Abstract
Abstract Background—Patients with long standing diabetes mellitus frequently have upper gut dysmotility. Gastroparesis has been well studied, whereas detailed data on duodenal motor function are limited. Aims—To characterise postprandial duodenal chyme transport in such patients. Methods—Intraluminal multiple impedance measurement, recently introduced as a novel technique for investigation of chyme transport, was used to study postprandial duodenal chyme flow in 10 patients with long standing insulin dependent diabetes mellitus with gastroparesis, and 10 healthy volunteers. Results—Four distinct transport patterns of chyme, termed bolus transport events (BTEs), were found in both groups and could be characterised as: short distance propulsive; simple long distance propulsive; retrograde; and complex long distance propulsive. Diabetic patients had significantly lower numbers of propulsive BTEs (p<0.01), and higher proportions of retrograde BTEs and complex long distance BTEs (p<0.05) than control subjects, whereas the proportion of simple long distance BTEs was significantly lower (p<0.05). The mean propagation velocities of the BTEs were similar in both groups. Conclusion—Abnormal postprandial duodenal chyme transport was found in patients with long standing insulin dependent diabetes mellitus. This is characterised by transport disorganisation and may result in disturbed chyme clearance.
Keywords: diabetic gastroparesis syndrome; postprandial chyme transport; intraluminal impedance measurement
Full Text
The Full Text of this article is available as a PDF (264.4 KB).
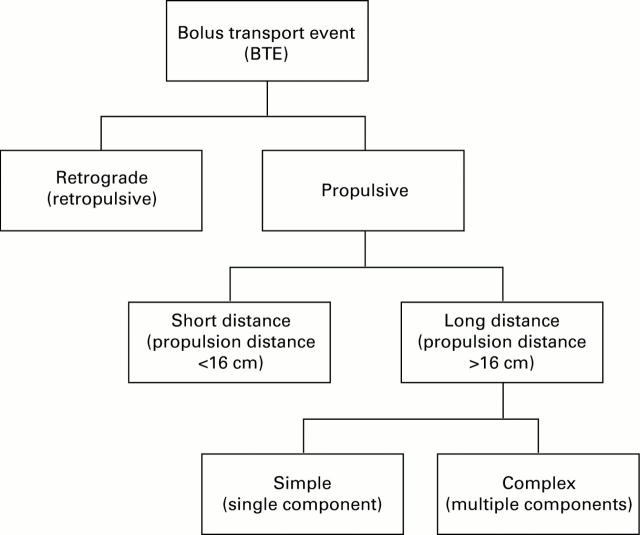
Figure 1 .
: Classification algorithm for the bolus transport events.
Figure 2 .
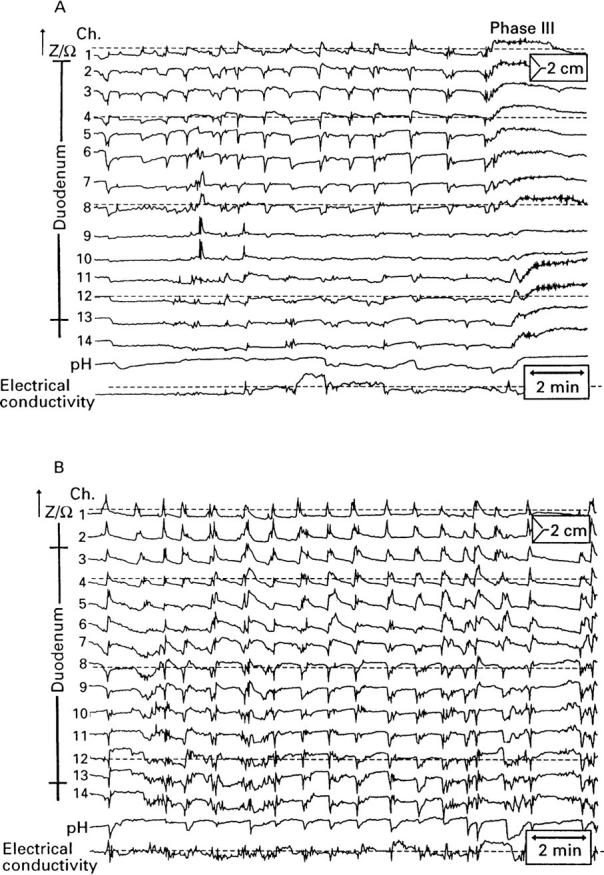
: Impedance tracings of two healthy subjects using a combined catheter device. Numbers and positions of measuring segments are shown at the left, channels 1-14, impedance; channel 15, luminal pH; channel 16, luminal electrical conductivity. (A) During the late phase II of a migrating motor complex cycle; (B) during the postprandial state.
