [PubMed] [Google Scholar]
- Haruma K., Kawaguchi H., Yoshihara M., Okamoto S., Sumii K., Kishimoto S., Kajiyama G. Relationship between Helicobacter pylori infection and gastric acid secretion in young healthy subjects. J Clin Gastroenterol. 1994 Jul;19(1):20–22. doi: 10.1097/00004836-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Iwao T., Toyonaga A., Kuboyama S., Tanikawa K. Effects of omeprazole and lansoprazole on fasting and postprandial serum gastrin and serum pepsinogen A and C. Hepatogastroenterology. 1995 Sep-Oct;42(5):677–682. [PubMed] [Google Scholar]
- Jerzy Glass G. B., Pitchumoni C. S. Structural and ultrastructural alterations, exfoliative cytology and enzyme cytochemistry and histochemistry, proliferation kinetics, immunological derangements and other causes, and clinical associations and sequallae. Hum Pathol. 1975 Mar;6(2):219–250. [PubMed] [Google Scholar]
- Karita M., Kouchiyama T., Okita K., Nakazawa T. New small animal model for human gastric Helicobacter pylori infection: success in both nude and euthymic mice. Am J Gastroenterol. 1991 Nov;86(11):1596–1603. [PubMed] [Google Scholar]
- Karita M., Li Q., Cantero D., Okita K. Establishment of a small animal model for human Helicobacter pylori infection using germ-free mouse. Am J Gastroenterol. 1994 Feb;89(2):208–213. [PubMed] [Google Scholar]
- Karnes W. E., Jr, Ohning G. V., Sytnik B., Kim S. W., Walsh J. H. Elevation of meal-stimulated gastrin release in subjects with Helicobacter pylori infection: reversal by low intragastric pH. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 8):S665–S670. doi: 10.1093/clinids/13.supplement_8.s665. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Pérez-Pérez G. I., Meuwissen S. G., Blaser M. J. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995 Dec 6;87(23):1777–1780. doi: 10.1093/jnci/87.23.1777. [DOI] [PubMed] [Google Scholar]
- Kuipers E. J., Uyterlinde A. M., Peña A. S., Roosendaal R., Pals G., Nelis G. F., Festen H. P., Meuwissen S. G. Long-term sequelae of Helicobacter pylori gastritis. Lancet. 1995 Jun 17;345(8964):1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- Lee A. H pylori-initiated ulcerogenesis: look to the host. Lancet. 1993 Jan 30;341(8840):280–281. doi: 10.1016/0140-6736(93)92624-3. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Goodwin C. S., Warren J. R., Murray R., Blincow E. D., Blackbourn S. J., Phillips M., Waters T. E., Sanderson C. R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988 Dec 24;2(8626-8627):1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Negrini R., Lisato L., Zanella I., Cavazzini L., Gullini S., Villanacci V., Poiesi C., Albertini A., Ghielmi S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology. 1991 Aug;101(2):437–445. doi: 10.1016/0016-5085(91)90023-e. [DOI] [PubMed] [Google Scholar]
- Negrini R., Savio A., Poiesi C., Appelmelk B. J., Buffoli F., Paterlini A., Cesari P., Graffeo M., Vaira D., Franzin G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology. 1996 Sep;111(3):655–665. doi: 10.1053/gast.1996.v111.pm8780570. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Peterson W. L., Barnett C. C., Evans D. J., Jr, Feldman M., Carmody T., Richardson C., Walsh J., Graham D. Y. Acid secretion and serum gastrin in normal subjects and patients with duodenal ulcer: the role of Helicobacter pylori. Am J Gastroenterol. 1993 Dec;88(12):2038–2043. [PubMed] [Google Scholar]
- Price A. B. The Sydney System: histological division. J Gastroenterol Hepatol. 1991 May-Jun;6(3):209–222. doi: 10.1111/j.1440-1746.1991.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Ross J. S., Bui H. X., del Rosario A., Sonbati H., George M., Lee C. Y. Helicobacter pylori. Its role in the pathogenesis of peptic ulcer disease in a new animal model. Am J Pathol. 1992 Sep;141(3):721–727. [PMC free article] [PubMed] [Google Scholar]
- Scheynius A., Engstrand L. Gastric epithelial cells in Helicobacter pylori-associated gastritis express HLA-DR but not ICAM-1. Scand J Immunol. 1991 Feb;33(2):237–241. doi: 10.1111/j.1365-3083.1991.tb03755.x. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N. The basis of autoimmunity: Part I. Mechanisms of aberrant self-recognition. Immunol Today. 1995 Feb;16(2):90–98. doi: 10.1016/0167-5699(95)80095-6. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Wee A., Teh M., Kang J. Y. Association of Helicobacter pylori with HLA-DR antigen expression in gastritis. J Clin Pathol. 1992 Jan;45(1):30–33. doi: 10.1136/jcp.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon A. C., Ortiz-Hidalgo C., Falzon M. R., Isaacson P. G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991 Nov 9;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Dixon M. F. Chronic gastritis--a pathogenetic approach. J Pathol. 1988 Feb;154(2):113–124. doi: 10.1002/path.1711540203. [DOI] [PubMed] [Google Scholar]
- Xiang Z., Censini S., Bayeli P. F., Telford J. L., Figura N., Rappuoli R., Covacci A. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect Immun. 1995 Jan;63(1):94–98. doi: 10.1128/iai.63.1.94-98.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]