Abstract
Background—Antisecretory factor (AF) is a recently identified regulatory protein which inhibits the intestinal fluid secretion induced by cholera toxin. Aims—To test the effect of AF on: (a) inflammation and hypersecretion induced by toxin A from Clostridium difficile; and (b) morphological changes and hypersecretion induced by okadaic acid (the blue mussel toxin) in rat intestinal mucosa. Methods—Morphological changes and fluid accumulation were observed in intestinal loops challenged with 1 µg of toxin A or 3 µg of okadaic acid administered before or after injection of 0.1 µg of recombinant AF (rAF). Results—The cytotoxic and inflammatory reaction caused by toxin A was abolished after treatment with rAF given either intraveneously or intraluminally prior to the toxin or one hour after the toxin. The intestinal fluid response induced by toxin A and okadaic acid was reduced 55-80% by rAF. However, the characteristic increase in goblet cells at the tips of villi in the okadaic acid treated mucosa was not inhibited by rAF. Conclusion—Results suggest that AF might be involved in protection against inflammation and in counteracting dehydration caused by enterotoxins. Both effects are probably mediated via the enteric nervous system.
Keywords: okadaic acid; Clostridium difficile toxin A; diarrhoea; neuropeptide; S5a; rat
Full Text
The Full Text of this article is available as a PDF (144.5 KB).
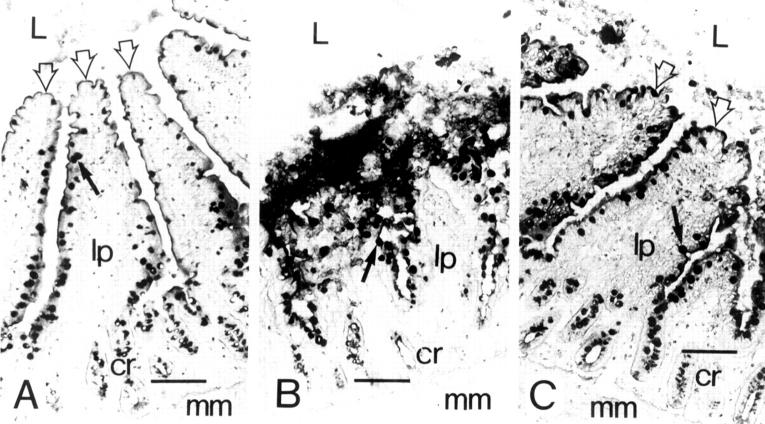
Figure 1 .
: (A) Control morphology in ligated jejunal loop challenged with PBS. The apical parts of the villi (open arrows) are distinctly lineated against the intestinal lumen (L). The lamina propria (lp) is intact, as are the crypt cells (cr) and muscularis mucosae (mm). The black arrow points to the black stained goblet cell. (B) Morphology in the ligated loop five hours after challenge with C difficile toxin A. The upper half of the villi is totally disintegrated, and the intestinal morphology in this part of the villi cannot be reconstructed. The intestinal lumen is full of cell debris. The basal part of the villi shows a more intact morphology, along with the crypt cells and muscularis mucosae. (C) The intact and preserved morphology in the ligated loop despite five hours of challenge with C difficile toxin A. Systemic injection of recombinant antisecretory factor inhibited substantially the cytotoxic action of toxin A. The upper half of the villi is morphologically intact; however, in the intestinal lumen some shed goblet and epithelial cells can be seen. The morphology further down the basal part of the villi and in the crypts and muscularis mucosae is identical to that of the control. Bars are 100 µm.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartlett J. G. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin Infect Dis. 1994 May;18 (Suppl 4):S265–S272. doi: 10.1093/clinids/18.supplement_4.s265. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I., LaMont J. T., Letourneau R., Kelly C., O'Keane J. C., Jaffer A., Theoharides T. C., Pothoulakis C. Neuronal involvement in the intestinal effects of Clostridium difficile toxin A and Vibrio cholerae enterotoxin in rat ileum. Gastroenterology. 1994 Sep;107(3):657–665. doi: 10.1016/0016-5085(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Chiou J. Y., Westhead E. W. Okadaic acid, a protein phosphatase inhibitor, inhibits nerve growth factor-directed neurite outgrowth in PC12 cells. J Neurochem. 1992 Nov;59(5):1963–1966. doi: 10.1111/j.1471-4159.1992.tb11034.x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Holmes C. F., Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990 Mar;15(3):98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- Edebo L., Lange S., Li X. P., Allenmark S. Toxic mussels and okadaic acid induce rapid hypersecretion in the rat small intestine. APMIS. 1988 Nov;96(11):1029–1035. doi: 10.1111/j.1699-0463.1988.tb00977.x. [DOI] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. B., Skadhauge E. New aspects of the pathophysiology and treatment of secretory diarrhoea. Physiol Res. 1995;44(2):61–78. [PubMed] [Google Scholar]
- Holmgren J., Lönnroth I., Månsson J., Svennerholm L. Interaction of cholera toxin and membrane GM1 ganglioside of small intestine. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2520–2524. doi: 10.1073/pnas.72.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson E., Lönnroth I., Lange S., Jonson I., Jennische E., Lönnroth C. Molecular cloning and expression of a pituitary gland protein modulating intestinal fluid secretion. J Biol Chem. 1995 Sep 1;270(35):20615–20620. doi: 10.1074/jbc.270.35.20615. [DOI] [PubMed] [Google Scholar]