Figure 3 .
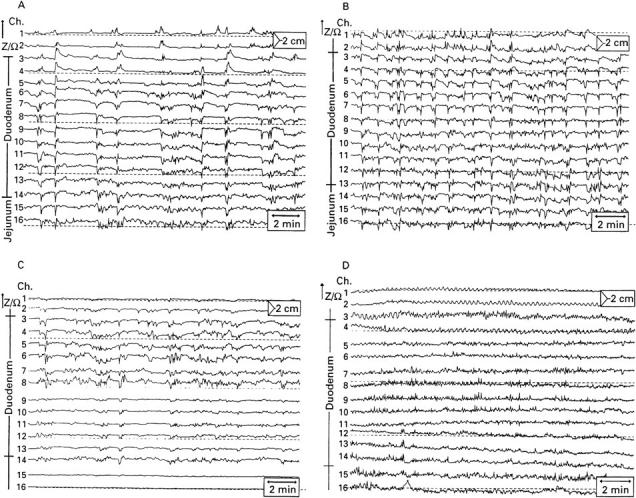
: Examples of postprandial impedance tracings, 10 minutes after the test meal using the standard catheter device. Numbers and positions of measuring segments are shown at the left. (A) Diabetic patient (30 year old man); (B) healthy subject (26 year old man); (C) patient with gastroparesis due to recurrent peptic ulcers (60 year old man); (D) patient with intestinal dysmotility due to progressive systemic sclerosis (55 year old woman).
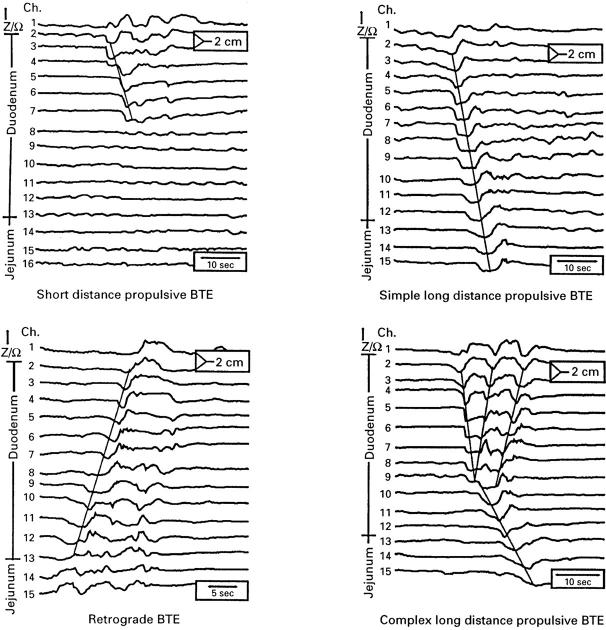
Figure 4 .
: Examples of the main BTEs, observed postprandially. The numbers (maximum 16) and positions of the measuring segments in duodenum or jejunum are shown at the left margin. Top left panel, short distance propulsive BTE originating from proximal duodenum; top right panel, a simple long distance propulsive BTE starting from proximal duodenum; bottom left panel, a retrograde BTE starting from proximal jejunum; bottom right panel, a complex long distance propulsive BTE featuring one long distance propulsive component with two additional retrograde components starting from mid-duodenum during bolus propulsion.
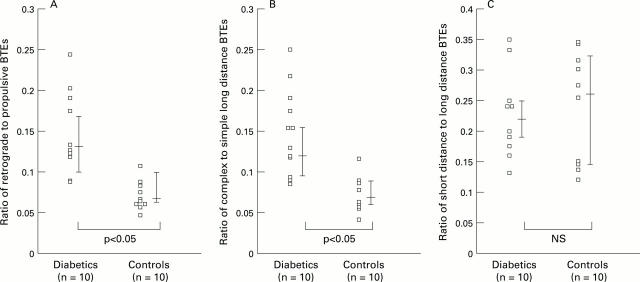
Figure 5 .
: Ratios of the different classes of BTE in diabetic patients compared with healthy controls. Bars are median and interquartile range.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahluwalia N. K., Thompson D. G., Barlow J., Heggie L. Human small intestinal contractions and aboral traction forces during fasting and after feeding. Gut. 1994 May;35(5):625–630. doi: 10.1136/gut.35.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M., Brown M. L., Malagelada J. R. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986 Jul;91(1):94–99. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- Camilleri M., Malagelada J. R. Abnormal intestinal motility in diabetics with the gastroparesis syndrome. Eur J Clin Invest. 1984 Dec;14(6):420–427. doi: 10.1111/j.1365-2362.1984.tb01206.x. [DOI] [PubMed] [Google Scholar]
- Cooke A. R., Clark E. D. Effect of first part of duodenum on gastric emptying in dogs: response to acid, fat, glucose, and neural blockade. Gastroenterology. 1976 Apr;70(4):550–555. [PubMed] [Google Scholar]
- Fass J., Silny J., Braun J., Heindrichs U., Dreuw B., Schumpelick V., Rau G. Measuring esophageal motility with a new intraluminal impedance device. First clinical results in reflux patients. Scand J Gastroenterol. 1994 Aug;29(8):693–702. doi: 10.3109/00365529409092496. [DOI] [PubMed] [Google Scholar]
- Feldman M., Schiller L. R. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983 Mar;98(3):378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- Frank J. W., Saslow S. B., Camilleri M., Thomforde G. M., Dinneen S., Rizza R. A. Mechanism of accelerated gastric emptying of liquids and hyperglycemia in patients with type II diabetes mellitus. Gastroenterology. 1995 Sep;109(3):755–765. doi: 10.1016/0016-5085(95)90382-8. [DOI] [PubMed] [Google Scholar]
- Fraser R. J., Horowitz M., Maddox A. F., Harding P. E., Chatterton B. E., Dent J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1990 Nov;33(11):675–680. doi: 10.1007/BF00400569. [DOI] [PubMed] [Google Scholar]
- Fraser R., Horowitz M., Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991 May;32(5):475–478. doi: 10.1136/gut.32.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser R., Horowitz M., Maddox A., Dent J. Dual effects of cisapride on gastric emptying and antropyloroduodenal motility. Am J Physiol. 1993 Feb;264(2 Pt 1):G195–G201. doi: 10.1152/ajpgi.1993.264.2.G195. [DOI] [PubMed] [Google Scholar]
- Frieling T., Hermann S., Kuhlbusch R., Enck P., Silny J., Lübke H. J., Strohmeyer G., Haeussinger D. Comparison between intraluminal multiple electric impedance measurement and manometry in the human oesophagus. Neurogastroenterol Motil. 1996 Mar;8(1):45–50. doi: 10.1111/j.1365-2982.1996.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Haba T., Sarna S. K. Regulation of gastroduodenal emptying of solids by gastropyloroduodenal contractions. Am J Physiol. 1993 Feb;264(2 Pt 1):G261–G271. doi: 10.1152/ajpgi.1993.264.2.G261. [DOI] [PubMed] [Google Scholar]
- Heddle R., Collins P. J., Dent J., Horowitz M., Read N. W., Chatterton B., Houghton L. A. Motor mechanisms associated with slowing of the gastric emptying of a solid meal by an intraduodenal lipid infusion. J Gastroenterol Hepatol. 1989 Sep-Oct;4(5):437–447. doi: 10.1111/j.1440-1746.1989.tb01741.x. [DOI] [PubMed] [Google Scholar]
- Heddle R., Dent J., Read N. W., Houghton L. A., Toouli J., Horowitz M., Maddern G. J., Downton J. Antropyloroduodenal motor responses to intraduodenal lipid infusion in healthy volunteers. Am J Physiol. 1988 May;254(5 Pt 1):G671–G679. doi: 10.1152/ajpgi.1988.254.5.G671. [DOI] [PubMed] [Google Scholar]