- Just I., Wilm M., Selzer J., Rex G., von Eichel-Streiber C., Mann M., Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995 Jun 9;270(23):13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S., Lönnroth I., Palm A., Hydén H. The effect of antisecretory factor on the permeability of nerve cell membrane to chloride ion. Pflugers Arch. 1987 Dec;410(6):648–651. doi: 10.1007/BF00581326. [DOI] [PubMed] [Google Scholar]
- Lange S., Lönnroth I., Skadhauge E. Effects of the antisecretory factor in pigs. Pflugers Arch. 1987 Jul;409(3):328–332. doi: 10.1007/BF00583485. [DOI] [PubMed] [Google Scholar]
- Lowe J., Mayer R. J. Ubiquitin, cell stress and diseases of the nervous system. Neuropathol Appl Neurobiol. 1990 Aug;16(4):281–291. doi: 10.1111/j.1365-2990.1990.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Lundgren O., Svanvik J., Jivegård L. Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci. 1989 Feb;34(2):264–283. doi: 10.1007/BF01536062. [DOI] [PubMed] [Google Scholar]
- Lyerly D. M., Saum K. E., MacDonald D. K., Wilkins T. D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985 Feb;47(2):349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth I., Jennische E. Reversal of enterotoxic diarrhoea by anaesthetic and membrane-stabilizing agents. Acta Pharmacol Toxicol (Copenh) 1982 Oct;51(4):330–335. doi: 10.1111/j.1600-0773.1982.tb01033.x. [DOI] [PubMed] [Google Scholar]
- Lönnroth I., Lange S., Skadhauge E. The antisecretory factors: inducible proteins which modulate secretion in the small intestine. Comp Biochem Physiol A Comp Physiol. 1988;90(4):611–617. doi: 10.1016/0300-9629(88)90675-5. [DOI] [PubMed] [Google Scholar]
- Qiu B., Pothoulakis C., Castagliuolo I., Nikulasson Z., LaMont J. T. Nitric oxide inhibits rat intestinal secretion by Clostridium difficile toxin A but not Vibrio cholerae enterotoxin. Gastroenterology. 1996 Aug;111(2):409–418. doi: 10.1053/gast.1996.v111.pm8690206. [DOI] [PubMed] [Google Scholar]
- Rapallino M. V., Cupello A., Lange S., Lönnroth I., Hydén H. Further studies on the effect of ASF factor on Cl- permeability across the Deiters' neurone plasma membrane. Int J Neurosci. 1989 Jun;46(3-4):93–95. doi: 10.3109/00207458908986244. [DOI] [PubMed] [Google Scholar]
- Reumaux D., Mézière C., Colombel J. F., Duthilleul P., Mueller S. Distinct production of autoantibodies to nuclear components in ulcerative colitis and in Crohn's disease. Clin Immunol Immunopathol. 1995 Dec;77(3):349–357. doi: 10.1006/clin.1995.1162. [DOI] [PubMed] [Google Scholar]
- Svensson B., Ekström P. A., Edström A. Okadaic acid and cultured frog sciatic nerves: potent inhibition of axonal regeneration in spite of unaffected Schwann cell proliferation and ganglionic protein synthesis. J Neurochem. 1995 Mar;64(3):1000–1007. doi: 10.1046/j.1471-4159.1995.64031000.x. [DOI] [PubMed] [Google Scholar]
- Tautz N., Meyers G., Thiel H. J. Processing of poly-ubiquitin in the polyprotein of an RNA virus. Virology. 1993 Nov;197(1):74–85. doi: 10.1006/viro.1993.1568. [DOI] [PubMed] [Google Scholar]
- Teneberg S., Lönnroth I., Torres López J. F., Galili U., Halvarsson M. O., Angström J., Karlsson K. A. Molecular mimicry in the recognition of glycosphingolipids by Gal alpha 3 Gal beta 4 GlcNAc beta-binding Clostridium difficile toxin A, human natural anti alpha-galactosyl IgG and the monoclonal antibody Gal-13: characterization of a binding-active human glycosphingolipid, non-identical with the animal receptor. Glycobiology. 1996 Sep;6(6):599–609. doi: 10.1093/glycob/6.6.599. [DOI] [PubMed] [Google Scholar]
- Torres J., Jennische E., Lange S., Lönnroth I. Clostridium difficile toxin A induces a specific antisecretory factor which protects against intestinal mucosal damage. Gut. 1991 Jul;32(7):791–795. doi: 10.1136/gut.32.7.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres J., Jennische E., Lange S., Lönnroth I. Enterotoxins from Clostridium difficile; diarrhoeogenic potency and morphological effects in the rat intestine. Gut. 1990 Jul;31(7):781–785. doi: 10.1136/gut.31.7.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka M., Asakura H., Hamada Y., Miura S., Kobayashi K., Morishita T., Morita A., Tsuchiya M. Inhibitory effect of somatostatin on cholera toxin-induced diarrhea and glycoenzyme secretion in rat intestine. Digestion. 1987;36(3):141–147. doi: 10.1159/000199411. [DOI] [PubMed] [Google Scholar]
- van Nocker S., Deveraux Q., Rechsteiner M., Vierstra R. D. Arabidopsis MBP1 gene encodes a conserved ubiquitin recognition component of the 26S proteasome. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):856–860. doi: 10.1073/pnas.93.2.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eichel-Streiber C., Boquet P., Sauerborn M., Thelestam M. Large clostridial cytotoxins--a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 1996 Oct;4(10):375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]