- Jebbink H. J., Bruijs P. P., Bravenboer B., Akkermans L. M., vanBerge-Henegouwen G. P., Smout A. J. Gastric myoelectrical activity in patients with type I diabetes mellitus and autonomic neuropathy. Dig Dis Sci. 1994 Nov;39(11):2376–2383. doi: 10.1007/BF02087654. [DOI] [PubMed] [Google Scholar]
- Jebbink R. J., Samsom M., Bruijs P. P., Bravenboer B., Akkermans L. M., Vanberge-Henegouwen G. P., Smout A. J. Hyperglycemia induces abnormalities of gastric myoelectrical activity in patients with type I diabetes mellitus. Gastroenterology. 1994 Nov;107(5):1390–1397. doi: 10.1016/0016-5085(94)90541-x. [DOI] [PubMed] [Google Scholar]
- Malagelada J. R., Rees W. D., Mazzotta L. J., Go V. L. Gastric motor abnormalities in diabetic and postvagotomy gastroparesis: effect of metoclopramide and bethanechol. Gastroenterology. 1980 Feb;78(2):286–293. [PubMed] [Google Scholar]
- Mearin F., Camilleri M., Malagelada J. R. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986 Jun;90(6):1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Meeroff J. C., Go V. L., Phillips S. F. Control of gastric emptying by osmolality of duodenal contents in man. Gastroenterology. 1975 May;68(5 Pt 1):1144–1151. [PubMed] [Google Scholar]
- Nguyen H. N., Silny J., Wüller S., Marschall H. U., Rau G., Matern S. Chyme transport patterns in human duodenum, determined by multiple intraluminal impedancometry. Am J Physiol. 1995 Apr;268(4 Pt 1):G700–G708. doi: 10.1152/ajpgi.1995.268.4.G700. [DOI] [PubMed] [Google Scholar]
- Orihata M., Sarna S. K. Contractile mechanisms of action of gastroprokinetic agents: cisapride, metoclopramide, and domperidone. Am J Physiol. 1994 Apr;266(4 Pt 1):G665–G676. doi: 10.1152/ajpgi.1994.266.4.G665. [DOI] [PubMed] [Google Scholar]
- Prather C. M., Camilleri M., Thomforde G. M., Forstrom L. A., Zinsmeister A. R. Gastric axial forces in experimentally delayed and accelerated gastric emptying. Am J Physiol. 1993 May;264(5 Pt 1):G928–G934. doi: 10.1152/ajpgi.1993.264.5.G928. [DOI] [PubMed] [Google Scholar]
- Rao S. S., Lu C., Schulze-Delrieu K. Duodenum as a immediate brake to gastric outflow: a videofluoroscopic and manometric assessment. Gastroenterology. 1996 Mar;110(3):740–747. doi: 10.1053/gast.1996.v110.pm8608883. [DOI] [PubMed] [Google Scholar]
- Rathmann W., Enck P., Frieling T., Gries F. A. Visceral afferent neuropathy in diabetic gastroparesis. Diabetes Care. 1991 Nov;14(11):1086–1089. doi: 10.2337/diacare.14.11.1086. [DOI] [PubMed] [Google Scholar]
- Scarpello J. H., Greaves M., Sladen G. E. Small intestinal transit in diabetics. Br Med J. 1976 Nov 20;2(6046):1225–1226. doi: 10.1136/bmj.2.6046.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skopnik H., Silny J., Heiber O., Schulz J., Rau G., Heimann G. Gastroesophageal reflux in infants: evaluation of a new intraluminal impedance technique. J Pediatr Gastroenterol Nutr. 1996 Dec;23(5):591–598. doi: 10.1097/00005176-199612000-00014. [DOI] [PubMed] [Google Scholar]
- Smout A., Horowitz M., Armstrong D. Methods to study gastric emptying. Frontiers in gastric emptying. Dig Dis Sci. 1994 Dec;39(12 Suppl):130S–132S. doi: 10.1007/BF02300393. [DOI] [PubMed] [Google Scholar]
- Spengler U., Stellaard F., Ruckdeschel G., Scheurlen C., Kruis W. Small intestinal transit, bacterial growth, and bowel habits in diabetes mellitus. Pancreas. 1989;4(1):65–70. doi: 10.1097/00006676-198902000-00010. [DOI] [PubMed] [Google Scholar]
- Thompson D. G., Wingate D. L. Effects of osmoreceptor stimulation on human duodenal motor activity. Gut. 1988 Feb;29(2):173–180. doi: 10.1136/gut.29.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems W. A., Seygal G. E. Fluid propulsion by cat intestinal segments under conditions requiring hydrostatic work. Am J Physiol. 1981 Feb;240(2):G147–G156. doi: 10.1152/ajpgi.1981.240.2.G147. [DOI] [PubMed] [Google Scholar]